Abstract
Background
The prognosis of metastatic colorectal cancer (mCRC) depends on the metastatic site and systemic therapy regimen. Peritoneal metastases are associated with a relatively unfavorable prognosis among patients with mCRC. In this article, we present the treatment outcomes of patients with peritoneal carcinomatosis (PC)-only, liver metastasis (LiM)-only, and lung metastasis (LuM)-only CRC.
Methods
Overall, 206 mCRC patients with single-site metastasis and who had received treatment from January 2014 to December 2018 were recruited. Among 206 patients with mCRC, 15 had PC-only mCRC, 145 had LiM-only mCRC, and 46 had LuM-only mCRC. They attended regular follow-ups until November 2020, and the median follow-up period was 24.7 months (5.1–41.3 months). Patients’ characteristics, including clinical data, gene mutation profiles, and clinical outcomes, were evaluated. All patients with PC-only CRC were treated with first-line bevacizumab and FOLFIRI, and the irinotecan dose escalation depended on UGT1A1 polymorphism.
Results
Of the 206 patients, no statistical difference was observed between the PC-only, LiM-only, and LuM-only groups in terms of age, primary tumor location, RAS mutation status, BRAF mutation status, and epidermal growth factor receptor overexpression (all P > 0.05). Patients with PC-only CRC had a median progression-free survival (mPFS) of 18.0 months and a median overall survival (mOS) of 24.6 months. Patients with LiM-only or LuM-only CRC had mPFS of 18.2 and 26.6 months and mOS of 25.0 and 44.5 months, respectively. No significant differences regarding PFS and OS (both P > 0.05) between the three groups of patients with mCRC were observed.
Conclusion
Our study revealed that in patients with PC-only mCRC treatment of first-line bevacizumab and FOLFIRI through irinotecan dose escalation according to UGT1A1 polymorphism could confer such patients with comparable outcomes to that of patients with LiM-only and LuM-only mCRC.
Keywords: prognosis, metastatic colorectal cancer, peritoneal carcinomatosis-only, UGT1A1, irinotecan dose escalation
Background
Colorectal cancer (CRC) is a major cause of cancer-related deaths worldwide; over 1 million new cases are diagnosed annually.1,2 Approximately 20–25% of patients with CRC present with metastases at their initial diagnosis, and the most common sites of metastases from CRC are the liver, lung, and peritoneum.3 The prognosis of metastatic colorectal cancer (mCRC) depends on the specific metastatic sites involved; a combination of systemic therapy and metastasectomy could provide the best survival rate.4,5 Peritoneal carcinomatosis (PC)-only CRC has a poor prognosis and is often considered to be a terminal condition. A comparison between isolated site metastasis between peritoneum, liver, and lung metastases reported median overall survival (OS) periods of 16.3 months in PC-only CRC, 19.1 months in liver metastasis CRC, and 24.6 months in lung metastasis CRC.6
Treatment of mCRC has significantly improved in terms of progression-free survival (PFS) and OS due to the use of new chemotherapeutic and molecular target agents. The association between clinical outcomes and the gene mutations of the KRAS, NRAS, and BRAF genes has become more clearly understood. RAS mutation status has been reported to exhibit a significant association with the survival outcomes of patients with liver metastasis (LiM)-only mCRC.7
For patients with mCRC, the current first-line systemic therapy includes fluoropyrimidine-based doublet chemotherapy—folinic acid + fluorouracil + oxaliplatin (FOLFIRI) or folinic acid + fluorouracil + irinotecan (FOLFOX)—plus targeted therapy.3 A retrospective study reported that patients with mCRC who received escalated doses of irinotecan according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping revealed favorable clinical responses and oncological outcomes.8 Recently, our prospective, randomized study also suggested that if patients with mCRC, regardless of their KRAS gene status, receive UGT1A1 genotyping, they can tolerate escalated doses of irinotecan and potentially achieve a more favorable clinical outcome without significantly increased toxicity.9,12 Furthermore, the oncological outcomes of patients with BRAF-mutated mCRC treated using bevacizumab and FOLFIRI with escalated doses of irinotecan as a first-line treatment are acceptable with tolerable adverse events; this approach may be a feasible therapy option for such patients.10 In the current retrospective study, we investigated the clinical outcomes of patients with PC-only mCRC who received first-line bevacizumab and FOLFIRI with irinotecan dose escalation according to UGT1A1 polymorphism.
Methods
Patients’ Characteristics
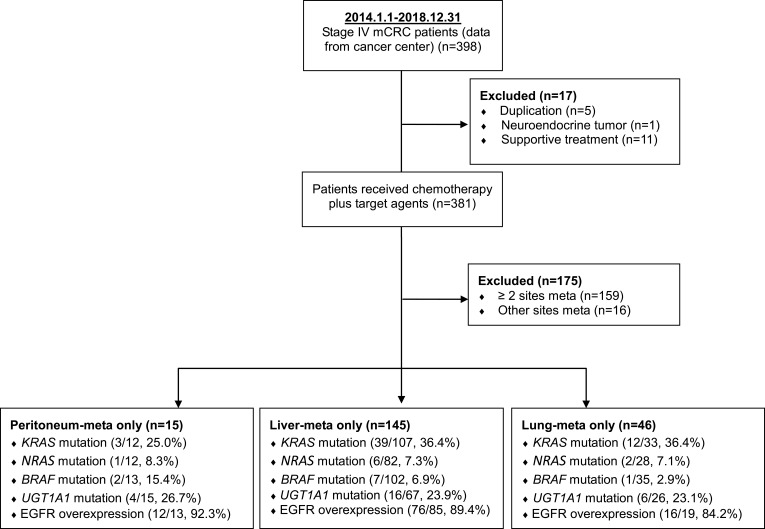
From a single institution, 398 patients with stage IV CRC who had received treatment between January 2014 and December 2018 were selected. Patients with record duplications (n = 5), neuroendocrine tumors (n = 1), or supportive treatment (n=11) were excluded, and those with two metastatic sites (n = 159) or other metastatic sites (n = 16) were also excluded. For the study, 15 (15/398, 3.8%) patients consecutively diagnosed as having PC-only CRC, 145 (145/398, 36.4%) patients diagnosed as having LiM-only CRC, and 46 (46/398, 11.6%) patients diagnosed were as having lung metastasis (LuM)-only CRC were recruited; the flow diagram of patient selection is presented in Figure 1. A case series analysis was performed using a routinely updated and maintained electronic medical record database. Demographic data included age at diagnosis, sex, primary tumor location, and gene mutation. All aspects of this study were approved by the institutional review board of Kaohsiung Medical University Hospital and complied with the Declaration of Helsinki. Finally, this study was a retrospective review of 206 patients with mCRC, and written informed consent was obtained from all patients (KMUHIRB-E(I)-20200036). The responses were classified by a radiologist according to RECIST Version 1.1.11 DNA extraction for direct sequencing of KRAS/NRAS and immunohistochemical (IHC) analysis of epidermal growth factor receptor (EGFR) expression were performed using the methods described in our previous study.9 FOLFIRI (folinic acid + fluorouracil + irinotecan) plus bevacizumab was the first-line treatment for patients with PC-only CRC, and irinotecan dose escalation was conducted according to UGT1A1 polymorphism. Patients with UGT1A1*1/*1 genotype, the initial dose of irinotecan is 180 mg/m2. If the adverse effects (AEs) are below grade 2, the dose will be gradually escalated in steps of 30 mg/m2. The estimated maximal dose of irinotecan is 260 mg/m2. With UGT1A1*1/*28 genotype, the initial dose of irinotecan is 180 mg/m2. The irinotecan dose will be increased in increments of 30 mg/m2 (180, 210, 240 mg/m2) every two cycles in the absence of grade 2 or worse AEs. The maximal dose of irinotecan will be 240 mg/m2. With UGT1A1*28/*28 genotype, the initial dose of irinotecan is 120 mg/m2. If the toxicities are below grade 2, the dose will be gradually escalated in steps of 30 mg/m2. The estimated maximal dose of irinotecan is 180 mg/m2.12
Figure 1.
CONSORT flow chart showing the 398 mCRC patients, which data were collected from cancer center (2014.1.1–2018.12.31).
Oncological Follow-Up
These patients were followed up for a median of 24.7 months (range, 5.1–41.3 months). Follow-ups included physical examinations and carcinoembryonic antigen measurements every 3 months for the first 2 years and twice a year thereafter. A computed tomography (CT) scan of the abdomen and thorax was arranged every 3 months for the first 3 years and every 3–6 months thereafter. We performed colonoscopies within 1 year after surgery and held follow-ups at 3-year intervals. Magnetic resonance imaging and positron emission CT were only used when necessary.
Statistical Analysis
Data were analyzed using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). PFS and OS rates were plotted using the Kaplan–Meier method. PFS was defined as the time from the start date of treatment until the date of any type of progression or the final follow-up, and OS was defined as the time from the beginning of treatment to death from any cause or to the final follow-up. PFS and OS were evaluated using the Kaplan–Meier method, and the Log rank test was used to compare time-to-event distributions. Statistical significance was set at P < 0.05.
Results
Patient Series, Tumor Characteristics, and Mutation Status
In total, 206 patients with single-site mCRC were included in the analysis. Of these, 15 (15/206, 7.3%) patients were subsequently diagnosed as having PC-only CRC, 145 (145/206, 70.4%) were were diagnosed as having LiM-only CRC, and 46 (46/206, 22.3%) were diagnosed as having LuM-only CRC. The median age was 65.0 years (range, 37–71 years) in the PC-only group, 62.0 years (range, 26–90 years) in the LiM-only group, and 65.5 years (range, 41–86 years) in the LuM-only group. The status of LiM-only or LuM-only patients was not related to primary tumor location (P = 0.191). Moreover, in 109 (75.1%) patients with LiM-only CRC and 33 (71.7%) patients with LuM-only CRC, the primary tumors originated from the left colon. A comparable rate of occurrence from the right or left colon in patients with PC-only CRC was observed; the primary tumors of eight (53.3%) patients with PC-only CRC originated from the left colon.
Genotyping of KRAS, NRAS, BRAF, and EGFR as well as overexpression analysis revealed no significant differences in metastatic site (Table 1). KRAS and NRAS mutations were evident in respectively 3 patients (25.0%) and 1 (8.3%) patient with PC-only CRC, 39 (36.4%) and 6 (7.3%) patients with LiM-only CRC, and 12 (36.4%) and 2 (7.1%) in patients with LuM-only CRC. BRAF and EGFR overexpression was respectively evident in 2 (15.4%) and 12 (92.3%) patients with PC-only CRC, 7 (6.9%) and 76 (89.4%) patients with LiM-only CRC, and 1 patient (2.9%) and 16 (84.2%) patients with LuM-only CRC. FOLFIRI (folinic acid + fluorouracil + irinotecan) plus bevacizumab was the first-line treatment for patients with PC-only CRC, and irinotecan dose escalation was conducted according to UGT1A1 polymorphism.8,12
Table 1.
Patients Characteristics at Diagnosis and Genes Mutation Profiles (n=206)
| Characteristic | Peritoneal Meta Only (n=15) | Liver Meta Only (n=145) | Lung Meta Only (n=46) | P value |
|---|---|---|---|---|
| Median age (years) | 65.0 (37–71) | 62.0 (26–90) | 65.5 (41–86) | 0.110 |
| Gender (M:F) | 11:4 | 90:55 | 20:26 | 0.041 |
| Primary tumor location | 0.191 | |||
| Right side | 7 | 36 | 13 | |
| Left side | 8 | 109 | 33 | |
| KRAS mutation | 3/12, 25.0% | 39/107, 36.4% | 12/33, 36.4% | 0.730 |
| NRAS mutation | 1/12, 8.3% | 6/82, 7.3% | 2/28, 7.1% | 0.991 |
| BRAF mutation | 2/13, 15.4% | 7/102, 6.9% | 1/35, 2.9% | 0.300 |
| EGFR over expression | 12/13, 92.3% | 76/85, 89.4% | 16/19, 84.2% | 0.741 |
| UGT1A1 mutant (*1/*28) | 4/15, 26.7% | 16/67, 23.9% | 6/26, 23.1% | 0.737 |
| PFS (months) | 18.0 | 18.2 | 26.6 | 0.194 |
| OS (months) | 24.6 | 25.0 | 44.5 | 0.191 |
Notes: Six TA repeats representing the most common allele of the UGT1A1 gene (UGT1A1*1, wild-type) and seven TA repeats representing a variant allele (UGT1A1*28, mutant type).
Abbreviations: EGFR, epidermal growth factor receptor; UGT1A1, uridine diphosphate glucuronosyI transferase 1A1; PFS, progression-free survival; OS, overall survival.
Survival Evaluation
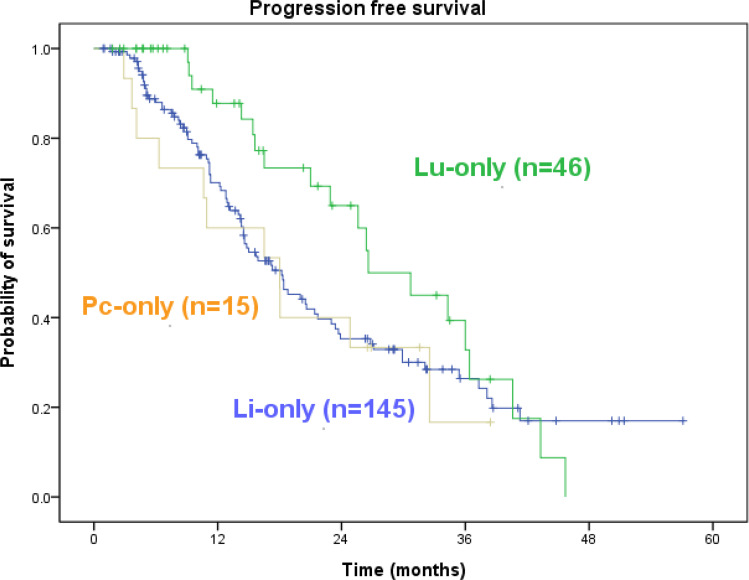
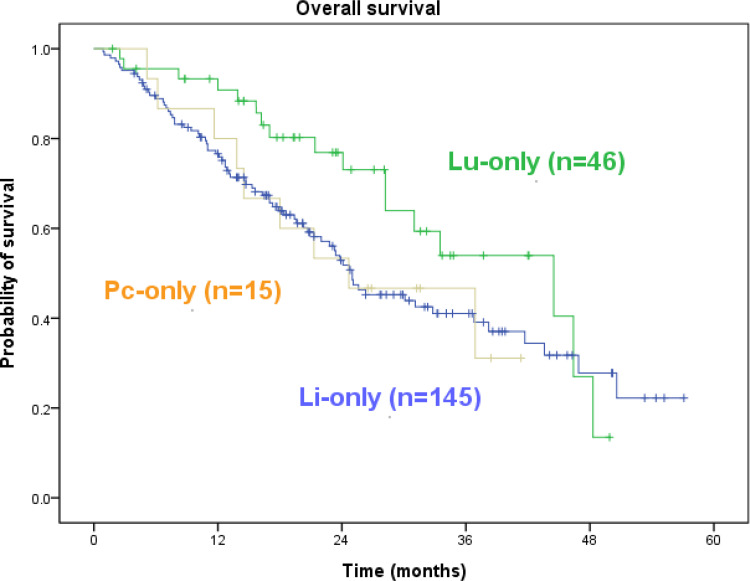
In patients with PC-only CRC, the median PFS was 18.0 months (range, 3.7–38.4 months), and the median OS was 24.6 months (range, 5.1–41.3 months). Among the 15 patients with PC-only CRC, six remained alive at the final follow-up visit conducted in November 2020. During the same period, the PFS of those with LiM-only and LuM-only CRC was 18.2 months and 26.6 months, and the OS of those with LiM-only and LuM-only CRC was 25.0 months and 44.5 months. Patients with PC-only mCRC did not have significantly inferior PFS or OS rates compared with patients with LiM-only or LuM-only mCRC (Figures 2 and 3, both P > 0.05).
Figure 2.
Kaplan–Meier survival analysis. Progression-free survival of all 206 cases. Pc-only vs Li-only vs Lu-only = 18.0 vs 18.2 vs 26.6 (p=0.194).
Figure 3.
Kaplan–Meier survival analysis. Overall survival of all 206 cases. Pc-only vs Li-only vs Lu-only = 24.6 vs 25.0 vs 44.5 (p=0.191).
Discussion
Approximately 20% of patients with CRC had metastases at their initial diagnosis. Furthermore, in almost 40% of the remaining patients, their (initially limited) diseases progressed to metastases during treatment.13 The tumor cell entrapment hypothesis explained how peritoneal metastases occurred through the shedding, binding, migration, and survival of cancer cells. They develop largely through transcoelomic spread, with a sequence of events that allow cells to first detach from primary tumours, survive in the peritoneal environment, attach to the peritoneal surface of organs and migrate into the submesothelial space to create a microenvironment conducive to metastatic growth.14 Peritoneal metastasis is associated with higher risks of death from all causes, and the reported median OS after 5-fluorouracil-only systemic chemotherapy is 5 to 7 months15,16 and varies between 13 and 34 months, depending on the chemotherapeutic and molecular targeting agents used.17–19 Because the plasma–peritoneal barrier reduces intraperitoneal drug penetration, systemic chemotherapy for PC-only CRC has only limited efficacy. According to a meta-analysis, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) provided survival benefits for select patients with PC from CRC.20 The meta-analysis of these 76 studies indicated that the median OS was approximately 29 months in the CRS plus HIPEC group. However, the patients were selected according to their favorable performance status and were able to receive surgical intervention, and the mean mortality and morbidity rates of those in the HIPEC program were 2.8% and 33.0%, respectively. In 2021, Quénet et al reported both the absence of an OS benefit after the addition of HIPEC to CRS and more frequent late postoperative complications; they suggested that CRS alone should be the cornerstone of therapeutic strategies with curative intent for colorectal peritoneal metastases.21 In our study, the median PFS and OS rates of 15 PC-only CRC patients given first-line treatment of FOLFIRI plus bevacizumab were 18.0 and 24.6 months, respectively, and irinotecan dose escalation depended on UGT1A1 polymorphism.
Irinotecan must be converted to 7-ethyl-10-hydroxycamptothecin (SN-38) by a carboxylesterase, and 7-ethyl-10-hydroxycamptothecin is actively cytotoxic and is detoxified by glucuronidation activity of uridine diphosphate glucuronosyltransferase (UGT). The number of repeats in the TATA box of the UGT1A1 promoter alters UGT1A1 activity, with six TA repeats representing the most common allele of the UGT1A1 gene (UGT1A1*1, wild-type) and seven TA repeats representing a variant allele (UGT1A1*28, mutant type). Therefore, the development of drug-associated adverse events (AEs) would be represented by the UGT1A1 genotype. In a genotype-directed irinotecan dose-escalating trial in mCRC patients given first-line FOLFIRI, patients with homozygous UGT1A1*28/*28 developed irinotecan-associated grade 3 and 4 AEs more frequently that did patients with UGT1A1*1/*1 or *1/*28 genotype.22 By contrast, the recommended initial irinotecan dose of 180 mg/m2 was generally well-tolerated in patients with UGT1A1*1/*1 or *1/*28, who received high doses of irinotecan to achieve a favorable objective response rate (ORR) without major toxicity.23–25 According to the pan-Asian-adapted European Society for Medical Oncology (ESMO) consensus guidelines, patients with UGT1A1 homozygous wild *1/*1 and heterozygous *1/*28 genotype can tolerate high-dose irinotecan without notable adverse events.26 In our research, 11 of the 15 patients with PC-only CRC had the UGT1A1*1/*1 genotype; three patients with the UGT1A1*1/*1 genotype received escalated irinotecan doses up to 260 mg/m2, and four patients with the UGT1A1*1/*1 genotype received escalated irinotecan doses up to 210 or 240 mg/m2 (Table 2).
Table 2.
Demographic and Clinicopathological Characteristics of 15 Patients with Peritoneal Metastases Only (N = 15)
| Case No. | Sex | Age (Years) | Performance Status | Gene Mutation | UGT1A1 Genotype | Irinotecan Dose Escalation | PFS (Months) | OS (Months) | Survival (Yes Or No) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | 1 | *1/*1 | 240 mg/m2 | 10.6 | 14.5 | No | |
| 2 | M | 65 | 0 | *1/*1 | 180 mg/m2 | 10.9 | 21.3 | No | |
| 3 | M | 66 | 1 | *1/*1 | 240 mg/m2 | 4.1 | 13.8 | No | |
| 4 | M | 44 | 0 | *1/*1 | 260 mg/m2 | 18.0 | 18.0 | No | |
| 5 | M | 37 | 0 | BRAF codon V600E mutation | *1/*1 | 240 mg/m2 | 6.3 | 11.6 | No |
| 6 | M | 73 | 0 | *1/*1 | 210 mg/m2 | 3.7 | 5.1 | No | |
| 7 | M | 55 | 0 | *1/*1 | 180 mg/m2 | 38.4 | 38.4 | Yes | |
| 8 | M | 77 | 1 | KRAS codon 12 mutation | *1/*28 | 180 mg/m2 | 32.5 | 41.3 | Yes |
| 9 | F | 71 | 1 | KRAS codon 12 mutation | *1/*28 | 150 mg/m2 | 26.5 | 26.5 | Yes |
| 10 | M | 64 | 1 | KRAS codon 13 mutation | *1/*1 | 195 mg/m2 | 18.0 | 36.9 | No |
| 11 | F | 65 | 1 | *1/*1 | 260 mg/m2 | 24.8 | 31.2 | Yes | |
| 12 | F | 60 | 0 | NRAS codon 12 mutation | *1/*28 | 180 mg/m2 | 16.5 | 36.9 | No |
| 13 | M | 68 | 0 | *1/*28 | 180 mg/m2 | 31.6 | 31.6 | Yes | |
| 14 | F | 50 | 0 | *1/*1 | 260 mg/m2 | 26.9 | 26.9 | Yes | |
| 15 | M | 83 | 1 | BRAF codon V600E mutation | *1/*1 | 180 mg/m2 | 2.9 | 6.2 | No |
Notes: Six TA repeats representing the most common allele of the UGT1A1 gene (UGT1A1*1, wild-type) and seven TA repeats representing a variant allele (UGT1A1*28, mutant type).
Abbreviations: Performance Status evaluation with the ECOG (Eastern Cooperative Oncology Group) scale; PFS, progression-free survival; OS, overall survival.
In patients with mCRC, the respective 5-year survival rates of those with resectable hepatic, pulmonary, and peritoneal metastases were approximately 60%, 40%, and 20%,27–31 respectively, and peritoneal metastases were associated with a 20% reduction in PFS and a 30% reduction in OS compared with nonperitoneal metastatic mCRC.32 Our results demonstrated that clinical outcomes were improved by escalated doses of irinotecan according to UGT1A1 polymorphisms in patients with PC-only mCRC treated with first-line FOLFIRI and bevacizumab; the median PFS and OS of patients with PC-only mCRC were 18.0 months and 24.6 months, respectively. No significant differences regarding PFS and OS (both P > 0.05) among the three groups of patients with mCRC were observed. Overall, in the present study, patients with PC-only mCRC had comparable PFS and OS to patients with LiM-only or LuM-only CRC. However, ours was only an observational study with a relatively small sample size; future investigations could have a longer follow-up and larger sample sizes to confirm the findings of our study.
Conclusions
Although chemotherapy regimens with new chemotherapeutic and molecular targeting agents improved mCRC outcomes, patients with PC-only mCRC were still considered to have poor clinical outcomes. In summary, our research suggest that patients with PC-only mCRC treated with first-line FOLFIRI plus bevacizumab, and given irinotecan dose escalation according to UGT1A1 polymorphism might obtain outcomes comparably favorable to those of patients with LiM-only or LuM-only mCRC. Further prospective randomized trials and comparison studies based on PC status should be conducted, and further research on the molecular and genetic profile may help patients with PC-only mCRC.
Funding Statement
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 109-2314-B-037-035, MOST 109-2314-B-037-040, MOST 109-2314-B-037-046-MY3, MOST110-2314-B-037-097) and the Ministry of Health and Welfare (MOHW109-TDU-B-212-134026, MOHW109-TDU-B-212-114006, MOHW110-TDU-B-212-1140026) and funded by the health and welfare surcharge of on tobacco products, and the Kaohsiung Medical University Hospital (KMUH110-0R37, KMUH110-0R38, KMUH110-0M34, KMUH110-0M35, KMUH110-0M36, KMUHSA11013, KMUH-DK(C)110010, KMUH-DK(B)110004-3) and KMU Center for Cancer Research (KMU-TC111A04-1) and KMU Center for Liquid Biopsy and Cohort Research Center Grant (KMU-TC109B05) and KMU Office for Industry-Academic Collaboration (S109036), Kaohsiung Medical University. In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, R.O.C.
Abbreviations
CRC, colorectal cancer; mCRC, metastatic colorectal cancer; PC, peritoneal carcinomatosis; LiM, liver metastasis; LuM, lung metastasis; OS, overall survival; PFS, progression-free survival; FOLFOX, folinic acid + fluorouracil + oxaliplatin; FOLFIRI, folinic acid + fluorouracil + irinotecan; UGT1A1, uridine diphosphate glucuronosyltransferase 1A1; IHC, immunohistochemical; CT, computed tomography; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; UGT, uridine diphosphate glucuronosyltransferase; SN-38, 7-ethyl-10-hydroxycamptothecin; AEs, adverse events; ORR, objective response rate; ESMO, European Society for Medical Oncology; UGT1A1*1, six TA repeats representing the most common allele of the UGT1A1 gene (wild-type); UGT1A1*28, seven TA repeats representing a variant allele of the UGT1A1 gene (mutant type).
Ethics Approval and Consent to Participate
All aspects of this study were approved by the institutional review board of Kaohsiung Medical University Hospital. Finally, this study was a retrospective review of 206 patients with mCRC, and written informed consent was obtained from all patients (KMUHIRB-E(I)-20200036).
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Author Contributions
All authors have reviewed and approved submission of the final version of the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
- 1.World Health Organization. Cancer today. Available from: https://gco.iarc.fr/today/home. Accessed February 1, 2021.
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Zarour LR, Anand S, Billingsley KG, et al. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):163–173. doi: 10.1016/j.jcmgh.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN. Colon cancer NCCN 2020 ver. 4; 2020.
- 5.Chang TK, Tsai HL, Su WC, et al. The clinicopathological variables to differentiate the nature of isolated pulmonary nodules in patients who received curative surgery for colorectal cancer. Asian J Surg. 2019;42(2):425–432. doi: 10.1016/j.asjsur.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Franko J. Therapeutic efficacy of systemic therapy for colorectal peritoneal carcinomatosis: surgeon’s perspective. Pleura Peritoneum. 2018;3(1):20180102. doi: 10.1515/pp-2018-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang SC, Huang CW, Chen YT, et al. Effect of KRAS and NRAS mutations on the prognosis of patients with synchronous metastatic colorectal cancer presenting with liver-only and lung-only metastases. Oncol Lett. 2020;20(3):2119–2130. doi: 10.3892/ol.2020.11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu CY, Huang CW, Hu HM, et al. Prognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line setting. Transl Res. 2014;164(2):169–176. doi: 10.1016/j.trsl.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 9.Tsai HL, Huang CW, Lin YW, et al. Determination of the UGT1A1 polymorphism as guidance for irinotecan dose escalation in metastatic colorectal cancer treated with first-line bevacizumab and FOLFIRI (PURE FIST). Eur J Cancer. 2020;138:19–29. doi: 10.1016/j.ejca.2020.05.031 [DOI] [PubMed] [Google Scholar]
- 10.Hsieh YC, Chang TK, Su WC, et al. UGT1A1 polymorphism for irinotecan dose escalation in patients with BRAF-mutated metastatic colorectal cancer treated with first-line bevacizumab and FOLFIRI. J Oncol. 2021;2021. doi: 10.1155/2021/6686517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 12.Yeh YS, Tsai HL, Huang CW, et al. Prospective analysis of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab plus FOLFIRI as the first-line setting: study protocol for a randomized controlled trial. Trials. 2016;17:46. doi: 10.1186/s13063-016-1153-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: potential place in therapy. Drugs. 2013;73(9):883–891. doi: 10.1007/s40265-013-0076-5 [DOI] [PubMed] [Google Scholar]
- 14.Narasimhan V, Ooi G, Michael M, Ramsay R, Lynch C, Heriot A. Colorectal peritoneal metastases: pathogenesis, diagnosis and treatment options - an evidence-based update. ANZ J Surg. 2020;90(9):1592–1597. doi: 10.1111/ans.15796 [DOI] [PubMed] [Google Scholar]
- 15.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x [DOI] [PubMed] [Google Scholar]
- 16.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–222. doi: 10.1097/01.sla.0000197702.46394.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized Phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–1355. doi: 10.1093/annonc/mdu141 [DOI] [PubMed] [Google Scholar]
- 18.Kerscher AG, Chua TC, Gasser M, et al. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer. 2013;108(7):1432–1439. doi: 10.1038/bjc.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240–2247. doi: 10.1200/JCO.2013.53.2473 [DOI] [PubMed] [Google Scholar]
- 20.Huang CQ, Min Y, Wang SY, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–55683. doi: 10.18632/oncotarget.17497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quénet F, Elias D, Roca L, et al.; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266. doi: 10.1016/S1470-2045(20)30599-4 [DOI] [PubMed] [Google Scholar]
- 22.Marcuello E, Páez D, Paré L, et al. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer. 2011;105(1):53–57. doi: 10.1038/bjc.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charles SF, John M, José B. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26(4):689–690. doi: 10.1200/JCO.2007.15.5390 [DOI] [PubMed] [Google Scholar]
- 24.Lu CY, Huang CW, Wu IC, et al. Clinical Implication of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab combined with FOLFIRI in the first-line setting. Transl Oncol. 2015;8(6):474–479. doi: 10.1016/j.tranon.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David P, María T, Julen FP, et al. Pharmacogenetic clinical randomised phase II trial to evaluate the efficacy and safety of FOLFIRI with high-dose irinotecan (HD-FOLFIRI) in metastatic colorectal cancer patients according to their UGT1A 1 genotype. Br J Cancer. 2019;120(2):190–195. doi: 10.1038/s41416-018-0348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738 [DOI] [PubMed] [Google Scholar]
- 27.Nordlinger B, Sorbye H, Glimelius B, et al.; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722. doi: 10.1097/01.sla.0000160703.75808.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yedibela S, Klein P, Feuchter K, et al. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol. 2006;13(11):1538–1544. doi: 10.1245/s10434-006-9100-2 [DOI] [PubMed] [Google Scholar]
- 30.Okumura S, Kondo H, Tsuboi M, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg. 1996;112(4):867–874. doi: 10.1016/S0022-5223(96)70085-5 [DOI] [PubMed] [Google Scholar]
- 31.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–3762. doi: 10.1002/cncr.25116 [DOI] [PubMed] [Google Scholar]
- 32.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]