Abstract
Background
Sepsis is a major cause of morbidity and mortality, especially in critical care patients. Developing tools to identify patients who are at risk of poor outcomes and prolonged length of stay in intensive care units (ICUs) is critical, particularly in resource-limited settings.
Objectives
To determine whether the quick sequential organ failure assessment (qSOFA) score based on bedside assessment alone was a promising tool for risk prediction in low-resource settings.
Methods
A retrospective cohort of adult patients admitted to the intensive care unit (ICU) at Edendale Hospital in Pietermaritzburg, South Africa (SA), was recruited into the study between 2014 and 2018. The association of qSOFA with in-ICU mortality was measured using multivariable logistic regression. Discrimination was assessed using the area under the receiver operating characteristic curve and the additive contribution to a baseline model using likelihood ratio testing.
Results
The qSOFA scores of 0, 1 and 2 were not associated with increased odds of in-ICU mortality (adjusted odds ratio (aOR) 1.24, 95% confidence interval (CI) 0.86 - 1.79; p=0.26) in patients with infection, while the qSOFA of 3 was associated with in-ICU mortality in infected patients (aOR 2.82; 95% CI 1.91 - 4.16; p<0.001). On the other hand, the qSOFA scores of 2 (aOR 3.25; 95% CI 1.91 - 5.53; p<0.001) and 3 (aOR 6.26, 95% CI 0.38 - 11.62, p<0.001) were associated with increased odds of in-ICU mortality in patients without infection. Discrimination for mortality was fair to poor and adding qSOFA to a baseline model yielded a statistical improvement in both cases (p<0.001).
Conclusion
qSOFA was associated with, but weakly discriminant, for in-ICU mortality for patients with and without infection in a resource-limited, public hospital in SA. These findings add to the growing body of evidence that support the use of qSOFA to deliver low-cost, high-value critical care in resource-limited settings
Contributions of the study
This study expanded the data supporting the use of qSOFA in resource-limited settings beyond the emergency department or ward to include patients admitted to the ICU. Additionally, this study demonstrated stronger predictive abilities in a population of patients admitted with trauma without suspected or confirmed infection, thus providing an additional use of qSOFA as a risk-prediction tool for a broader population.
Keywords: sepsis, quick sequential organ failure assessment score (qSOFA), resource-limited setting, low- and middle-income countries (LMIC), global critical care
Background
The global burden of sepsis poses significant human and economic costs. Recent studies reported 31.5 million cases of sepsis, leading to 5.3 million sepsis-related deaths annually worldwide.[1–4] This burden is disproportionately felt in developing countries, which are often limited in access to both diagnostics and therapeutics.[5]
The third international consensus definitions for sepsis and septic shock (Sepsis-3) proposed the sequential (sepsis-related) organ failure assessment (SOFA) as an updated definition of sepsis to replace the systemic inflammatory response syndrome (SIRS; Sepsis-2) criteria. [6] Additionally, they introduced the quick sequential (sepsis-related) organ failure assessment score (qSOFA) as a novel risk score to identify patients with infection who are at risk of poor outcomes including in-hospital mortality and long intensive care unit (ICU) length of stay.[2–4] In the Sepsis-3 validation study for predicting in-hospital mortality, qSOFA was equivalent to SOFA and superior to SIRS among patients outside the ICU, and inferior to SOFA but equivalent to SIRS among patients in the ICU[4]. As with many risk predictors, qSOFA was initially derived and validated only in well-resourced settings.[3,4] In response, a large retrospective secondary analysis evaluated qSOFA in 10 developing countries across sub-Saharan Africa, Asia and the Americas, and reported that qSOFA was associated with hospital mortality but with variable predictive validity. [7] Other smaller studies have reported similar findings.[8,9]
qSOFA is of particular immediate interest for under-resourced settings because it relies on bedside physical exam findings alone, without requiring laboratory studies such as those needed for SOFA and SIRS calculations, among other risk scores which may be unavailable or prohibitively expensive. An inexpensive and accessible tool to guide risk stratification could prove useful in optimising allocation of scarce acute and critical care resources such as ICU beds, and in identifying patients at high risk for poor outcomes early in their treatment course.[10] The present study sought to further elucidate the use of qSOFA in resource-limited settings by examining its association with and discrimination for mortality in: (i) the South African public health system, a previously unstudied resource-limited setting; (ii) an ICU population; and (iii) in both infection and non-infection populations.
Methods
The integrated critical care electronic database is the first published multicentre database of its kind in South Africa (SA)[11,12]] and includes all referrals and admissions for ICU care at two public hospitals within the KwaZulu-Natal Department of Health. This study evaluated ICU admissions at Edendale Hospital, a regional hospital with 900 in-patient beds and one mixed medical-surgical ICU that admits adult and paediatric patients with any aetiology of critical illness and has a closed, high-intensity staffing model.
The integrated critical care electronic database and the study protocol were approved by the Biomedical Research Ethics Administration of the University of KwaZulu-Natal (ref. no. BCA211/14, BE156/19). The study protocol was approved by the Institutional Review Board of the University of Pennsylvania (ref. no. 824688).
All adult patients (≥18 years) admitted to the ICU at Edendale Hospital from September 2014 to August 2018 were included in this study.
A retrospective cohort study of the association and discrimination of qSOFA for in-ICU mortality was performed. For comparison, parallel analyses using the SIRS criteria were also performed.[13] Comparison with the full SOFA score was not possible owing to data availability. Both inferential and predictive analytical approaches were used to align this study with prior recent work evaluating qSOFA in resource-limited settings.[7]
The study population was stratified based on the presence or absence of confirmed or suspected infection. Confirmed or suspected infection was defined as a primary ICU admission diagnosis of infection, as recorded prospectively by the clinical team or an antibiotic order at the time of ICU admission. Antibiotic orders have been used in prior studies as a component of confirmed or suspected infection,[14,15] but culture orders, used to remove antibiotics other than for acute infection, were not currently available in the database. This potential source of bias is addressed in sensitivity analyses.
The primary exposure variable was qSOFA at the time of the ICU admission. qSOFA includes one point each for altered mental status (Glasgow Coma Score <15), systolic blood pressure ≤100 mmHg, and respiratory rate ≥22 breaths/minute. Data were collected at the time of ICU referral (e.g. from the emergency department (ED), wards or another hospital) and updated at the time of ICU admission. SIRS criteria included exposure in a secondary analysis. qSOFA and SIRS calculations used the worst values for each component collected during this referral to admission window. Owing to the low frequency of qSOFA scores of 0 (4.8%) and SIRS criteria of 0 (0.3%) in this ICU population, qSOFA scores and SIRS criteria of 0 and 1 were pooled as the reference levels.
The primary outcome was in-ICU mortality, defined as death in the ICU or a palliative discharge from the ICU. The database does not follow patients longitudinally after ICU discharge and, as a result, hospital and other longer-term outcomes were not available.[15]
To align with prior validation studies of qSOFA in developing countries, a priori covariates in baseline risk models included age, sex and HIV status.[7]
Descriptive statistical analyses were performed on the total cohort of patients admitted to the ICU. Multivariable logistic regression was used to evaluate the association with in-ICU mortality first of a baseline risk model including only the a priori covariates age, sex and HIV status, and then of the baseline risk model plus qSOFA score or SIRS criteria. Discrimination of each model was assessed using area under the receiver operating characteristic curve (AUROC). Finally, the additive contribution of qSOFA or SIRS was assessed by performing likelihood ratio (LR) testing between the baseline model and the baseline plus qSOFA or SIRS model. All of the above analyses were stratified by infection status.
The qSOFA exposure variable, in-ICU mortality outcome variable, and all adjustment variables had either complete data or were missing in less than 1.5% of patients, allowing for complete case analyses. SIRS criteria used in the secondary comparative analyses were missing in 14.6% of patients, driven almost entirely by the sole laboratory input of white blood cell count (missing in 14.6% of patients compared with 0.8 - 3.4% missing for the non-white blood cell count SIRS criteria). White blood cell count derangements are likely to correlate with derangements in the other SIRS criteria that were missing at low levels; thus, a complete case approach was performed for these secondary analyses.
To reduce the risk of misclassifying patients into the suspected or confirmed infection groups who were receiving antibiotics for peri-procedural prophylaxis but who did not have a true active or suspected infection, qSOFA analyses were repeated, further restricting the suspected or confirmed infection group to patients referred to the ICU by a medical rather than surgical service, and to patients with a primary ICU admission diagnosis of infection as recorded prospectively by the ICU team. All analyses were done using Stata v14.1 (StataCorp LP, USA).
Results
The study population characteristics and infection status are illustrated in Table 1.
Table 1. Study population characteristics by infection status (N=2 119).
| Characteristics |
Confirmed
or suspected infection (n=1 190), n(%)* |
No confirmed
or suspected infection (n=929), n(%)* |
| Age (years), mean (SD) | 40.7 (15.9) | 37.7 (15.6) |
| Female gender | 570 (47.9) | 378 (40.7) |
| Black race | 1 142 (96.0) | 869 (93.5) |
| Referring specialty | ||
| Surgical | 875 (73.5) | 684 (73.6) |
| Medical | 287 (24.1) | 217 (23.4) |
| Unknown | 28 (2.4) | 28 (3.0) |
| Pre-ICU hospital LOS, median days (IQR) | 1 (0 - 3) | 0 (0 - 1) |
| Invasive mechanical ventilation at time of ICU admission | 710 (59.7) | 518 (55.8) |
| HIV-positive | 321 (27.0) | 175 (18.8) |
| On HAART | 245 (76.3) | 143 (81.7) |
| qSOFA score at ICU admission | ||
| 0 - 1 | 412 (34.6) | 406 (43.7) |
| 2 | 509 (42.8) | 384 (41.3) |
| 3 | 257 (21.6) | 120 (12.9) |
| Missing | 12 (1.0) | 19 (2.1) |
| SIRS criteria at ICU admission | ||
| 0 - 1 criteria | 27 (2.3) | 35 (3.8) |
| 2 criteria | 184 (15.5) | 165 (17.8) |
| 3 criteria | 439 (36.9) | 345 (17.1) |
| 4 criteria | 382 (32.1) | 232 (25.0) |
| Missing | 158 (13.3) | 152 (16.4) |
| Sepsis | ||
| qSOFA score definition | 766 (64.4) | n/a |
| SIRS criteria definition | 1 005 (84.5) | n/a |
| ICU LOS, median days (IQR) | 2.6 (1.2 - 5.2) | 2.0 (1.0 - 4.3) |
| In-ICU mortality | 232 (19.5) | 110 (11.8) |
SD = standard deviation
ICU = intensive care unit
LOS = length of stay
IQR = interquartile range
qSOFA = quick sequential organ failure assessment
SIRS = systemic inflammatory response syndrome
* Unless otherwise specified
Association of qSOFA and in-ICU mortality among patients with confirmed or suspected infection
Unadjusted predicted in-ICU mortality based on qSOFA is shown in Fig. 1. qSOFA scores of 0, 1 and 2 were not associated with in-ICU mortality (adjusted odds ratio (aOR) 1.24; 95% confidence interval (CI) 0.86 - 1.79; p=0.26) in patients with confirmed or suspected infection, but a qSOFA score of 3 was associated with increased odds of in-ICU mortality in these patients (aOR 2.82; 95% CI 1.91 - 4.16; p<0.001) (Table 2).
Fig. 1.
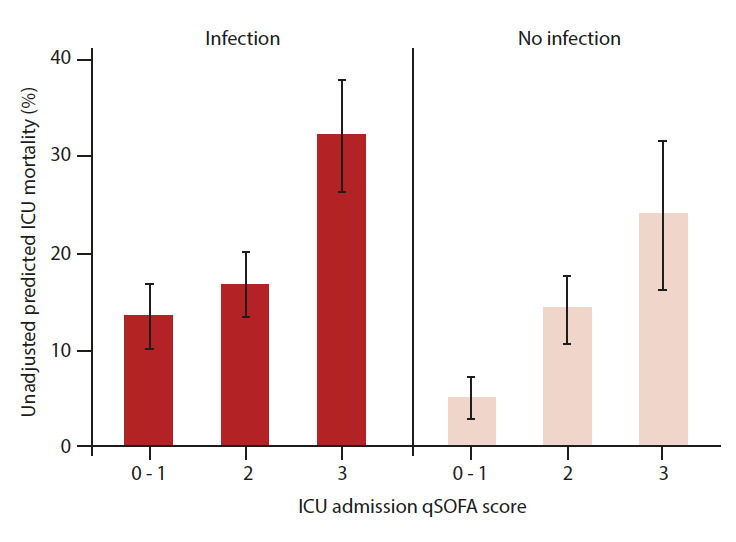
qSOFA score and ICU mortality by infection status
ICU = intensive care unit
qSOFA = quick sequential organ failure assessment
Table 2. Association of qSOFA with in-ICU mortality.
| Characteristics |
Confirmed
or suspected infection (n=1 175); aOR (95% CI), p-value |
No confirmed
or suspected infection (n=906); aOR (95% CI), p-value |
|
qSOFA
(Ref: 0 - 1 points) |
||
| 2 points | 1.24 (0.86 - 1.79), 0.26 | 3.25 (1.91 - 5.53), <0.001* |
| 3 points | 2.82 (1.91 - 4.16), <0.001* | 6.26 (3.38 - 11.62), <0.001* |
| Age | 1.02 (1.01 - 1.0), 0.001* | 1.03 (1.01 - 1.04), <0.001* |
| Male sex | 0.66 (0.49 - 0.90), 0.008* | 1.12 (0.72 - 1.74), 0.61 |
| HIV positive | 1.33 (0.95 - 1.86), 0.09 | 0.61 (0.33 - 1.15), 0.13 |
qSOFA = quick sequential organ failure assessment
aOR = adjusted odds ratio
CI = confidence interval
Discrimination for mortality was poor for both the baseline model (AUROC 0.61; 95% CI 0.58 - 0.65) and the baseline plus qSOFA model (AUROC 0.66; 95% CI 0.62 - 0.70), but the addition of qSOFA to the baseline model made a statistical improvement (LR test χ² 30.8; p<0.001) (Table 3, http://sajcc.org.za/public/sup/433.zip).
Table 3. qSOFA as a predictor of in-ICU mortality.
| AUROC (95% CI) | LR χ² (p-value) | |
|
Confirmed or
suspected infection (n=1 175) |
||
| Baseline factors | 0.61 (0.58 - 0.65) | 30.8 (<0.0001) |
| qSOFA + baseline factors | 0.66 (0.62 - 0.70) | |
|
No confirmed or
suspected infection(n=906) |
||
| Baseline factors | 0.61 (0.55 - 0.67) | 39.6 (<0.0001) |
| qSOFA + baseline factors | 0.71 (0.66 - 0.77) |
qSOFA = quick sequential organ failure assessment
AUROC = area under the receiver operating characteristic curve
CI = confidence interval
LR = likelihood ratio test
Association of qSOFA and in-ICU mortality among patients without confirmed or suspected infection
The adjusted for baseline risk factors qSOFA scores of 2 (aOR 3.25; 95% CI 1.91 - 5.53; p<0.001) and 3 (aOR 6.26; 95% CI 3.38 - 11.62; p<0.001) were associated with increased odds of in-ICU mortality in patients without confirmed or suspected infection (Table 2). Discrimination for mortality was poor for the baseline model (AUROC 0.61; 95% CI 0.55 - 0.67), fair for the baseline plus qSOFA model (AUROC 0.71; 95% CI 0.66 - 0.77) and the addition of qSOFA to the baseline model made a statistical improvement (LR test χ² 39.6; p<0.001) (Table 3, http://sajcc.org.za/public/sup/433.zip).
Association of SIRS and in-ICU mortality
In both the infection and non-infection subgroups, SIRS criteria were not statistically associated with in-ICU mortality in multivariable logistics regression adjusted for baseline risk factors. Discrimination for mortality was poor for both the baseline and the baseline plus SIRS models, and the addition of SIRS to the baseline model did not yield a statistical improvement (Tables 1 and 2)).
Sensitivity analyses
The qSOFA score was unable to discriminate mortality among patients with confirmed or suspected infection who were referred by a medical service (AUROC 0.63; 95% CI 0.56 - 0.71) and those who had infection as the primary ICU admission diagnosis (AUROC 0.66; 95% CI 0.60 - 0.71). However, the addition of qSOFA to the baseline model resulted in a statistically significant difference between both cases (Table 3).
Discussion
In this retrospective cohort study in a resource-limited public hospital in SA, qSOFA score was highly associated with, but weakly discriminant for in-ICU mortality, improved upon a baseline risk model, and was superior to SIRS criteria. These findings are consistent with prior multicentre retrospective studies in other sub-Saharan African countries.[7–9]
The present study expanded the data supporting the use of qSOFA in resource-limited settings in two notable ways: firstly, this study evaluated the performance of qSOFA for ICU patients at the time of ICU admission, rather than in patients in the ED or on the ward. The superior performance of qSOFA compared to SIRS in our setting differed from the Sepsis-3 validation studies which showed that qSOFA was superior to SIRS for ward patients but comparable with SIRS for ICU patients.[3,4] While ICUs in resource-limited settings may by definition have greater resources than nearby wards, they may still benefit from access to a well-performing risk predictor that does not require laboratory testing for use in guiding management decisions and allocating scarce resources, including ICU beds. Secondly, this study evaluated the performance of qSOFA for patients both with and without suspected or confirmed infection and showed even stronger predictive abilities in the non-infection population. The potential addition of qSOFA to the risk-prediction toolkit for all patients admitted to the ICU, including those admitted for trauma, who make up large proportions of admissions to typically mixed medical-surgical ICUs in resource-limited settings,[12] would be further beneficial.
The results of this study should be interpreted in the context of several important limitations. Firstly, this study was performed at a single hospital and the results should be interpreted within the greater body of emerging literature evaluating qSOFA at multiple facilities across diverse countries. Furthermore, South Africa is a developing country[16] that has immense income and wealth inequality and an under-resourced public healthcare system that serves a very low-income population,[17] so the results may not apply to extremely low-resourced settings where systems for critical care delivery are largely absent.
Secondly, because microbial culture orders are not available in the electronic database,[15] the primary definition of suspected or confirmed infection in this study, which includes patients with an antibiotic order alone at the time of ICU admission, certainly misclassifies some patients into the suspected or confirmed infection group who were receiving antibiotics for peri-procedural prophylaxis but who did not have a true active or suspected infection. This issue is addressed with sensitivity analyses using more specific definitions for infection that show a consistent but attenuated result, consistent with a stronger qSOFA-mortality relationship observed in non-infected patients.
Thirdly, while both inferential (association) and predictive analytic (discrimination) methods were used in an effort to align this study with recent studies evaluating qSOFA in resource-limited settings,[7] this approach could have produced over-fit models and provided overly optimistic performance assessments from a predictive standpoint. Results should be interpreted in this context and future studies may wish to adhere to a strictly predictive statistical framework.
Finally, this study could not make a useful comparison between qSOFA and the full SOFA score owing to unavailability or lack of laboratory data. Future studies should further compare qSOFA with other risk predictors, including those developed and validated in resource-limited settings such as the Rwanda mortality probability model (R-MPM).[10] Additionally, because this database does not follow patients longitudinally after ICU discharge, hospital and other longer-term outcomes were not explored.
Conclusion
The qSOFA was associated with, but weakly discriminant for, in-ICU mortality for patients both with and without infection. These findings add to the growing body of evidence that support the use of qSOFA to deliver low-cost, high-value critical care in resource-limited settings. Future studies should explore the validity of qSOFA in comparison with other mortality prediction models in both medical and surgical populations.
Acknowledgments
None.
References
- 1.Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 2.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:775–7787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob ST, Lim M, Banura P, et al. Integrating sepsis management recommendations into clinical care guidelines for district hospitals in resource-limited settings: The necessity to augment new guidelines with future research. BMC Medicine. 2013;11:107. doi: 10.1186/1741-7015-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/nejmc1506819. [DOI] [PubMed] [Google Scholar]
- 7.Rudd KE, Seymour CW, Aluisio AR, et al. Association of the quick sequential (sepsis-related) organ failure assessment (qSOFA) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA. 2018;319:2202–2211. doi: 10.1001/jama.2018.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huson MA, Kalkman R, Grobusch MP, van der Poll T. Predictive value of the qSOFA score in patients with suspected infection in a resource limited setting in Gabon. Travel Med Inf Dis. 2017;15:76–77. doi: 10.1016/j.tmaid.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Huson MAM, Katete C, Chunda L, et al. Application of the qSOFA score to predict mortality in patients with suspected infection in a resource-limited setting in Malawi. Infection. 2017;45:893–896. doi: 10.1007/s15010-017-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riviello ED, Kiviri W, Fowler RA, et al. Predicting mortality in low-income country ICUs: The Rwanda mortality probability model (R-MPM). PLoS One. 2016;11:e0155858. doi: 10.1371/journal.pone.0155858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allorto NL, Wise RD. Development and evaluation of an integrated electronic data management system in a South African metropolitan critical care service. South Afr J Anaesth Analg. 2015;21(6):173–177. doi: 10.1080/22201181.2015.1115607. [DOI] [Google Scholar]
- 12.Anesi GL, Gabler NB, Allorto NL, et al. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: A 2-Hospital Study in South Africa. J Intensive Care Med. 2018;p.088506661881580 doi: 10.1177/0885066618815804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;29(4):530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 14.Anesi GL, Liu VX, Gabler NB, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc. 2018;15:1328–1335. doi: 10.1513/annalsats.201804-241oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Filbin MR, Massaro AF, et al. Compliance with the national SEP-1 quality measure and association with sepsis outcomes: A multicentre retrospective cohort study. Crit Care Med. 2018;46:1585–1591. doi: 10.1097/ccm.0000000000003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Bank Country Lending Groups [Internet] The World Bank; https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 2 October 2019) [Google Scholar]
- 17.Naidoo S. The South African national health insurance: A revolution in healthcare delivery! J Public Health. 2012;34(1):149–150. doi: 10.1093/pubmed/fds008. [DOI] [PubMed] [Google Scholar]


