Abstract
The activities of cefotaxime and minocycline against Aeromonas hydrophila were investigated. Cefotaxime (4 times the MIC) plus minocycline (0.75 times the MIC) elicited an inhibitory effect for 48 h in a time-kill study, and more infected mice treated with both drugs survived (91%) than survived after treatment with cefotaxime (9%) or minocycline (44%) alone, suggesting that cefotaxime and minocycline act synergistically against A. hydrophila.
Although not common isolates from the clinical microbiology laboratory, Aeromonas species can cause bacteremia (4, 5, 8, 9, 11), spontaneous bacterial peritonitis (11), and invasive soft-tissue infections (6) in immunocompromised hosts. The outcome of invasive Aeromonas infection usually is poor. Despite the empirical administration of a β-lactam agent active in vitro with or without an aminoglycoside, 36% of 59 episodes of Aeromonas bacteremia were associated with fatality in the hospital (9). Cefotaxime, a broad-spectrum cephalosporin, is active in vitro against Aeromonas species, but there is a potential risk of emergence of derepressed mutants during β-lactam therapy for Aeromonas infections (10, 15). The combination of cefotaxime and minocycline has been demonstrated to be synergistic against Vibrio vulnificus in vitro (2) and in experiments with mice (3). Such a regimen has not been studied for invasive Aeromonas infections, and thus the in vitro activity of cefotaxime and minocycline in combination against Aeromonas hydrophila and the therapeutic potential of combination therapy in murine Aeromonas infections were examined.
Clinical bacteremic strain A136 from a patient with a fire burn was used. It was susceptible to cefotaxime (MIC by E test: 0.75 μg/ml) and minocycline (MIC by E test: 4 μg/ml), as previously described (10). Nineteen clinical strains of A. hydrophila HG 1 were randomly collected from National Cheng Kung University Hospital. The susceptibility testing of the isolates was done by the agar dilution method (13). The MICs for the surviving subpopulation of A136 after 48 h of coculture with antibiotics were determined by E-test strips (AB Biodisk, Solna, Sweden). The method of the time-kill study was as previously described (2). Briefly, the overnight bacterial suspension was diluted to 5 × 105 CFU/ml in 50 ml of fresh Luria-Bertani (LB) broth. The suspensions containing various drug concentrations were incubated at 35°C. Bacterial counts were measured at 2, 4, 8, 12, 24, and 48 h on LB agar. All the experiments were performed at least twice.
A136 was incubated in Mueller-Hinton broth overnight, and after 3 h of incubation in sterile broth, the pellet obtained after centrifugation was diluted to the anticipated turbidity for mouse experiments. The antibiotic suspensions prepared from commercial vials of cefotaxime and minocycline were freshly diluted and were injected into the peritoneums of inbred BALB/c mice weighing 20 g on average and 5 to 6 weeks old. Following intraperitoneal injection of A136, mice develop severe peritonitis with fatal sepsis if untreated (7). Therefore, cefotaxime at 150 mg/kg of body weight every 6 h, minocycline at 20 mg/kg every 12 h, and both in combination were given 2 h after the intraperitoneal bacterial inoculation. Antibiotics were administered for 48 h. In the first 72 h after the initiation of Aeromonas infection, the number of surviving mice was recorded four times daily. There were two control groups. Mice in one group were infected by A136 without treatment; those in the other group were not infected and received cefotaxime, minocycline, or both in combination intraperitoneally. For comparison of categorical variables, the χ2 test or the two-tailed Fisher exact test was employed and a P value <0.05 was considered to be statistically significant.
The MICs of cefotaxime, minocycline, and tetracycline for 20 clinical strains and a control strain, Escherichia coli ATCC 25922, are shown in Table 1. The MICs of cefotaxime (0.5 μg/ml) and minocycline (2 μg/ml) by the agar dilution method were one dilution variation from or identical to those determined by E tests (0.75 and 4 μg/ml, respectively). There were no colonies grown within the zones of inhibition around E-test strips. However, the MICs for A136 of cefotaxime and minocycline were 16 and 2 μg/ml, respectively, by the broth dilution method. For the clone obtained from the broth with A136 and cefotaxime at 0.75 or 3 μg/ml alone or in combination with minocycline at 3 μg/ml after 48 h of incubation, the MIC of cefotaxime was >256 μg/ml and the MIC of minocycline was the same as that for the initial strain (Table 2). The phenotypic expression of cefotaxime resistance was stable even after repeated passage of resistant subpopulations. In contrast, the MICs of cefotaxime for the A136 subpopulation after coculture with 3 μg of minocycline/ml did not increase but the MIC of minocycline increased fourfold.
TABLE 1.
MICs of cefotaxime, minocycline, and tetracycline determined by agar dilution for 20 clinical strains of A. hydrophila HG 1, including A136, and E. coli ATCC 25922
| Antimicrobial agent | MIC (μg/ml)a for:
|
|||
|---|---|---|---|---|
|
A. hydrophila
|
E. coli ATCC 25922 | |||
| Range | 50% | 90% | ||
| Cefotaxime | <0.03–128 | <0.03 | 128 | 0.06 |
| Minocycline | 1–4 | 4 | 4 | 1 |
| Tetracycline | 1–16 | 8 | 16 | 2 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
TABLE 2.
MICs for A136 surviving subpopulation isolated after 48-h cocultures with antimicrobial agent
| Antimicrobial agent (concn [μg/ml]) | MIC (μg/ml)a of:
|
|
|---|---|---|
| Cefotaxime | Minocycline | |
| No antibiotic exposure | 0.75 | 4 |
| Cefotaxime (0.75) | >256 | 4 |
| Cefotaxime (3) | >256 | 4 |
| Minocycline (3) | 0.5 | 16 |
| Cefotaxime (0.75) + minocycline (3) | >256 | 4 |
| Cefotaxime (3) + minocycline (3) | >256 | 4 |
MICs were determined by E test.
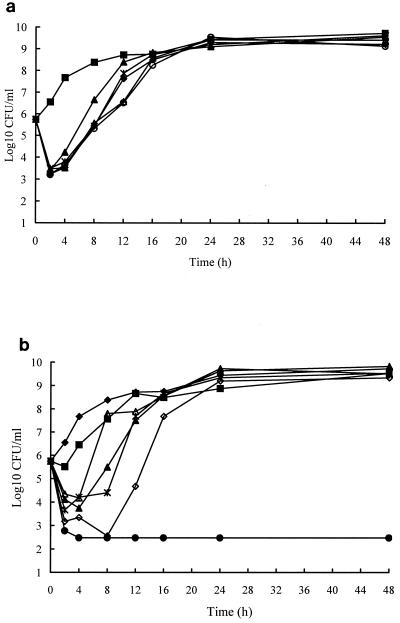
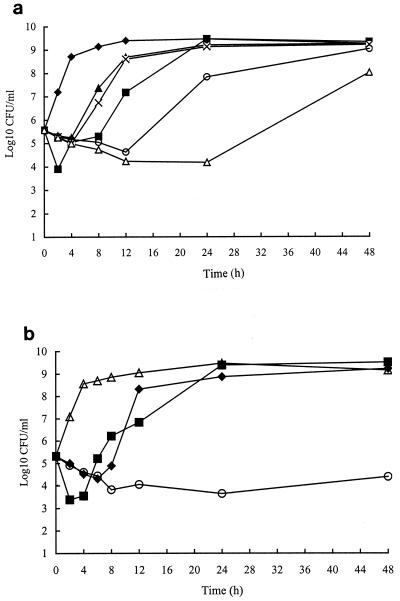
In time-kill studies, cefotaxime ranging from 0.37 to 6 μg/ml exhibited an inhibitory effect for 2 h and thereafter the bacteria began to regrow (Fig. 1a). In contrast, with minocycline concentrations at less than three times of the MIC before 24 h, the magnitude and duration of the inhibitory effect were proportional to the minocycline concentration, but sustained bactericidal activity was found until 48 h with a minocycline concentration of 12 μg/ml, three times the MIC (Fig. 1b). Minocycline at 3 μg/ml combined with cefotaxime at 0.75 μg/ml resulted in a reduction of viable bacterial colonies by at least 2 orders of magnitude at 24 h (Fig. 2a), compared with either drug alone, but regrowth occurred after 24 h. However, a higher cefotaxime concentration, 3 μg/ml, combined with minocycline at 3 μg/ml caused a reduction by at least 4 orders of magnitude compared to either of the two drugs alone (Fig. 2b).
FIG. 1.
Time-kill curves for A. hydrophila A136, with cefotaxime (a) ranging from 0.37 to 6 μg/ml (■, control; ▴, 0.37 μg/ml; ⧫, 0.75 μg/ml; ✻, 1.5 μg/ml; ○, 3 μg/ml; ▵, 6 μg/ml) and minocycline (b) ranging from 0.75 to 12 μg/ml (⧫, control; ■, 0.75 μg/ml; ▵, 1.5 μg/ml; ▴, 2 μg/ml; ✻, 4 μg/ml; ◊, 6 μg/ml; ●, 12 μg/ml).
FIG. 2.
Time-kill curves for A. hydrophila A136 with cefotaxime (CTX) and minocycline (MIN). (a) ⧫, control; ■, CTX at 0.75 μg/ml; ▴, MIN at 2 μg/ml; ×, MIN at 3 μg/ml; ○, CTX at 0.75 μg/ml plus MIN at 2 μg/ml; ▵, CTX at 0.75 μg/ml plus MIN at 3 μg/ml. (b) ▵, control; ■, CTX at 3 μg/ml; ⧫, MIN at 3 μg/ml; ○, CTX at 3 μg/ml plus MIN at 3 μg/ml.
All mice infected with A136 without antibiotic therapy died within 24 h. Intraperitoneal administration of cefotaxime, minocycline, or a combination of the two for 48 h did not cause mortality or a decline in physical activity. With the same intraperitoneal inoculum and regular antimicrobial therapy initiated 2 h after bacterial inoculation, mice treated with cefotaxime and minocycline were more likely to survive than those treated with cefotaxime or minocycline alone (Table 3).
TABLE 3.
Numbers of surviving mice observed at 5 days in four groups with or without 48 h of antimicrobial therapya
| Bacterial inoculum (CFU); no. of mice | No. of mice (%)b surviving at 5 days after treatment withc:
|
|||
|---|---|---|---|---|
| CTX, 150 mg/kg, i.p. q6h | MIN, 20 mg/kg, i.p. q12h | CTX, 150 mg/kg, i.p. q6h + MIN, 20 mg/kg, i.p. q12h | Control | |
| 3.8 × 107; 10 | 0 | 6 | 10 | 0 |
| 3.9 × 107; 10 | 1 | 3 | 9 | 0 |
| 4.5 × 107; 12 | 2 | 5 | 10 | 0 |
| Total | 3 (9.4) | 14 (43.8) | 29 (90.6) | 0 (0) |
Mice were infected by intraperitoneal (i.p.) injection of A. hydrophila A136.
For treatment with cefotaxime (CTX) versus treatment with minocycline (MIN), P = 0.001; for treatment with MIN versus treatment with CTX plus MIN, P < 0.0001; for treatment with CTX versus treatment with CTX plus MIN, P < 0.00001.
q6h, every 6h; q12h, every 12 h.
Rapid regrowth of A136 occurred even with cefotaxime at a concentration of eight times the MIC. Cefotaxime-resistant (MIC, >256 μg/ml) and minocycline-susceptible (MIC, 4 μg/ml) Aeromonas strains were isolated from the broth containing A136 and a subinhibitory concentration of cefotaxime alone or combined with minocycline after 48 h of coculture. These findings suggest that a preexisting resistant subpopulation would be selected by cefotaxime alone and could be killed in the presence of cefotaxime and minocycline. Given the rapid regrowth of A136 in the broth containing 6 μg of cefotaxime/ml and the discrepancy between the cefotaxime MICs by the broth dilution method and the agar dilution method, it is likely that the biomass contacting the antimicrobial agent in broth studies is larger than that in agar plates and thus the trend of selection of resistant clones would be more evident in broth studies. Minocycline has been shown to be capable of inhibition of total protein and β-lactamase synthesis in β-lactamase-producing Staphylococcus aureus (1). The in vitro effect of minocycline on β-lactamase production in gram-negative bacteria was not reported. The hypothesis that the inhibition of β-lactamase production by minocycline contributes to the incremental activity of cefotaxime against Aeromonas remains plausible.
The peak serum cefotaxime concentrations after administration of 100 and 200 mg/kg to mice were 94 and 180 μg/ml (14), respectively, which are close to 100- and 200-μg/ml serum levels in humans after parenteral administration of 1 (15 mg/kg) and 2 g (30 mg/kg), respectively, of cefotaxime (12). According to the relevant pharmacokinetics data in mice, the dosage of cefotaxime (150 mg/kg) in our study could achieve the same serum drug levels in mice as the recommended dosage (30 mg/kg) for children did. The pharmacokinetics information for minocycline in mice was very limited in that the dosage of minocycline, 20 mg/kg, that we used in the present experiment with mice was five times the usual dosage for children (4 mg/kg), as was the selected cefotaxime dosage.
The mouse model of Aeromonas peritonitis has been clearly demonstrated to cause invasive infections in mice (7), mimicking Aeromonas bacteremia in humans as our control mice with bacterial inoculation alone died within 24 h. With this murine model of Aeromonas infection, the therapeutic superiority of the combination regimen was found. Therefore, such a combination regimen can be the empirical treatment for suspected Vibrio or Aeromonas necrotizing fasciitis in cases with severe soft-tissue infections following exposure to contaminated water or marine creatures.
Acknowledgments
This study was supported by a grant (NSC 88-2314-B-006-027) from the National Science Council, Taiwan, Republic of China.
We thank Yu V.L. for his critical review of the manuscript.
REFERENCES
- 1.Chopra I, Anderson M A. Inhibition of β-lactamase synthesis in Staphylococcus aureus by minocycline. J Antimicrob Chemother. 1985;16:17–21. doi: 10.1093/jac/16.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Chuang Y C, Liu J W, Ko W C, Lin K Y, Wu J J, Huang K Y. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob Agents Chemother. 1997;41:2214–2217. doi: 10.1128/aac.41.10.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang Y C, Ko W C, Wang S T, Liu J W, Kuo C F, Wu J J, Huang K Y. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob Agents Chemother. 1998;42:1319–1322. doi: 10.1128/aac.42.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duthie D, Ling T W, Cheng A F B, French G L. Aeromonas septicaemia in Hong Kong: species distribution and associated disease. J Infect. 1995;30:241–244. doi: 10.1016/s0163-4453(95)90808-0. [DOI] [PubMed] [Google Scholar]
- 5.Funada H, Matsuda T. Aeromonas bacteremia in patients with hematologic diseases. Intern Med. 1997;36:171–174. doi: 10.2169/internalmedicine.36.171. [DOI] [PubMed] [Google Scholar]
- 6.Gold W I, Salit I E. Aeromonas hydrophila infections of skin and soft tissue: report of 11 cases. Clin Infect Dis. 1993;16:69–74. doi: 10.1093/clinids/16.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Janda J M, Clark R B, Brenden R. Virulence of Aeromonas species as assessed through mouse lethality studies. Curr Microbiol. 1985;12:163–168. [Google Scholar]
- 8.Janda J M, Guthertz L S, Kokka R P, Shimada T. Aeromonas species in septicemia: laboratory characteristics and clinical observations. Clin Infect Dis. 1994;19:77–83. doi: 10.1093/clinids/19.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Ko W C, Chuang Y C. Aeromonas bacteremia: review of 59 episodes. Clin Infect Dis. 1995;20:1298–1304. doi: 10.1093/clinids/20.5.1298. [DOI] [PubMed] [Google Scholar]
- 10.Ko W C, Wu H M, Chang T C, Yan J J, Wu J J. Inducible β-lactam resistance in Aeromonas hydrophila: therapeutic challenge for antimicrobial therapy. J Clin Microbiol. 1998;36:3188–3192. doi: 10.1128/jcm.36.11.3188-3192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko W C, Lee H C, Chuang Y C, Liu C C, Wu J J. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteremia. J Infect. 2000;40:267–273. doi: 10.1053/jinf.2000.0654. [DOI] [PubMed] [Google Scholar]
- 12.Luthy R, Blaser J, Bonetti A, Simmen H, Wise R, Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981;20:567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing for bacteria that grow aerobically, 4th ed. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Soriano F, Ponte C, Nieto E, Parra A. Correlation of in-vitro activity and pharmacokinetic parameters with in-vivo effect of amoxycillin, co-amoxiclav and cefotaxime in a murine model of pneumococcal pneumonia. J Antimicrob Chemother. 1996;38:227–236. doi: 10.1093/jac/38.2.227. [DOI] [PubMed] [Google Scholar]
- 15.Walsh T R, Stunt R A, Nabl J A, MacGowan A P, Bennett P M. Distribution and expression of β-lactamase genes among Aeromonas spp. J Antimicrob Chemother. 1997;40:171–178. doi: 10.1093/jac/40.2.171. [DOI] [PubMed] [Google Scholar]