Abstract
The Pseudomonas aeruginosa nfxB mutant lacking mexAB-oprM showed hypersusceptibility to 9 out of 24 β-lactams tested. This hypersusceptibility was found for the nfxB mutant lacking mexAB-oprM-mexXY (N108) but not for the nfxB mutant lacking both mexAB-oprM-mexXY and ampC. The level of the AmpC β-lactamase induction was reduced in N108. Thus, the reduced AmpC induction must be the cause of the hypersusceptibility.
A series of multicomponent efflux systems, each made up of three components, play important roles in the intrinsic and acquired resistance of gram-negative bacteria (20, 21, 22). Four of these efflux systems, MexAB-OprM (11, 24), MexCD-OprJ (25), MexEF-OprN (9), and MexXY-OprM (1, 19, 26), have been characterized for Pseudomonas aeruginosa. MexAB-OprM and MexXY-OprM contribute to both intrinsic and acquired resistance, whereas MexCD-OprJ and MexEF-OprN contribute to only acquired resistance. MexAB-OprM is slightly expressed in wild-type strains, and a nalB mutation causes an overexpression of the efflux system. The expression of MexCD-OprJ and MexEF-OprN is strictly suppressed in wild-type strains, and mutations in nfxB and nfxC cause overexpression of MexCD-OprJ and MexEF-OprN, respectively. The expression of MexXY, which is not detectable in the wild-type strain, is induced by several antimicrobial agents such as tetracycline, erythromycin, and gentamicin (17). MexXY is associated with OprM and contributes to the intrinsic resistance to these agents. While nfxB mutants show resistance to quinolones, tetracycline, erythromycin, chloramphenicol, and expanded-spectrum cephems such as cefpirome, they show hypersusceptibility to penicillins, carbapenems, and aminoglycosides (15). A characterization of mutants lacking the mexAB-oprM region demonstrated that the hypersusceptibility to β-lactams such as carbenicillin and aztreonam is caused by the reduced expression of MexAB-OprM in the nfxB mutants (4).
To investigate whether the hypersusceptibility of the nfxB mutants to β-lactams is generally attributable to this mechanism, in this study, we compared the susceptibilities of the isogenic mutants, i.e., the MexCD-OprJ-producing KG2259 (ΔMexAB-OprM of COR6 [4]) and the non-MexCD-OprJ-producing KG2239 (ΔMexAB-OprM of PAO1 [4]), to the 24 β-lactams. Table 1 shows the MICs determined by the usual twofold agar dilution technique with Mueller-Hinton II agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.). The results indicated that these β-lactams can be classified into three groups. The first group, which consists of piperacillin, cloxacillin, nafcillin, cefpirome, cefepime, cefozopran, and cefoselis, showed 8- to 64-fold-lower activities against KG2259 than against KG2239. The second group, which consists of penicillin G, cefuroxime, cefmetazole, ceftazidime, cefsulodin, meropenem, S-4661 (7), and aztreonam, showed almost the same activities against KG2239 and KG2259. The third group, which consists of sulbenicillin, cefpodoxime, ceftriaxone, moxalactam, flomoxef, imipenem, panipenem, biapenem, and R-95867 (an active form of a new oral carbapenem, CS-834 [3]), showed 4- to 32-fold-higher activities when MexCD-OprJ was expressed and MexAB-OprM was not. These results suggest that there is at least one other mechanism responsible for the hypersusceptibility to the third group of β-lactams independent of the decreased expression of MexAB-OprM with accompanying expression of MexCD-OprJ.
TABLE 1.
Susceptibilities of isogenic MexAB-OprM mutants with or without MexCD-OprJ expression
| Group and antimicrobial agent | MIC (μg/ml) for straina:
|
|
|---|---|---|
| KG2239 | KG2259 | |
| Group I | ||
| Piperacillin | 0.25 | 2 |
| Cloxacillin | 256 | >4,096 |
| Nafcillin | 32 | 256 |
| Cefpirome | 1 | 16 |
| Cefepime | 0.06 | 4 |
| Cefozopran | 0.13 | 8 |
| Cefoselis | 0.5 | 4 |
| Group II | ||
| Penicillin G | 2,048 | 2,048 |
| Cefuroxime | 512 | 256 |
| Cefmetazole | 2,048 | 1,024 |
| Ceftazidime | 0.25 | 0.5 |
| Cefsulodin | 0.25 | 0.5 |
| Meropenem | 0.13 | 0.25 |
| S-4661 | 0.25 | 0.13 |
| Aztreonam | 0.06 | 0.13 |
| Group III | ||
| Sulbenicillin | 2 | 0.25 |
| Cefpodoxime | 1,024 | 64 |
| Ceftriaxone | 64 | 8 |
| Moxalactam | 8 | 1 |
| Flomoxef | 8,192 | 1,024 |
| Imipenem | 1 | 0.13 |
| Panipenem | 4 | 1 |
| Biapenem | 0.25 | 0.03 |
| R-95867 | 16 | 0.5 |
KG2239 was MexAB-OprM deficient. KG2259 produced MexCD-OprJ but was MexAB-OprM deficient.
The deletion of mexXY from KG2239 (N103 [17]) and KG2259 (N108 [18]) did not affect their susceptibilities to the third group of β-lactams, whereas the deletion of mexCD-oprJ from N108 (KG4507 [N. Gotoh, unpublished data]) eliminated these hypersusceptibilities (Table 2). These results suggest that MexCD-OprJ expression is directly related to hypersusceptibility.
TABLE 2.
Susceptibilities of isogenic strains to β-lactams
| Strain | Genotypea
|
MIC (μg/ml) of drugb:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nfxB | ABM | XY | CDJ | ampC | SBPC | CPD | CRO | MOX | FMOX | IPM | PAPM | BIPM | R-95867 | |
| KG2239 | W | − | + | + | + | 2 | 1,024 | 64 | 8 | 8,192 | 1 | 4 | 0.25 | 16 |
| KG2259 | M | − | + | + | + | 0.25 | 64 | 8 | 1 | 1,024 | 0.13 | 1 | 0.03 | 0.5 |
| N103 | W | − | − | + | + | 2 | 1,024 | 64 | 8 | 8,192 | 1 | 4 | 0.25 | 16 |
| N108 | M | − | − | + | + | 0.25 | 128 | 8 | 1 | 1,024 | 0.25 | 1 | 0.03 | 0.5 |
| KG4507 | M | − | − | − | + | 2 | 1,024 | 64 | 8 | 8,192 | 1 | 4 | 0.25 | 16 |
| N106 | W | − | − | + | − | 0.25 | 0.25 | 0.25 | 0.5 | 0.13 | 0.13 | 0.13 | 0.06 | 0.06 |
| N119 | M | − | − | + | − | 0.25 | 32 | 1 | 0.5 | 2 | 0.06 | 0.25 | 0.03 | 0.25 |
nfxB, wild type (W) or mutated (M); ABM, mexAB-oprM possessing (+) or deficient (−); XY, mexXY possessing (+) or deficient (−); CDJ, mexCD-oprJ possessing (+) or deficient (−); ampC, ampC possessing (+) or deficient (−).
SBPC, sulbenicillin; CPD, cefpodoxime; CRO, ceftriaxone; MOX, moxalactam; FMOX, flomoxef; IPM, imipenem; PAPM, panipenem; BIPM, biapenem.
Given that OprJ and other outer membrane components of the multicomponent efflux systems of P. aeruginosa are assumed to form a channel, expression of OprJ might enhance the permeability of the P. aeruginosa outer membrane to the agents. To examine this hypothesis, we introduced an OprJ expression plasmid into N103. Although we confirmed the expression of OprJ by immunoblot assay using an OprJ-specific antibody (5), the susceptibilities of these strains to the agents were not affected by this expression (data not shown).
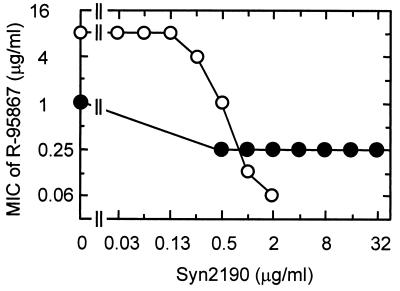
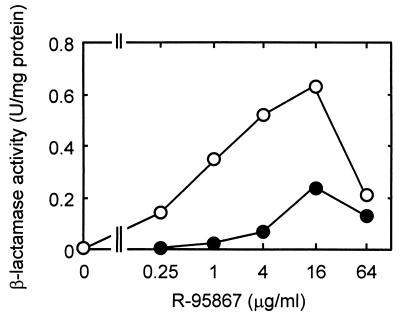
We previously demonstrated that the chromosomal AmpC β-lactamase acts as one of the factors causing the intrinsic resistance of P. aeruginosa to β-lactams via its interplay with the efflux system (16). To examine the possibility that the AmpC β-lactamase is involved in hypersusceptibility, we compared the susceptibilities of N119 (ampC::Ω of N108 [18]) and N106 (ampC::Ω of N103 [18]). N108 was 8- to 32-fold more susceptible than was N103 to R-95867, cefpodoxime, ceftriaxone, and flomoxef, whereas N119 was 4- to 128-fold less susceptible than N106 to these agents (Table 2). We also evaluated the susceptibilities of N103 and N108 to R-95867 in the presence of various concentrations of Syn2190 (23), an AmpC β-lactamase inhibitor. MICs of Syn2190 were >4,096, 8, and 128 μg/ml against PAO1, N103, and N108, respectively. Figure 1 shows the effect of subinhibitory concentrations of Syn2190 on the susceptibilities of N103 and N108. The susceptibility of N103 to R-95867 increased as the concentration of Syn2190 increased, while the subinhibitory concentrations of Syn2190 had little effect on the susceptibility of N108 to R-95867. Syn2190 did not induce β-lactamase activity at 0.03 to 2 μg/ml in N103 and at 0.5 to 128 μg/ml in N108, although it did induce slight β-lactamase activity at the higher concentrations in N103 (data not shown). The presence of the intact ampC gene imparted R-95867 resistance to N103 (compared with N106) but not to N108 (compared with N119) (Table 2). In addition, the inhibition of AmpC β-lactamase had little effect on the susceptibility of N108, a strain that produces MexCD-OprJ (Fig. 1). Since these results suggested a defect in the AmpC expression of N108, we examined the β-lactamase activity induced by R-95867 in N103 and N108. These strains were incubated with various concentrations (0.25 to 64 μg/ml) of R-95867 for 1 h, and the induced β-lactamase was quantified by a spectrophotometric assay with 50 μM cephaloridine used as a substrate, as described previously (14). N108 produced a lower amount of β-lactamase than did N103 (Fig. 2), suggesting that the decreased level of AmpC expression is the cause of the hypersusceptibility to R-95867 in the MexCD-OprJ-producing strain.
FIG. 1.
Effect of Syn2190 on susceptibilities of P. aeruginosa N103 (○) and N108 (●) to R-95867. MICs were determined by the microdilution broth method.
FIG. 2.
Induction of β-lactamase in P. aeruginosa N103 (○) and N108 (●) by R-95867.
Although R-95867 is stable in response to hydrolysis by AmpC (3), a synergistic effect between the slow inactivation of the agent by AmpC and the low level of permeability of the outer membrane might contribute to the resistance in P. aeruginosa, imparting the same mode of resistance seen when this organism is exposed to imipenem and meropenem (12, 16). The nfxB mutant showed hypersusceptibility to only certain kinds of β-lactams (15) (Table 2). A balance between the reduced expression of AmpC and the extrusion of each β-lactam by MexCD-OprJ might determine the phenotype, i.e., hypersusceptibility or resistance. The induction mechanism of AmpC β-lactamase has been well studied for Enterobacter cloacae (2, 6, 8). The inhibition of cell wall synthesis by β-lactam results in the accumulation of precursors, N-acetylglucosamyl-1,6-anhydromuropeptides, in the periplasm. These precursors are transported via AmpG into the cytoplasm, where they are converted into 1,6-anhydromuropeptides by a cytosolic β-N-acetylglucosaminidase. The 1,6-anhydromuropeptides convert the transcriptional regulator AmpR into an activator of AmpC expression. Given that AmpG and AmpR were also reported for P. aeruginosa (10, 13), a similar induction mechanism must be present in P. aeruginosa. MexCD-OprJ might extrude some of the N-acetylglucosamyl-1,6-anhydromuropeptides or 1,6-anhydromuropeptides and reduce the β-lactamase expression. In a previous paper (15), we reported that nfxB mutants isolated from β-lactamase-deficient strains were also more susceptible than were their parent strains to carbenicillin, imipenem, moxalactam, and aztreonam. This discrepancy can be explained by the MexAB-OprM expression of the β-lactamase-deficient strains. The reduced level of MexAB-OprM must have caused the hypersusceptibility of the nfxB mutants isolated from the AmpC-deficient strains.
Our results suggest that the reduction of AmpC induction is the cause of the hypersusceptibility, although further experiments are needed to elucidate the mechanism of the reduction of AmpC induction.
Acknowledgments
This research was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Aires J R, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz H, Pfeifle D, Wiedemann B. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob Agents Chemother. 1997;41:2113–2120. doi: 10.1128/aac.41.10.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuoka T, Ohya S, Utsui Y, Domon H, Takenouchi T, Koga T, Masuda N, Kawada H, Kakuta M, Kubota M, Ishii C, Ishii C, Sakagawa E, Harasaki T, Hirasawa A, Abe T, Yasuda H, Iwata M, Kuwahara S. In vitro and in vivo antibacterial activities of CS-834, a novel oral carbapenem. Antimicrob Agents Chemother. 1997;41:2652–2663. doi: 10.1128/aac.41.12.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotoh N, Tsujimoto H, Nomura A, Okamoto K, Tsuda M, Nishino T. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1998;165:21–27. doi: 10.1111/j.1574-6968.1998.tb13122.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanson N D, Sanders C C. Regulation of inducible AmpC β-lactamase expression among Enterobacteriaceae. Curr Pharm Des. 1999;5:881–894. [PubMed] [Google Scholar]
- 7.Iso Y, Irie T, Nishino Y, Motokawa K, Nishitani Y. A novel 1 beta-methylcarbapenem antibiotic, S-4661. Synthesis and structure-activity relationships of 2-(5-substituted pyrrolidin-3-ylthio)-1-beta-methylcarbapenems. J Antibiot (Tokyo) 1996;49:199–209. doi: 10.7164/antibiotics.49.199. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs C, Frère J-M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;886:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 9.Köhler T, Michéa-Hamzepour M, Henze U, Gotoh N, Curty L K, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 10.Langaee T Y, Gagnon L, Huletsky A. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob Agents Chemother. 2000;44:583–589. doi: 10.1128/aac.44.3.583-589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livermore D M. Interplay of impermeability and chromosomal β-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:2046–2048. doi: 10.1128/aac.36.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodge J, Busby S, Piddock L. Investigation of the Pseudomonas aeruginosa ampR gene and its role at the chromosomal ampC promoter. FEMS Microbiol Lett. 1993;111:315–320. doi: 10.1111/j.1574-6968.1993.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 14.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda N, Gotoh N, Ohya S, Nishino T. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:909–913. doi: 10.1128/aac.40.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda N, Gotoh N, Ishii C, Sakagawa E, Ohya S, Nishino T. Interplay between chromosomal β-lactamase and the MexAB-OprM efflux system in intrinsic resistance to β-lactams in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:400–402. doi: 10.1128/aac.43.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Contribution of the MexX-MexY-OprM system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:2242–2246. doi: 10.1128/aac.44.9.2242-2246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:3322–3327. doi: 10.1128/aac.44.12.3322-3327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis. 1998;27:S32–S41. doi: 10.1086/514920. [DOI] [PubMed] [Google Scholar]
- 22.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishida K, Kunugita C, Uji T, Higashitani F, Hyodo A, Unemi N, Maiti S N, Phillips O A, Spevak P, Atchison K P, Salama S M, Atwal H, Micetich R G. In vitro and in vivo activities of Syn2190, a novel β-lactamase inhibitor. Antimicrob Agents Chemother. 1999;43:1895–1900. doi: 10.1128/aac.43.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 26.Westbrock-Wadman S, Sherman D R, Hickey M J, Coulter S N, Zhu Y Q, Warrener P, Nguyen L Y, Shawar R M, Folger K R, Stover C K. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–2983. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]