Abstract
Growing evidence indicates that hyperinflammatory syndrome and cytokine storm observed in COVID-19 severe cases are narrowly associated with the disease’s poor prognosis. Therefore, targeting the inflammatory pathways seems to be a rational therapeutic strategy against COVID-19. Many anti-inflammatory agents have been proposed; however, most of them suffer from poor bioavailability, instability, short half-life, and undesirable biodistribution resulting in off-target effects. From a pharmaceutical standpoint, the implication of COVID-19 inflammation can be exploited as a therapeutic target and/or a targeting strategy against the pandemic. First, the drug delivery systems can be harnessed to improve the properties of anti-inflammatory agents and deliver them safely and efficiently to their therapeutic targets. Second, the drug carriers can be tailored to develop smart delivery systems able to respond to the microenvironmental stimuli to release the anti-COVID-19 therapeutics in a selective and specific manner. More interestingly, some biosystems can simultaneously repress the hyperinflammation due to their inherent anti-inflammatory potency and endow their drug cargo with a selective delivery to the injured sites.
Keywords: SARS-CoV-2, Inflammation, Drug delivery, Nanomedicine, Nanodecoys, Bioresponsive, Biomimetics, Mesenchymal stem cells
Graphical abstract
Drug delivery systems can be harnessed to fight against SARS-CoV-2 induced hyperinflammation by improving the properties of anti-inflammatory agents, smartly responding to the inflammatory site to selectively deliver their cargo, and may possess inherent anti-inflammatory potency.
1. Introduction
As of February 16th,2022, about 41661409 infection cases of the novel coronavirus disease 2019 (COVID-19) have been reported on https://www. worldometers.info, including 5859611 deaths and 71278547 infected patients around the world. COVID-19 infection is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single positive-strand RNA virus belonging to β coronaviruses. The pathogenesis of the virus is mainly mediated by its envelope (E), membrane (M) and nucleocapsid (N) structural proteins, as well as its spike (S) glycoprotein facilitating its cell entry through the binding to angiotensin-converting enzyme-2 (ACE2) receptors highly expressed on pulmonary epithelial cells [[1], [2], [3], [4]]. Although the pathogenesis of this disease is still not completely understood, growing evidence indicates that a dysregulated inflammatory syndrome is narrowly associated with COVID-19 severity and poor prognosis [[5], [6], [7], [8]]. In some cases, the SARS-CoV-2 may induce an exaggerated immune response, resulting in an overproduction of pro-inflammatory mediators resulting in acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation, and multi-organ failure [7,9].
To prevent SARS-CoV-2 infection, several vaccine candidates are currently in use around the world, such as Pfizer-BioNTech (USA/Germany), Moderna (USA), Johnson/Janssen (USA), Sinopharm(China), Sinovac (China), AstraZeneca (UK), and Gamaleya (Russia) [10,11]. Also, multiple treatments, including antimalarial agents (hydroxychloroquine and its analog chloroquine), antiviral drugs (remdesivir, lopinavir-ritonavir, favipiravir), interferons (IFNs), azithromycin, angiotensin-converting enzyme inhibitors (captopril, enalapril), as well as convalescence plasma transfusion, have shown positive clinical responses at the early phases of SARS-CoV-2 infection.However, most of them lack efficiency in targeting the late-stage COVID-19-associated cytokine storm [[12], [13], [14], [15]]. Therefore, besides preventing or blocking the virus entry, more focus should be on the attenuation of COVID-19-induced hyperinflammation. For this purpose, many immunomodulators and anti-inflammatory drugs have been proposed, including corticosteroids, interleukin-6 or interleukin-1 antagonists, anti-TNF-α agents, and Janus kinase inhibitors. Still, no therapies have succeeded in circumventing the exacerbated immune response in severe COVID-19 patients [[16], [17], [18]]. This may be partly attributed to the complexity of cytokine interactions and the multiplicity of inflammatory pathways orchestrated by many molecules, rendering the inhibition of one or a few of them not enough to reverse the inflammatory syndrome. Also, this may be ascribed to the lack of specificity, stability, and undesirable kinetics profiles of these agents [19]. Therefore, together with identifying new therapeutic targets and testing different anti-inflammatory agents, the development of suitable drug delivery systems (DDS) is needed, especially after the recent advances of nanotechnology in inflammatory diseases [[20], [21], [22]]. DDS can be leveraged to load conventional anti-inflammatory drugs or gene therapies to deliver them safely and efficiently to their therapeutic targets [[23], [24], [25]]. Moreover, the inflammation can be used as a cue to develop intelligent delivery systems able to respond to the inflammation site stimuli or attach to specific inflammatory markers to selectively release the anti-COVID-19 therapeutics. More importantly, mesenchymal stems cells and biomimicry using immune cells can simultaneously alleviate the hyperinflammation, due to their inherent anti-inflammatory potency, and endow their drug cargo with a selective delivery to the injured sites [[26], [27], [28]].
In this review, we begin with an overview of the inflammatory profile of COVID-19. Next, we offer insights on how to harness the drug delivery strategies to tackle this inflammation by improving the therapeutic efficiency of conventional anti-inflammatory drugs or by directly adsorbing proinflammatory cytokines. Also, we highlight the possibility of using inflammatory microenvironment features as a trigger for drug release. Then, we provide an overview of cell membrane-based nanoplatforms and mesenchymal stem cell-derived systems that can be leveraged to treat and target inflammation. Finally, we conclude with future perspectives on these approaches.
2. The inflammatory profile of COVID-19
Chronologically, SARS-CoV-2 infection may be divided into 3 stages: viral invasion phase, pulmonary immune-inflammatory phase and hyperinflammatory phase. Herein, the SARS-CoV-2-induced inflammation plays a dual role that may protectively contribute to virus removal and healing process (in more than 85% of patients) or harmfully lead to ARDS development and disease worsening (in 10 to 15% of patients) [29].
2.1. Virological invasion phase
This phase starts with the attachment of the virus to ACE2+/TMPRSS2+ cells (mainly type II pneumocytes but also ciliated epithelium of the nasopharynx and upper respiratory tract, macrophages, and endothelial cells) by the receptor-binding domain (RBP) of its spike (S) protein. Upon endocytosis, the virus replicates in its target cells and assembles before release [[29], [30], [31]]. In this early phase which may last for 8 days as a maximum (incubation period), SARS-CoV-2 proliferates without inducing severe or specific symptoms [32]. However, the cytopathic effect of the virus can directly induce injury and pyroptosis of infected cells, causing the liberation of PAMPs (pathogens associated molecular pattern) and DAMPs (damage-associated molecular pattern) molecules that are recognized by the PRRs (pattern-recognition receptors) of the adjacent epithelial cells and the resident alveolar macrophages, and then activating innate immunity in the following phase [[33], [34], [35]]. Simultaneously, SARS-CoV-2 has been reported to downregulate the cellular expression of ACE2 [30], thereby inhibiting the renin-angiotensin system resulting in increased inflammation and vascular permeability [36].
2.2. Pulmonary inflammatory phase
In this second stage, the virus continues its multiplication, but a localized inflammation progresses. This has been revealed by the bilateral infiltrates and ground-glass opacities in CT and chest X-ray imaging, the high levels of cytokines and chemokines in both BALF and plasma, as well as the highly recruited immune cells to the lung [30,31,37,38]. Early after viral infection, the antiviral immune-inflammatory response begins as a result of the DAMPS/PAMPs released in the alveolar space and/or the SARS-CoV-2-induced ACE2 downregulation [36]. The detection of PAMPs, such as the viral RNA, by the PRRs receptors of innate immune cells, triggers the expression of proinflammatory cytokines and transcription factors, such as NF-kB, as well as the activation of type I interferon (IFN-I) known for its antiviral immunity [29,39]. Similarly, the recognition of DAMPs molecules, such as ATP, heat shock proteins, and HMGB1 (high mobility group box 1 protein) by the neighboring epithelial cells, endothelial cells (ECs), and alveolar macrophages, induces more secretion of pro-inflammatory mediators, such as IL-1β, IL-2, IL-6, IL-7, IL-8, GM-CSF, TNF-a, IFN-γ, CXCL10 (IP-10), CCL2 (MCP-1), CCL3 (MIP-1a), and CCL4 (MIP-1b), and the overexpression of adhesion molecules (i.e., ICAM-1, VCAM-1, and PECAM-1) by the activated ECs, recruiting more monocytes, neutrophils and dendritic cells to the site of infection [39,40]. Also, SARS-CoV-2 has been shown to activate the lectin and classical pathways of the complement system, leading to the generation of C3a and C5a and promoting the immune cell recruitment [41]. The increased secretion of cytokines and chemokines will subsequently lead to the pulmonary recruitment of lymphocytes, which may explain the SARS-CoV-2-associated lymphopenia [30]. Antigen-presenting cells present antigen peptides to T and B cells to activate the cytotoxic T cells to destroy infected alveolar cells, as well as the B cell immunity generating neutralizing antibodies (mainly targeting S protein) to facilitate the recognition and phagocytosis of neutralized viruses and apoptotic cells by macrophages [30,33,39]. Also, regulatory cells, such as Tregs, can produce anti-inflammatory cytokines like IL-10 and TGF-β to antagonize overactivated immune responses [40]. Usually, these innate and adaptive immune responses lead to the virus clearance and gradually, the virus-induced inflammation resolves. However, a dysregulated immune response such as an impaired IFN-I response may lead to phase 3 of the hyper-inflammatory syndrome and cytokine storm observed in COVID-19 severe cases [42].
2.3. Hyper-Inflammatory phase and cytokine storm
In severe cases, the mediators secreted by the recruited immune cells could activate more immune cells via positive feedback breaking the balance between pro-inflammatory and anti-inflammatory cytokines [40]. For example, NF-κB signaling induces the production of pro-inflammatory cytokines such as IL-6, IL-2, TNF-α, and IFN-γ that may themselves upregulate the NF-κB signaling [43]. Anti-inflammatory mediators such as IL-10 are also produced but are insufficient to antagonize the hyperinflammation [40]. Considering the protective role of IFN-I response in viral clearance, an impaired or delayed IFN-I found in severe COVID-19 patients has been incriminated to potentialize the continuous recruitment of circulating monocytes into the lung and their differentiation into proinflammatory macrophages, intensifying the inflammation [33,40]. This can probably be ascribed to a direct inhibition of IFN-I signaling via the viral proteins, that is M protein, N protein, open reading frame 3a (ORF3a) protein, and ORF6 protein [40]. Besides the impaired IFN-I pathway, the inflammation can be exacerbated by M1 macrophages and CD4+ T cells overproducing cytokines [37]. The activated neutrophils that contribute to the fight against the virus by fabricating neutrophil extracellular traps (NETs) may also damage the surrounding cells when excessively releasing leukotrienes and reactive oxygen species (ROS) [40]. Also, the generated neutralizing antibodies are not always protective (depending on the targeted viral antigen) and may over-activate the M1 macrophages-hypersecretion of proinflammatory mediators [36,40]. Further, the high presence of hyperactivated T cells in the alveolar space, such as the proinflammatory T helper 17 (Th17) cells and cytotoxic CD8+ T cells, may promote the hyperinflammation [30].
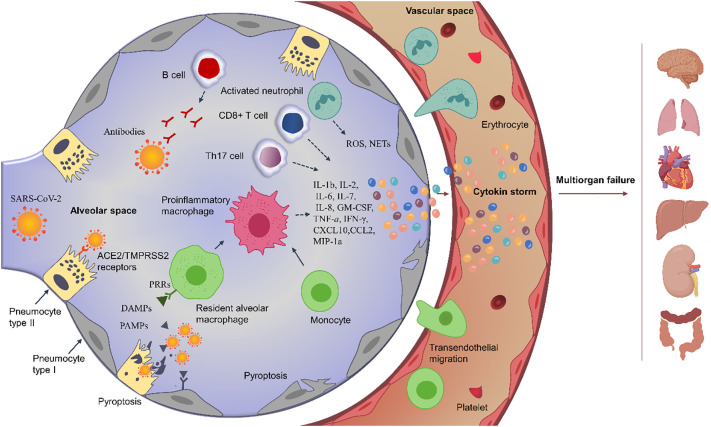
Even if the viral load may diminish, the overproduced pro-inflammatory cytokines would eventually damage the lung infrastructure, which causes hypoxemia and acute respiratory distress syndrome, and circulate to distant organs resulting in an extrapulmonary systemic hyperinflammation, disseminated intravascular coagulation, and multi-organ failure (Fig. 1 ) [30,35,41]. The systemic hyperinflammation has been revealed by the high load of systemic infection markers in the serum of severe COVID-19 subjects, such as procalcitonin, CRP, ferritin, D-dimer, IL-1, IL-2, IL-6, IL-7, IL-8, and IL-10, TNF-α, G-CSF, GM-CSF, IP10, IFN-γ, MCP-1 and MIP-1a [31,34,40,44]. This exacerbated host immune response named cytokine storm has been reported to be more damaging than the viral infection itself [45,46]. Therefore, anti-inflammatory/immunosuppressive therapies are justified in this phase contrarily to the first phases, where the inflammation itself was beneficial [32].
Fig. 1.
Schematic illustration of COVID-19-induced hyperinflammation
3. Implications of inflammation in COVID-19 therapies and drug delivery
From a pharmaceutical standpoint, the implication of inflammation in COVID-19 can be exploited as a therapeutic target and/or as a targeting strategy against the pandemic. Accordingly, the drug delivery systems (DDS) can be harnessed to improve the physicochemical properties of anti-inflammatory agents, especially those suffering from poor bioavailability and instability, and deliver them in a safe and efficient manner to their therapeutic targets. Also, the drug carriers can be tailored to develop smart carriers able to respond to the microenvironmental stimuli to carry the anti-covid 19 therapeutics, either antiviral or immunomodulator, in a selective and specific manner. More interestingly, some biosystems could simultaneously repress the hyperinflammation due to their inherent anti-inflammatory potency and endow their drug cargo with a selective delivery to the injured sites [47,48].
3.1. The inflammation as a therapeutic target for DDS
Targeting the inflammatory pathways to tackle the inflammation in its primary site before becoming systemic seems to be a rational therapeutic strategy against COVID-19 [49]. More importantly, neutralizing the hyperinflammation syndrome is independent of the virus mutation possibilities constituting the primary hurdle for antiviral drugs or vaccine efficiency [50]. Although promising, targeting inflammation constitutes a significant challenge in itself due to the complexity of cytokines interactions and the multiplicity of inflammatory pathways orchestrated by a multitude of molecules rendering the inhibition of one or a few of them not enough to reverse the inflammatory syndrome in the clinic [19]. For this, a plethora of anti-inflammatory agents has been proposed. For instance, corticosteroids such as dexamethasone, cytokines blockers such as tocilizumab (IL-6 receptor antagonist) and anakinra (IL-1 receptor antagonist), and signaling pathways inhibitors such as ruxolitinib and baricitinib (JAK-inhibitors), as well as several plant-derived agents such as colchicine and curcumin, have shown positive results in treating COVID-19 [[51], [52], [53], [54], [55], [56], [57]]. Still, most of them suffer from instability, poor bioavailability, short half-life, lack of efficacy in some COVID-19 patients, and undesirable biodistribution resulting in off-target effects. Together with identifying new therapeutic targets and testing different anti-inflammatory agents, the development of suitable drug delivery systems is needed, especially after the recent advances in nanotechnology in inflammatory diseases. DDS can be leveraged to load conventional anti-inflammatory drugs or gene therapies downregulating the cytokines expression to protect them, improve their physicochemical properties, control their release, optimize their pharmacokinetic profiles, and enhance their accumulation in the injury site [[58], [59], [60]]. More interestingly, novel DDS such as nanodecoys can directly bind proinflammatory cytokines antagonizing their action [[61], [62], [63]].
3.1.1. Anti-inflammatory drugs-loaded DDS against COVID-19
Numerous anti-inflammatory drugs have been proposed for the treatment of SARS-CoV-2. Some were newly proposed, and others were repositioned. However, the promising in vitro SARS-CoV-2 inhibitory effects have been shown inefficient when tested clinically. This may be partly attributed to these agents' poor bioavailability and kinetic profiles, leading to insufficient drug concentrations in the site of action that may induce toxicity if increased to the required levels. Several delivery strategies have been investigated in preclinical trials to optimize the therapeutic efficiency of conventional anti-inflammatory drugs against COVID-19 (Table 1 ) [[64], [65], [66], [67], [68]].
Table 1.
Drug delivery strategies of conventional anti-inflammatory drugs investigated in preclinical studies against COVID-19.
| Drug carrier | Therapeutic cargo | DDS properties | In vivo model | Administration route | Advantages | Reference |
|---|---|---|---|---|---|---|
| Liposomes (DPPC and cholesterol) | Hydroxychloroquine | Not defined | WT rats | Pulmonary route | Higher and prolonged exposure. Lower cardiotoxicity |
[64] |
| 25-HC@DDAB lipid nanovesicles | 25-hydroxycholesterol (25-HC) | 126.5 nm +93.26 mV | CLP-induced septic mice | Intravenous | Improved cellular uptake of 25-HC and enhanced pulmonary accumulation of NPs. Alleviated lung inflammation and reduced cytokines production (i.e., IL-1β and IL-6, IL-8, and TNF-α). |
[65] |
| Glycyrrhizic acid NPs | Glycyrrhizic acid | 70.65 nm, −32.7 mV | MHV-A59-infected mice LPS-induced endotoxemia |
Intravenous | Relieved systemic and lung inflammation with reduced production of inflammatory factors, such as IL-1α, IL-1β, IL-6, and IL-12, TNF-α, TGF-β, IFN-γ, IP-10, G-SCF, and MCP-1 | [66] |
| Polydopamine-modified PEG-PLGA NPs | DNase-1 | 220 nm, −12.0 mV | CLP-induced septic mice | Intravenous | Enhanced stability and prolonged circulation of DNase-1 Reduced NETosis factors neutralize the activity of neutrophils. |
[67] |
| Squalene lipid NPs | α-tocopherol adenosine | 71.2 nm −14.29 mV |
LPS-induced endotoxemia | Intravenous | Enhanced accumulation in inflamed lungs. Enhanced IL-10 levels and reduced ROS and pro-inflammatory cytokines production (i.e., TNF-α, MCP-1, and IL-6) |
[68] |
In this regard, Tai et al. [64] formulated hydroxychloroquine in liposomes and evaluated their pharmacokinetic behavior after direct lung administration in a murine model. As desired, the liposomes displayed higher and longer pulmonary exposure compared to the typical drug administered intravenously or intratracheally, as well as lower systemic and cardiac concentrations, reducing the risks of the systemic drug toxicity, especially the cardiotoxicity [64].
A decreased cholesterol level in viral infections, namely 25-hydroxycholesterol (25-HC), has been believed to contribute to the COVID-19 associated inflammation through the upregulation of NF-κB-pathway and SREBP2 (sterol regulatory element-binding protein 2)-mediated inflammation [69,70]. Thus, a nanoformulation composed of 25-HC and DDAB (di dodecyl dimethylammonium bromide) has been proposed to suppress the COVID-19-induced cytokine storm [65]. Besides its stabilizing role and amphiphilic character in forming lipid nanovesicles, DDAB could assure lung targeting owing to its cationic nature triggering a transient agglutination with the anionic erythrocytes encountered during the NPs circulation, and thereby forming large aggregates that would be likely retained in the pulmonary capillaries [65]. However, this agglutination may also make them vasotoxic, which was disregarded in this study, considering only the absence of cytotoxicity on endothelial cells in vitro and the hematotoxicity in vivo as evidence. Efficiently, the lung accumulation of the i.v. delivered NPs, with a size of 126.5 nm and surface charge of + 93.26 mV, enhanced the survival rate to 50% in CLP (cecal ligation and puncture)-induced septic mice model and dampened the cytokines production, such as IL-1β and IL-6, IL-8, and TNF-α in COVID-19 patient-derived PBMCs [65].
A plethora of plant-derived agents known for their anti-inflammatory potency could be investigated against COVID-19. However, most of these agents displayed poor solubility and bioavailability, which required using the advantages of drug delivery systems. In this respect, curcumin has been encapsulated into nanomicelles and orally delivered to COVID-19 patients [71]. Successfully, this therapy reduced the expression of IL-6 and IL-1β formerly increased, but not IL-18 or TNF-a. The clinical manifestations have been improved, and the survival rate also increased to 80% compared to 60% in the placebo group [71]. In another research, nanocurcumin could reduce the Th17 cells count and downregulate the expression of Th17-mediated cytokines (IL-17, IL-21, IL-23, and GM-CSF) in COVID-19 patients [72]. These two trials used the same nanomicellar formulation, named SinaCurcumin®, previously developed by Hatamipour et al. [73] to enhance the oral bioavailability of curcuminoids. Glycyrrhizic (GA) acid is another naturally derived agent proposed for COVID-19 treatment owing to its ability to impede SARS-CoV-2 replication and undesired inflammatory responses [74]. Besides its therapeutic benefits, GA per se is a potential drug carrier due to its ability of self-association in aqueous solutions and complexation with other drugs [75]. In a study reported by Zhao et al.2, glycyrrhizic acid (GA) nanoparticles (size: 70.65 nm, surface charge: −32.7 mV) have shown a potent anti-inflammatory effect by dwindling the release of cytokines and chemokines, such as IL-1α, IL-1β, IL-6, and IL-12, TNF-α, TGF-β, IFN-γ, IP-10, G-SCF, and MCP-1, in addition to their antiviral and antioxidant properties. This was observed both in vitro and in vivo after i.v injection of the NPs into mice infected either with the coronavirus mouse hepatitis virus A59 (MHV-A59), inducing similar respiratory damage to that of SARS-CoV-2, or LPS as a non-viral inflammatory stimulator to prove the direct inflammatory of the prepared NPs [66].
Interestingly, Lee et al. [67] synthesized polydopamine-modified PLGA NPs decorated with PEG and DNase-1 (size: 220nm, surface charge: −12.0 mV) to compensate the low endogenous levels of DNase-1 in COVID-19 cases and get rid of the cell-free DNA, one of the NETosis factors of neutrophils that are highly produced in SARS-CoV-2 sepsis and may exacerbate the tissue damage. Successfully, the designed NPs, tested in plasma samples of COVID-19 patients and in CLP-induced septic mice, reduced the NETosis markers, neutralizing the neutrophils' activity and thereby reducing the expression of NF-κB and secretion of IL-1β, IL-6, IFN-γ, and TNF-a. Remarkably, less efficiency was observed with non-formulated DNase-1, which may be explained by its short half-life and instability in blood after i.v.administration [67].
Another promising advantage of NPs is the possibility of combination therapies [76]. In this regard, Dormont et al. [68] combined the antioxidant α-tocopherol with the modulator of inflammation adenosine previously conjugated to the endogenous lipid squalene in one nanoplatform (size: 71.2 nm, surface charge: −14.29 mV) for synergistic effects against COVID-19 hyperinflammation. As expected, the in vitro incubation of NPs with LPS-activated macrophages reduced reactive nitrogen species production and inhibited the expression of pro-inflammatory factors. Successfully, the hybrid NPs, delivered intravenously, could accumulate in the inflamed lung, enhance IL-10 levels, and reduce the cytokines production such as TNF-α, MCP-1, and IL-6 in the LPS-induced endotoxemia murine model [68].
3.1.2. Nanodecoys against COVID-19-associated inflammation
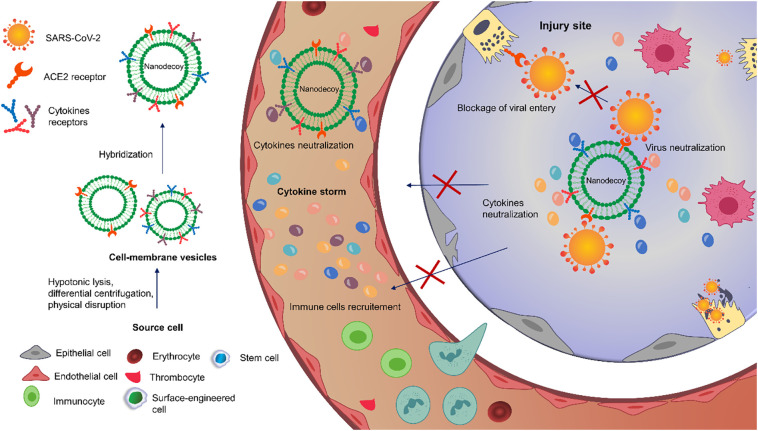
Cell-membrane mimics have been broadly applied in multiple diseases, including infectious and inflammatory disorders, for different purposes such as drug delivery, detoxification, immunomodulation, and diagnosis [[77], [78], [79]]. Commonly, the cell-membrane vesicles are derived from erythrocytes, thrombocytes, immunocytes, cancer cells, and stem cells, but also other cells such as bacteria, endothelial or epithelial cells [79,80]. Frequently, these source cells are lysed using hypotonic conditions, then the cytomembranes are separated from other cellular components by differential centrifugation, and finally physically disrupted (i.e., sonication, homogenization, nitrogen cavitation, extrusion with polycarbonate membrane) to produce vesicles of the desired size [81]. Recently, different types of cells have been fused to obtain hybrid vesicles inheriting the properties of both parental cells [81]. The cellular vesicles are either used as therapeutic entities themselves, loaded with drugs to act as drug carriers, or leveraged to cloak NPs core generally by co-extrusion, electroporation, or electrostatic interactions between the core material and the vesicle [78,81,82].
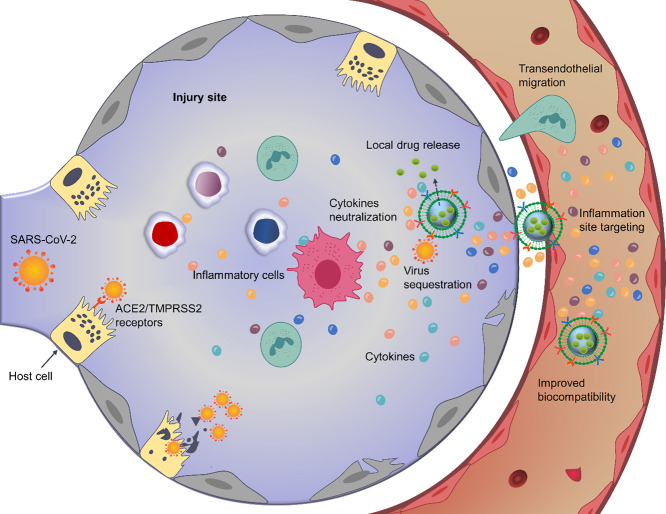
Interestingly, the ability of cell-membrane mimics, either empty or drug-loaded, with NPs core or not, to display the same membrane receptors as the mother cells, makes them a potential arm to attach toxins, viruses, membrane proteins, or signaling molecules and divert them away from their targets, giving an emerging class of biomimetics termed nanodecoys or nanosponge [79,[83], [84], [85]]. Further, hybridization and emerging genetic engineering technologies can be employed to quantitively and qualitatively change their antigenic profile [77]. Besides being nanodecoys, this biomimetics have been originally used to camouflage drug-loaded NPs owing to the virtues they offer to the nanomaterial core, involving high biocompatibility and biodegradability, prolonged circulation time, and improved targeting efficiency due to the homing tendency of their cell membrane proteins [78,86,87]. Therefore, biomimetic nanodecoys constitute a potential therapeutic strategy against COVID-19 infection (Fig. 2 ) [77,85].
Fig. 2.
Schematic illustration of the therapeutic potential of biomimetic nanodecoys against COVID-19
Primarily, nanodecoys have been used as host cell mimics to adsorb the SARS-CoV-2. For instance, Li et al. [88] exploited the resident lung cells inherently expressing ACE2 receptors to produce 320 nm nanodecoys able to bind SARS-CoV-2 to protectively impede its attachment to the target cells and trigger its phagocytosis and clearance [88]. Similarly, Zhang et al. [89] designed 100 nm PLGA (polylactic-co-glycolic acid) NPs covered with cellular membrane isolated from human pneumocytes type II to induce SARS-CoV-2 sequestration [89]. Interestingly, similar antiviral neutralization was obtained when PLGA core was wrapped in a human macrophages-derived membrane, probably through the attachment of the virus to CD147 proved to be highly expressed on the macrophages nanovesicles by Western blot analysis [89]. Moreover, the macrophages decoys may possess additional abilities over the pneumocytes regarding the vital role they play in the COVID-19 hyperinflammatory pathways and their capacity to adsorb proinflammatory cytokines (i.e., IL-6 by CD126 and CD130, TNF-a by CD120) impeding them from potentiating the cytokine storm [90].
Inspiringly, cell membrane-based NPs can be leveraged for inflammation neutralization and/or the loading of inflammatory drugs besides the virus adsorption. For instance, 220 nm HUVEC membrane-camouflaged PLGA NPs, initially loaded with hydroxychloroquine, were used to adsorb proinflammatory cytokines such as TNF-α and IL-1β since the endothelial cells inherently express TNF-α and IL-1β receptors [91]. In this study, the cells were transfected to express tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to induce the apoptosis of M1 inflammatory macrophages after attachment of TRAIL to the death receptor-5 (DR5) overexpressed on the surface of inflamed macrophages and thereby reduce the production of cytokines and the recruitment of other immune cells [91].
More importantly, hybrid nanodecoys that can capture both the inflammatory cytokines and the virus can be produced by the fusion of nanovesicles obtained from different cells. In this respect, Rao et al. [92] developed a nanodecoy by fusion of cell membrane-derived nanovesicles isolated from human embryonic kidney 293T cell transfected with ACE2 receptors (293T/ACE2) and human monocytes THP-1 cell. The resultant 100 nm hybrid nanovesicles holding both ACE2 and cytokine receptors (i.e., CD130 for IL-6 and CD116 for GM-CSF) could effectively neutralize SARS-CoV-2 in vitro, displaying its ability to protectively compete with covid-19 target cells for virus attachment on ACE2 receptors and neutralize the inflammatory cytokines when incubated with LPS-activated THP-1 cells. These findings were confirmed in vivo using LPS-induced ALI murine model. Significantly, the decoy NPs delivered intratracheally reduced the levels of cytokines and alleviated the lung injury. Interestingly, the designed system attaching both the virus and cytokines exhibited better antiviral capacity than the nanovesicles obtained from 293T/ACE2 alone [92]. Recently, THP-1 membrane-derived PLGA NPs of approximatively 100 nm were also evaluated for their SARS-CoV-2 inhibition, but the main focus was the effect of surface modification of the nanosponges with heparin on the virus neutralization [93]. However, the potency of THP-1 mimetics of cytokines neutralization shouldn’t be disregarded. Fluorescence spectra using labeled antibodies, such as anti-CD130, anti-CD120a, and anti-CD119 receptors (for IL-6, TNF-α, and IFN-γ, respectively) showed that the surface modification didn’t alter the ability of the monocytes to trap cytokines [93].
Inspired by these studies and SARS-CoV-2 mechanism, Wang et al. [94] fused cell membranes of a genetically engineered ACE2 receptor-overexpressing HEK293 cells and proinflammatory macrophages (IFN-γ and LPS-activated Raw264.7) to camouflage the methacrylate hyaluronic acid hydrogel core and obtain microspheres destinated to be delivered via inhalation to be distributed in all the respiratory system, including the upper tract and nasopharynx neglected by nanoscale vesicles. The efficiency was evaluated both in vitro and in vivo. The presence of ACE receptors enabled the system to compete with the virus, enhancing the antiviral activity 10 times more than the blank. Concurrently, the co-existence of cytokines receptors, including IL-1, IL-6, and TNF-a receptors, confirmed by WB, endowed the microspheres with an anti-inflammatory potency revealed by neutralized proinflammatory markers, alleviated edema, reduced lung infiltration of immune cells, reduced pneumocytes apoptosis, lowered CD3+T cells, CD8+ T cells, and CD19+ B cells with increased anti-inflammatory Foxp3+ regulatory T cells in spleen and lymph nodes, as well as an improved survival ratio after intratracheal administration to LPS-induced ALI mice model [94].
3.2. The inflammation as a targeting strategy for DDS
The overcome the shortcomings of anti-COVID-19 therapeutics, particularly the nonspecific biodistribution leading to undesirable side effects, and enhance their concentrations in the diseased area, the inflammation site features can be exploited to achieve a site-specific drug delivery. This can be achieved by developing smart drug carriers responsive to inflammatory biochemical signals or by exploiting the endothelial dysfunction in the injury site [95].
3.2.1. Inflammation-bioresponsive DDS
In order to deliver the anti-COVID-19 agents, either immunomodulator or not, in a selective and specific manner to the injury site, drug delivery systems can be modified to smartly respond to the specific stimuli of the inflammatory microenvironment by releasing their cargo (Table 2 ) [[96], [97], [98], [99]]. The inflammatory biochemical stimulus can be the low pH, the high level of reactive oxygen species (ROS), or the overexpressed inflammatory enzymes [95,100].
Table 2.
Bioresponsive DDS used for site-specific targeting of COVID-19-induced inflammation.
| Drug carrier | Therapeutic cargo | DDS properties | Targeting mechanism | Animal model | Administration route | Reference |
|---|---|---|---|---|---|---|
| ICAM-1 decorated biotinylated (PEG-PAE)- NPs | TPCA-1 | 100 nm | Targeting of the inflamed endothelium by the anti-ICAM-1 antibody. Specific drug release in the acidic inflammatory site by the pH-responsive copolymer (biotinylated PEG- PAE) |
LPS-induce ALI | Intravenous | [96] |
| Mannose-decorated PEI-NPs | Dexamethasone | 115 nm +31 mV |
Targeting of proinflammatory alveolar macrophages by mannose modification. pH-responsive release of dexamethasone due to its linkage to PEI polymer. |
LPS-induce ALI | Intravenous | [97] |
| Tempol-phenylboronic acid pinacol- β-cyclodextrin NPs | Tempol | 109 nm −16 mV |
Enhanced hydrolysis of PBAP (phenylboronic acid pinacol ester) group in the presence of high concentrations of ROS due to its oxidation-labile units | LPS-induce ALI | Intravenous | [99] |
| Poly(thioketal) polymeric NPs | Dexamethasone | 307 nm −22 mV |
Cleavage of thioketal bonds by the high level of ROS in the injury site. | LPS-induce ALI | Intravenous | [98] |
pH-responsive DDS synthesized with materials cleavable in acidic pH have been widely investigated in cancer and inflammatory diseases [101]. The low pH in the inflamed area, probably due to the anaerobic and the infiltration of inflammatory cells, can be exploited to trigger the release of different therapeutics against COVID-19 [95]. For example, Zhang et al. [96] developed an endothelium targeting pH-responsive polymeric NPs (size ~100 nm) as a drug carrier for acute lung injuries. To assure a low pH-triggering release, they used biotinylated polyethylene glycol (PEG)-poly(β-aminoester) (PAE) as pH-sensitive copolymer to encapsulate the anti-inflammatory agent TPCA-1(5-p-Fluorophenyl-2-ureido-thiophene-3-carboxamide). The pH-dependent drug release was confirmed in vitro by more than 90 % of TPCA-1 liberated at pH 6.5 after 15 h, compared to less than 20 % released after 1 day at physiological pH. Then, the NPs were decorated with anti-ICAM-1 antibody to target the ICAM-1 receptors overexpressed on inflamed endothelium. In vivo efficiency, revealed by the reduced infiltration of immune cells in the lung and the decreased levels of proinflammatory cytokines (i.e., TNF-α and IL-6) in the LPS-induced ALI murine model, confirmed the efficient targeting of the i.v delivered bioresponsive NPs mediated by the anti-ICAM-1 and followed by the specific drug release in response to acidic pH of the inflammatory site. However, the pulmonary targeting in this study was mainly attributed to anti-ICAM-1 moiety. The acidic pH-triggered release alone might not be enough to specifically target the inflammatory lungs [96]. Similarly, Su and coworkers have linked dexamethasone (Dexa) with branched PEI to obtain a pH-responsive prodrug that was further modified with mannose molecules to target the proinflammatory alveolar macrophages during lung injury. As desired, the obtained NPs (size: 115 nm, surface charge: +31 mV) displayed a pH-dependent release in vitro and repressed the lung inflammation in LPS-induced ALI murine model [97]. Although prodrug NPs without mannose modification haven’t been tested in the in vivo efficacy study, the enhanced therapeutic effect was probably due to targeting mannose receptors rather than the specific pH release. Recently, PEGylated halloysite/spinel ferrite nanocomposites have been proposed to deliver dexamethasone in a pH-responsive manner to the acidic inflamed lungs. The DDS released 17.5% at acidic media while less than 5% of Dexa was liberated after 168 h at neutral pH [102]. Still, their trafficking benefits, efficiency and toxicity weren’t inspected in vivo.
The oxidative stress, mainly resulting from the infiltration of neutrophils releasing reactive oxygen species (ROS), is another biochemical stimulus that can be used to trigger the release of therapeutics in inflammation sites during lung injuries [103,104]. In addition to the selective drug release, ROS materials may scavenge oxygens species contributing to inflammation. In this regard, Li et al. [99] reported ROS-responsive NPs (size:109 nm, surface charge: −16 mV) composed of Tempol as the therapeutic agent and PBAP (phenylboronic acid pinacol ester) group as a catalase-mimetic moiety eliminating hydrogen peroxide, both conjugated with β-cyclodextrin. The oxidation-labile units of PBAP successfully assured the ROS-triggering release in the injury site leading to an enhanced accumulation of the NPs in the LPS-injured lungs, reduced oxidative stress and less production of TNF-α and IL-1β compared to free tempol [99]. It is worth noting that tempol has been stated as a drug candidate against COVID-19 for its antioxidant and anti-cytokine activities proved in vitro [105]. Currently, a randomized, double-blind clinical study (NCT04729595) is evaluating its efficiency against COVID-19. Also, Zhai et al. [98] employed PPADT (poly(1,4- phenyleneacetonedimethylene thioketal)) and PTKU (polythioketal urethane) polymers to encapsulate dexamethasone. The high level of ROS in the inflammatory sites could cause the cleavage of thioketal bonds of the polymeric DDS, leading to a local release of their cargo and improved anti-inflammation against LPS-induced ALI. Besides the enhanced lung accumulation, the decreased ROS level due to their interaction with NPs contributed to the resolution of the injury [98].
3.2.2. Endothelium-mediated targeting DDS
The endothelial dysfunction and enhanced vascular permeability effect (EPR) observed in inflammation tissues also contribute to the site-specific delivery [95]. In addition to passive targeting, overexpressed adhesion molecules on endothelial cells such as VCAM-1, ICAM-1, and PECAM-1, can be used to actively target the injury site. This can be achieved by surface modification of DDS with specific antibodies, such as the work of Zhang et al. [96] reviewed above, or by exploiting the inherent inflammation-responsiveness of immune cells to develop inflammation targeting biomimetics as discussed in the section below [106]. In contrast to the bioresponsive DDS, endothelium-mediated targeting DDS is more important for systemic administration routes than local pulmonary delivery [95]
3.3. The inflammation as both a targeting strategy and therapeutic target for DDS
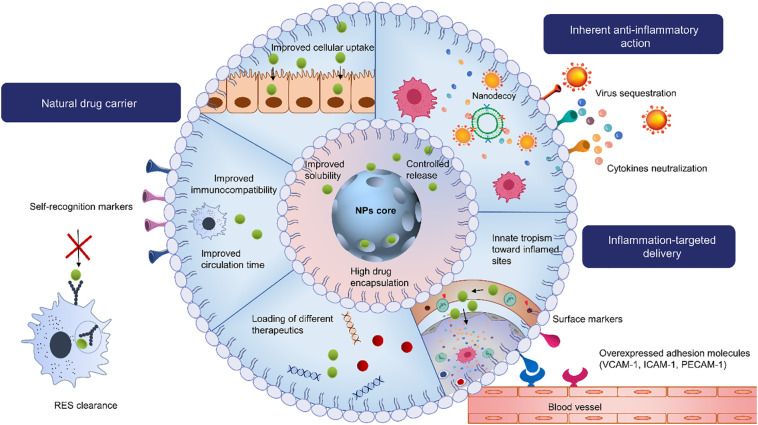
In spite of the benefits offered by nanoscaled delivery systems, they are still considered exogenous materials that tend to be rapidly cleared by the reticuloendothelial system (RES) [107]. Synthetic DDS lacks biocompatibility and stability, and may even cause inflammation which is highly undesirable in inflammatory diseases. Promisingly, biomimetic DDS sprung out as a potent strategy to disguise the exogenous profile of nanomaterials, thereby improving their immunocompatibility and circulation time. Combining both the natural advantages of cell membranes, such as their biocompatibility and inherent ability to target tissues and evade immune clearance, with the artificial attributes of nanomaterials, that is their controlled release, high drug encapsulation, and solubilization potency, yields promising biosystems that may be exploited to deliver different therapeutics to treat various disorders, including COVID-19. Further, these systems may be wisely exploited for targeted delivery purposes. Various cell types can be used as source cells to mimic the interactions happening in the inflammation microenvironment and assure inflammation-targeted drug delivery (Fig. 3 ) [108]. If no cell sources cells with the desired properties are natively available, they can be tailored or genetically modified to display the desired trafficking or targeting properties [109].
Fig. 3.
Schematic illustration of the delivery and therapeutic potentials of cell-derived DDS against COVID-19
Interestingly, some native cells, including immune cells, thrombocytes, and mesenchymal stem cells, have an inherent affinity for inflammation tissues and/or anti-inflammatory properties [110]. Their derived systems can be employed as therapeutics by themselves, as they hold multiple bioactive molecules (e.g., miRNA and proteins), or can be leveraged for anti-inflammatory and/or antiviral agents’ delivery against COVID-19, as they express a multitude of targeting molecules on their surface [111]. For these purposes, either the cell’s membranes or their naturally secreted extracellular vesicles (EVs) can be used. EVs, including exosomes (size:30–150 nm), microvesicles (size:150–1000 nm), and apoptotic bodies (random size >1000 nm), are nucleus-free membranous structures charged with biocompounds (nucleic acids, proteins, and lipids), secreted by various kinds of cells in vivo as intercellular communication mediators, but also in vitro in response to external stimulus. EVs inherit many features from their source cells making them promising DDS and therapeutic candidates for many diseases, particularly their smallest subtype exosomes [[112], [113], [114], [115]].
3.3.1. Leukocyte-derived DDS
Although the immune system is usually considered a barrier to DDS, it can be leveraged to improve drug delivery in several diseases. Under inflammatory conditions, leukocytes, composed of monocytes, lymphocytes, and granulocytes (neutrophils, basophils, and eosinophils) [116], are recruited from the bloodstream to the inflammation tissue by interactions between their surface proteins (i.e., VLA-4, LFA-1, Mac-1) and the adhesion molecules of activated endothelial cells (i.e., VCAM-1, ICAM-1, PECAM-1 receptors) [117,118]. Therefore, leukocyte-derived membranes have been utilized to endow their cargo with an active targeting to inflammatory sites against different diseases-associated inflammation, especially SARS-CoV-2 (Table 3 ) [[119], [120], [121], [122], [123], [124]]. Additionally, they can protect the DDS from RES clearance by extending its lifetime. Claudia and coworkers have investigated the tendency of leukocyte-like liposomes and bare liposomes to adsorb blood components forming the so-called “protein corona” and its influence on their immune clearance. Although both NPs have been opsonized by the same proteins (mainly IgG, coagulation and complement factors), revealed by an increase of their diameter from ∼170 nm before in vivo administration to ∼350 nm 1h post-injection, biomimetic NPs were less taken up by macrophages [125]. This has been elucidated by the possibility that blood proteins can attach to leukocyte-like NPs in a manner that the recognition domains of those proteins are oriented toward the biomimetic surface inherently expressing macrophage receptors, which masks them from phagocytes recognition, whereas proteins adsorbed on their non-biomimetic counterparts can be randomly oriented [125]. Besides, the leukocytes nanovesicles per se may have anti-inflammatory activities independently of their cargo [119]. Commonly, leukocyte-based DDS can be obtained by a top-down strategy through the coating of the leukocyte-derived membrane onto the NP generating a core-shell structure named “nanoghost”, or by incorporating their extracted surface proteins into a phospholipid bilayer to obtain the so-called “leukosome” combining the bottom-up and top-down approaches [[126], [127], [128]].
Table 3.
Leukocytes-derived DDS against COVID-19-induced inflammation.
| Source cell+/-phospholipids | Nanomaterial core | Cargo | DDS properties | In vivo model | Advantages | Reference |
|---|---|---|---|---|---|---|
| Macrophage (J774) DPPC, DOPC, and cholesterol (molar ratio: 4:3:3) |
None | Dexamethasone | 150 nm −20 mV |
LPS-induced endotoxemia | The leukosome alleviated the systemic inflammation more potentially than the free drug. | [119] |
| Monocytes (THP-1 cells) | PLGA NPs | Lopinavir | 102.2 nm −12.4 mV |
MHV-infected mice model | Potentialized anti-antiviral effect due to the targeted delivery of the antiviral drug lopinavir to the infection site. Neutralization of inflammatory mediators by the cytokine receptors present on the surface of nanovesicles. |
[120] |
| Macrophages (RAW264.7 cells) | Nanostructured lipid carrier | Cepharanthine | 152.48 nm −16.93 mV |
LPS-induced ALI | Improved bioavailability of cepharanthine provided by the lipid core. Prolonged circulation and efficient homing to the injured lung resulting in improved attenuation of LPS-induced inflammation was assured by the biomimetic shell. |
[121] |
| Neutrophils (DMSO-activated HL-60 cells) |
None | TPCA-1 | ~200 nm −16 mV |
LPS-induced ALI | Selective delivery of the anti-inflammatory drug to the injured tissues was assured by interactions between ICAM-1 upregulated on activated endothelial cells and the integrin β2 expressed on neutrophils nanovesicles. | [122] |
| Neutrophils (DMSO-activated HL-60 cells) |
None | Piceatannol | ~200 nm −18 mV |
LPS-induced ALI | [123] | |
| Genetically modified leukocytes (leukemia cells C1498) | PLGA NPs | Dexamethasone | 175 nm −20 mV |
LPS-induced ALI | Selective delivery of dexamethasone to the injured tissues was assured by interactions between VCAM-1 overexpressed on inflamed endothelial cells and VLA-4 of the leukocyte membrane. | [124] |
3.3.1.1. Macrophage-derived DDS
Monocytes are the largest leukocytes circulating in the bloodstream but can infiltrate into tissues in response to the received signals, where they differentiate into macrophages or dendritic cells [117]. Due to their abundance in inflammatory environments, macrophages have been widely investigated as source cells for biomimetic DDS design [129,130]. As mentioned above, macrophage-like nanoparticles have been utilized as nanodecoys to trap the pathogen particles and proinflammatory factors. Furthermore, macrophages' membrane-derived vesicles may bestow their cargo with immune escape by their self-recognition markers (i.e., CD47 and CD45) and selective delivery due to their innate tropism toward inflamed sites mediated by their membrane proteins (i.e., CD18, CD11a, and CD11b) [125,131]. Moreover, macrophage membrane-based-nanovesicles exhibited potent intrinsic anti-inflammatory attributes by inducing proinflammatory macrophages to secrete anti-inflammatory cytokines (IL-10 and TGF-β) and repressing their release of pro-inflammatory cytokines (TNF-a, IL-6 and IL-1β) [132].
To mitigate the COVID-19-cytokine storm, Molinaro and coworkers have loaded dexamethasone (Dexa) in macrophage-like nanovesicles designed by mixing J774 macrophage membranes with the lipids DPPC, DOPC, and cholesterol (4:3:3 molar ratio) using a microfluidic platform [119,133]. The Dexa-leukosomes (size:150 nm, surface charge: −20 mV) incubated with LPS-activated endothelial or macrophages cells showed similar inhibitory effects on the pro-inflammatory cytokines IL-6 and TNF-α as free Dexa. However, they caused more decrease of IL-1β in endothelial cells and more increase of the anti-inflammatory cytokine IL-10 in macrophages, compared to the free drug. The ameliorated anti-inflammatory effect provided by leukosomes to the corticosteroid was further assessed in mice with LPS-induced endotoxemia. As established by the reduction of plasmatic levels of pro-inflammatory cytokines (ICAM-1, IL-1α, IL-16, TNF-α, and M-CSF), chemokines (CXCL13, CCL5, and CCL2), and C5-C5a complement factor, the intravenously injected Dexa-loaded leukosomes alleviated the systemic inflammation more potentially than the non encapsulated Dexa [119]. Nevertheless, neither stability nor pharmacokinetics/biodistribution studies, which would confirm the improved targeting and extended circulation time, were investigated in this work. The same nanovesicles were employed by Boada et al. [134] to deliver the rapamycin, an inhibitor of the mTOR signaling pathway, against atherosclerosis. Despite the decreased inflammation markers by drug-loaded leukosomes (Diameter:108 nm, surface charge: −15.4 mV) over free rapamycin, this cannot be merely ascribed to the targeted delivery of inflamed endothelium since empty leukosomes were not used in their in vivo study.
Likewise, Tan et al. [120] used monocytes (THP-1 cells)-derived nanovesicles to simultaneously take advantage of their anti-inflammatory properties and assure a targeted delivery of PLGA NPs loaded with the antiviral drug lopinavir thought to be effective against COVID-19. The biomimetics could neutralize proinflammatory cytokines, mainly IL-6 and IL-1β, by their receptors IL-6R and IL-1R, suppressing the activation of macrophages and neutrophils revealed by downregulation of STAT3 and NETs, respectively. Besides, the presence of ACE2 receptors on their surface, as analyzed by western blot, and their targeting efficacy of the infected sites, as revealed by the biodistribution study, potentialized their antiviral activity. These advantages have been validated in vivo using MHV (mouse hepatitis virus)-infected mice as a model of severe COVID-19 infection [120].
With the same strategy and the same aim, Lu and coworkers loaded cepharanthine, another drug candidate repurposed to treat COVID-19 due to its inflammatory and antiviral potency, in nanostructured lipid carriers (NLCs) and then coated them with macrophage membranes (derived from RAW264.7 cells) to obtain biomimetic systems with a size of 152.48 nm and a surface charge of −16.93 mV [121,135,136]. Synergistically, the lipid core provided the alkaloid cepharanthine, suffering from poor solubility and bioavailability, with the desired physicochemical properties, while the biomimetic shell assured its prolonged circulation and efficient homing to the injured lung to mitigate the LPS-induced inflammation [121]. However, the enhanced therapeutic effect was merely attributed to the improved kinetics and the selective delivery provided by macrophages membranes proteins (mainly CD44 and CD11b) without considering the intrinsic anti-inflammatory activity of macrophages membranes, since they used neither free drug-NLC coated with macrophages membranes nor empty macrophages nanovesicles as controls in their in vivo efficacy study.
3.3.1.2. Neutrophil-derived DDS
Neutrophils or polymorphonuclear leukocytes (PMNs) are the most abundant circulating white blood cells. Under the guidance of cytokines and chemokines, PMNs migrate and infiltrate into the site of inflammation within a few minutes [87,137]. Like other immune cells, the extravasation of neutrophils is facilitated by multiple interactions with the endothelium, such as the interaction between intercellular adhesion molecule 1 (ICAM-1) upregulated on the inflamed endothelium and the integrin β2 expressed on neutrophils [138]. To take advantage of this interaction to actively deliver the anti-inflammatory drug TPCA-1 (5-p-Fluorophenyl-2-ureido-thiophene-3-carboxamide) to inflamed endothelial cells, Gao et al. [122] used neutrophils nanovesicles obtained by nitrogen cavitation, ultracentrifugation, and extrusion of HL 60 cells, as a delivery system. The human promyelocytic leukemia cells HL-60 cells were selected due to their ability to differentiate into neutrophils-like cells overexpressing integrin β2 after activation by DMSO [112]. As expected, neutrophil nanovesicles were more efficiently internalized by TNF-α-activated HUVECs overexpressing ICAM-1 compared to red blood cells nanovesicles. The selective interaction with inflamed vasculature was confirmed by intravital microscopy in a TNF-α treated live mouse. Further, the selectively delivered TPCA-1 to the activated endothelial cells could downregulate their ICAM-1 expression through its NF-κB inhibitory action. When intravenously delivered into the LPS-induced ALI mice model, the drug-loaded nanovesicles that remarkably accumulated in the lung alleviated the pulmonary edema and reduced infiltration of neutrophils and cytokine production, mainly TNF-α and IL-6 [122]. Similar results were found by the same group but by using the anti-inflammatory drug piceatannol instead of TPCA-1. Further, they confirmed that nitrogen cavitation allowed a high-yield generation of nanovesicles, similar to the naturally secreted EVs in structure and composition, but with fewer sub-cellular organelles and genetic materials that may cause safety issues [123]. Here, the adhesion molecule ICAM-1 was investigated as both a therapeutic target for TPCA-1/piceatannol and a delivery target for the neutrophil-based NPs to mitigate acute lung inflammation. Despite the absence of material genetic in the nanovesicles obtained by nitrogen cavitation, non-malignant human neutrophils should be used as source cells. A more recent report used neutrophil membrane derived from human neutrophils instead of the differentiated leukemia cells HL-60 to actively deliver Resolvin D2 to inflamed brain endothelium [139].
More interestingly, PMN-derived nanovesicles can be employed to co-deliver several therapeutic agents to concurrently target inflammation and/or pathogens. Gao et al. [140] exploited the previously developed human neutrophil-isolated nanovesicles to co-load the hydrophobic anti-inflammatory drug Resolvin D1 (on the surface of NVs) and the hydrophilic antibiotic ceftazidime (inside the NVs) to tackle the inflammation and bacterial infection against bacterium-induced peritonitis. Successfully, the therapeutic efficacy was increased compared to the non-coated drugs, mainly due to the efficient targeting of inflamed tissues [140]. It is worth noting that the inflammation resolving agents resolvins (Rvs), biosynthesized from omega-3 polyunsaturated fatty acids (Rvs E from Eicosapentaenoic acid and Rvs D from docosahexaenoic acid) such as Rvs D1 and D2, have been previously speculated to mitigate inflammation in COVID-19 patients through inhibiting several inflammatory signaling pathways [141,142].
3.3.1.3. Other leukocyte-derived DDS
Lymphocytes, including specific immune cells (T and B cells) and innate immune cells (natural killer cells), constitute promising cell sources for membrane coating strategies in several biomedical applications, especially cancer, inflammation, and infectious diseases [117]. Frequently, T cells have been investigated for their biomimicry merits. For instance, T cell membranes derived from the EL4 mouse lymphoma cell line have been used to camouflage anticancer drug-loaded PLGA NPs and actively target the inflamed endothelium in malignant tissues overexpressing ICAM-1 through lymphocyte function-associated antigen-1 (LFA-1) expressed on their surface [143]. Also, to selectively deliver grapefruit-derived NPs, carrying either doxorubicin or curcumin as an anti-inflammatory model, Wang et al. [144] exploited activated T-lymphoma EL4 cell-isolated membranes as a coating. The presence of LFA-1 on their surface enabled the biomimetic system to preferentially deliver their cargo to the inflammation tissues [144].
Another important interaction to recruit more leukocytes to the inflammation site is mediated by VCAM-1 (vascular cell adhesion molecule–1) highly expressed on inflamed endothelial cells and VLA-4 (very late antigen–4) inherently expressed on leukocytes membranes. Park et al. [124] proposed to leverage this interaction to specifically deliver anti-inflammatory drugs to inflamed lungs. For this, they generated cellular membranes overexpressing VLA-4 (composed of a4 and B1 integrins) by genetically modifying the mouse leukemia cells C1498 (naturally expressing a4 integrin) to co-express B1 integrins and used them to coat PLGA cores carrying Dexa as a model of anti-inflammatory payload. The resultant biomimetic (size: 175 nm, surface charge: −20 mV) could specifically bind to VCAM-1-expressing cells in vitro and majorly accumulate in the inflamed lungs compared to the non-targeted NPs when injected intravenously in LPS-induced ALI murine model, resulting in an improved anti-inflammation [124]. Nevertheless, only malignant mice cells were used in these studies, far from clinical translation.
3.3.2. Thrombocyte-derived DDS
Platelets (PLTs) or thrombocytes are small (2–4 μm in diameter) anucleate discoid cells circulating in the blood in a nonadherent state as sentinels of vascular integrity [118,145]. PLTs can rapidly change their shape and increase their adhesiveness when encountering exposed sub-endothelium upon vascular injury [146,147]. Besides their hemostatic functions, thrombocytes play a major role in inflammation and immune responses. PLTs can be recruited to the inflamed sites and activated by the locally released proinflammatory mediators, including chemokines and platelet-activating factor (PAF) [148,149].
The selective adherence of PLTs to injured vasculatures, their relatively short lifespan of 7–10 days, as well as their high storage and release capacities inspired the design of several platelet-mimicking platforms as drug delivery systems [118,150]. Platelet membrane-camouflaged NPs inherent the protein profile of platelets surface giving them a targeting ability towards injury sites assured by specific PLTs/cells interactions, such as the binding of their glycoprotein Iba (GP Iba) to the P-selectin overexpressed on the surface inflamed endothelial cells [146,148]. Further, the cloaking of NPs with PLTs membrane decreases the NPs immunogenicity and RES clearance due to the interaction between CD47 present on the platelet membrane and the immunoglobulin SIRPα of the phagocytic cells downregulating their phagocytosis and increasing their circulation time [148]. PLTs membranes, usually extracted using freeze-thawing processes, can be fused onto the pre-prepared NPs by sonication. For instance, He et al. [151] coated PLGA NPs with platelet membrane to endow them with inflammation targeting ability. The resultant NPs (size:122.3 nm) inherited the platelet-surface receptors increasing the binding of NPs to inflamed endothelium in vitro and their accumulation in inflammation sites in a mouse model of rheumatoid arthritis [151].
Unfortunately, directly implementing platelets as DDS may induce inflammation since these cells can release cytokines and chemokines [145]. To deal with this issue, researchers preferred using platelet extracellular vesicles (PEVs) that inherent the targeting and trafficking ability of PLTs but do not exacerbate inflammation [152]. PEVs, constituting more than half of the total EVs in circulation, are plasma membranes fragments naturally and largely produced in vivo by thrombocytes undergoing activation, stress, or apoptosis, but also can be obtained by activation of platelets in vitro [[153], [154], [155]]. PEVs may include microparticles or nanometric exosomes with different proteomic profiles depending on the isolation and activation techniques, the agonist used (i.e., thrombin, collagen, von Willebrand factor, adenosine diphosphate (ADP), and platelet-activating factor), and the stimulation duration [155]. The drug is either preloaded in PLTs before the generation of PEVs or post-loaded in the isolated PEVs, passively via incubation or actively through sonication, electroporation, or extrusion [149]. Fortunately, PEVs inherit the same surface profile of the parent platelets, such as the glycoproteins GPIIbIIIa, GPIaIIa, GPIba, P-selectin, PECAM-1, and CD40L, and thereby display the same tissue affinity, trafficking, and immune properties [149,154]. Nowadays, 2 clinical trials utilizing PEVs are reported on www. clinicaltrials.gov. One study, identified NCT04281901 for the treatment of post-surgical inflammation of the temporal bone, has been completed; and the second, identified NCT04761562 for chronic middle ear infections, is recruiting.
Promisingly, Li et al. [156] proposed platelet-derived vesicles-camouflaged NPs loaded with TPCA-1 (5-p-Fluorophenyl-2-ureido-thiophene-3-carboxamide) to repress the cytokine storm of patients with pneumonia such as in severe SARS-CoV-2 cases. The drug was first encapsulated into the thrombocytes, and then the platelets were activated with thrombin to generate a large number of 100–150 nm vesicles loaded with TPCA-1, which were finally isolated by ultracentrifugation. Herein, the antiinflammation effect was assured by TPCA-1 which can selectively inhibit IkB kinases (IKK) in the NF-kB pathway reducing the production of inflammatory factors such as TNF-a, IL-6, and IL-8. The targeting strategy of the inflammation site was based on the intrinsic affinity of platelet-derived vesicles to adhere to the activated vessel wall via CD40L, glycoproteins Iba, aIIb, and VI, and P-selectin, to reach the inflamed tissue. Successfully, the thrombocyte biomimetic nanocarriers could selectively target the site of injury upon intravenous administration and sustainably liberate TPCA-1, to alleviate the lung edema and infiltration of inflammatory cells, and significantly reduce the cytokines production in LPS-induced ALI mice model better than the free drug-treated group [156].
For an efficient anti-inflammatory effect, Ma et al. [157] synthesized PEVs-camouflaged NPs loaded with MCC950, an NLRP3-inflammasome inhibitor. Antagonizing NLRP3-inflammasome has been reported as a potent strategy to combat COVID-19 uncontrolled inflammation [158,159]. In this study, the targeting efficacy of the biomimetic NPs has been proved in vitro by the enhanced affinity toward oxidized low-density lipoprotein-treated macrophages/LPS-activated-HUVECs compared to the inactivated cells. The reduced inflammation over the free drug has been also confirmed both in vitro by the reduced IL- 1β and NLRP3 expression in LPS-stimulated macrophages and endothelial cells and in vivo against atherosclerosis-associated vascular inflammation. Safely, the pro-inflammatory potency of PEVs has been discarded by the absence of cytokine secretion [157]. In another study, 121 nm platelet membrane-camouflaged NPs were utilized to weaken the inflammatory response [160]. The core of NPs was constituted of a porous metal-organic framework prepared by glycyrrhizic acid and calcium ions and then loaded with hydrocortisone. In addition to the anti-inflammatory effect of their cargo, platelet vesicles might compete with endogenously activated platelets to bind and aggregate in the inflammatory site, thereby hampering their degranulation and liberation of inflammatory mediators [160].
Also, Jin et al. [161] developed PEV-coated PEG-PLGA NPs for targeted delivery of berberine to the inflammatory lungs. Berberine is a natural alkaloid that fights against COVID-19 by downregulating MAPKinase and NFκB pathways [162]. However, the therapeutic efficiency of berberine may be hampered by its low bioavailability and off-targeting properties in vivo [161]. Efficiently, the PEV coating enhanced the accumulation of berberine NPs (size:400 nm, surface charge:−23 mV) in inflammatory lungs and alleviated lung inflammation in house dust mite-induced murine inflammation model, revealed by decreased inflammatory cells and cytokines (such as IL-4, IL-5, and IL-13) compared with the free drug or non coated NPs, mainly via regulating Th1/Th2 balance and enhancing IL-12 expression [161].
3.3.3. Mesenchymal Stem Cell-derived DDS
Stem cells, including pluripotent, hematopoietic, and mesenchymal stem cells, are non-differentiated or partially differentiated cells capable of self-renewal and differentiation into various specific cells. Mainly, mesenchymal stem cells (MSCs), isolated from the umbilical cord, placenta, bone marrow, adipose tissue, or dental pulp, have been investigated for their therapeutic effect against multiple diseases, including COVID-19, due to their immunomodulatory and regenerative capabilities [163,164]. Their ability to proliferate and differentiate into various cells, including type II pneumocytes and decrease the apoptosis of alveolar cells by transferring their mitochondria, as well as their liberation of growth factors (hepatocyte growth factor HGF, vascular endothelial growth factor VEGF, and keratinocyte growth factor KG and angiopoietin 1) is beneficial for the recovery of COVID-19-induced epithelial and endothelial injury [163,165,166]. Besides, MSCs have been reported to increase alveolar fluid clearance and decrease inflammation [167].
According to the stimuli received from their microenvironment, MSCs can differentiate into pro-inflammatory (MSC1) or anti-inflammatory phenotypes (MSC2). Under the COVID-19 cytokine storm, MSCs polarized to MSC2 to suppress the hyperinflammation by interacting with innate and adaptive immunity [5,[168], [169], [170]]. They induce the differentiation of M2 macrophages into their anti-inflammatory phenotype M1 releasing the anti-inflammatory cytokines like IL-10 and TGF-β and thereby lowering the recruitment of neutrophils [166,[170], [171], [172]]. Also, they may inhibit NK cells proliferation via secretion of prostaglandin E2 (PGE2), indolamine 2,3-dioxygenase (IDO), and human leukocyte antigen G5 (HLA-G5), suppress T and B lymphocytes proliferation and promote T cell differentiation to T reg cells [163,166,173,174]. Altogether, this would eventually suppress the cytokine storm caused by COVID-19 [175].
The high level of cytokines in the injured site and the upregulated adhesion molecules may play a chemoattractant role in attracting MSCs to the disease area [166,170]. Further, surface markers such as CD44 CD29, CD18, CD24, and chemokine receptors such as CXCR4, CXCR7, and CCR2 on MSCs mediate their adhesiveness on endothelial cells expressing different ligands such as the stem cell-derived factor (SDF)-1 [5,176]. However, this migration is relatively low and depends on the route of administration. When intravenously delivered, MSCs highly accumulated in the lungs due to their relatively large size causing their entrapment in the pulmonary microvessels [166]. Surface modification or conjugation with antibodies (anti-VCAM-1 or ICAM-1) may promote their movement to the inflamed endothelium [176]. Safely, MSCs express only a few molecules of major histocompatibility complex class I (MHCI) and class II (MHCII) that lower their immunogenicity and do not express ACE2/TMPRSS2, protecting them from the virus infection [165,173,177].
The therapeutic effects of MSCs are partly mediated by their paracrine activity and especially by their extracellular vesicles. MSC-derived exosomes contain a panel of proteins, lipids, and microRNAs such as miR-124-3p, 21-5p, 146a, 34a, 122, 124, and 127 involved in suppressing inflammation and pulmonary cell apoptosis, enabling them to exert similar immunomodulatory and regenerative potential as their mother cells [113,178]. Moreover, MSC-derived exosomes may offer several advantages over MSCs, such as higher stability, lower immunogenicity, and the ability to bypass physiological barriers [179]. Further, exosomes as nucleus-free therapeutics are devoid of tumorigenic and mutational risks [170]. Also, exosomes with their nanometric size (30-150 nm) can be lyophilized and delivered through inhalation, being more suitable for COVID-19 pneumonia treatment [173,180,181]. In this respect, three clinical trials, two completed (NCT04276987 and NCT04313647) and another enrolling by invitation (NCT04602442), utilized inhalation for the MSC-derived exosome delivery. Additionally, exosomes can be used as DDS of several drugs, including antiviral and anti-inflammatory agents, as well as RNAi therapeutics, against SARS-CoV-2 [175,179,180,182]. Besides the passive targeting, exosomes can be modified to improve their targeting [175].
The beneficial effects of MSCs and MSC-derived EVs on acute respiratory syndrome have been proved preclinically and clinically [176]. Commonly, MSC-derived EVs negatively regulated pro-inflammatory factors levels and potentialized the anti-inflammation in different ALI/ARDS models. For example, Li and coworkers demonstrated the protective immunomodulatory role of MSC-derived exosomes on pulmonary epithelial cells, attributed to their miRNA-21-5p cargo in the ischemia/reperfusion injury mouse model [183]. Similarly, Wang et al. [184] found that MSCs, as well as their EVs, could mitigate LPS-induced ALI, partly by transferring their miR-27a-3p to M2 macrophage and promoting their polarization [184]. Similarly, Li et al. [185] confirmed the ability of these exosomes to alleviate LPS-induced ALI probably through Nrf-2/ARE and NF-kB signaling pathways. Consistently, Xiao et al. [186] reported that the inactivated NF-κB signaling pathway and the beneficial effect of MSC-exosomes on LPS-injured cells were related to their miRNA payload, namely miR-182-5p and miR-23a-3p.
Up to 10th February 2022, 88 clinical trials investigating the safety and efficiency of MSCs or their derived vesicles are reported on www. clinicaltrials.gov, including 17 not yet recruiting, 30 recruiting, 10 actives not recruiting, and 19 completed assays. For example, bone marrow-MSCs derived exosomes labeled ExoFlo®, investigated by Sengupta et al. [187] in a nonrandomized prospective study, have safely and efficiently relieved COVID-19- pneumonia and downregulated the cytokine storm. Currently, 2 not yet recruiting clinical trials, identified NCT05125562 and NCT05116761, are ongoing to evaluate the safety and efficacy of intravenous administration of ExoFlo® in the treatment of mild-moderate COVID-19 and Post-Acute/Chronic COVID-19 syndrome, respectively. However, these therapeutics need to bypass several hurdles regarding their purification, standardization, large-scale manufacturing, stability, and transportation issues [179]. Also, the thrombogenic risk should be considered, especially when thromboembolic accidents are frequent in COVID-19 patients [176,180].
4. Conclusions and future perspectives
The COVID-19 severity has been narrowly linked to the virus's inflammatory profile. Therefore, potential therapeutic and delivery strategies are required to tackle the COVID-19-associated hyperinflammation and cytokine storm besides preventing and eliminating SARS-CoV-2. This is of utmost importance for patients suffering from other comorbidities and immune disorders. Also, the efficiency of targeting the host genes and/or proteins is independent of the virus mutations, unlike vaccines and antiviral drugs. A plethora of anti-inflammatory therapeutics have been repurposed for COVID-19, and some of them are currently under clinical evaluation, including small molecular-weight drugs, monoclonal antibodies, and cell-based therapies. Still, they suffered from multiple side effects, and some of them yielded poor or controversial outcomes. This may be partly ascribed to the difficulty of completely targeting the inflammation regarding the complexity of cytokine interactions and the multiplicity of cytokine targets. Also, this may be attributed to the lack of specificity, stability, and undesirable biodistribution profiles of these agents. Further, the timing of administration of anti-inflammatory therapeutics is another important factor that should be considered. In SARS-CoV-2 infection, the inflammation can be regarded as a double-edged phenomenon. In the early stage, the inflammation is a beneficial host response that may contribute to virus removal. Therefore, an early administration may hinder the virus clearance by the immune system and promote its proliferation. In the late stages, the inflammatory response becomes detrimental, leading to disease worsening in 10 to 15% of patients. Therefore, timely delivery of anti-inflammatory agents at the beginning of the cytokine storm may efficiently prevent the development of ARDS and multiorgan failure. The inflammation, either during the early stage or the hyperinflammatory syndrome, can be positively used from a pharmaceutical standpoint. In the first case, the inflammation can be leveraged as a cue for specific delivery of anti-COVID-19 drugs, especially antivirals, to reduce the high viral load. In the second case, it can be exploited as a strategy for drug delivery and/or regarded as a detrimental process that should be targeted and stopped.
Together with identifying new therapeutic targets and testing different anti-inflammatory agents, the development of suitable drug delivery systems is needed, especially after the recent advances in nanotechnology in inflammatory disorders. Drug delivery systems can be harnessed to load conventional anti-inflammatory drugs or gene therapies to improve their physicochemical and in vivo trafficking properties and deliver them safely and efficiently to their therapeutic targets. Also, smart delivery systems may be developed to specifically attach to the inflammatory markers and/or smartly respond to the inflammation site stimuli to assure a selective release of the anti-COVID-19 therapeutics. Despite the benefits offered by synthetic delivery systems, they lack biocompatibility and may even cause inflammation which is highly undesirable in inflammatory diseases. Promisingly, biomimicry using cell membranes and extracellular vesicles sprung out as a potential arm against COVID-19 infection. Cloaking synthetic nanomaterials with cell membranes allow the combination of the natural advantages of the source cells, such as their biocompatibility and inherent ability to target tissues, with the artificial attributes of nanomaterials such as the high drug loading, solubilization, and controlled release. Interestingly, some biomimetics have been used as nanodecoys to simultaneously trap the virus and cytokines. Further, these systems may be wisely exploited for targeted delivery purposes. Various cell types can be used as source cells to mimic the interactions happening in the inflammation microenvironment and assure inflammation-targeted drug delivery. If no source cells with the desired properties are natively available, they can be tailored or genetically modified to display the desired trafficking or targeting properties. Also, hybrid systems derived from different types of cells can be endowed with multiple functions inherited from the parental cells. Recently, mesenchymal stem cells are emerging as promising therapeutics for their immunomodulatory and regenerative capabilities. Hopefully, a multitude of clinical trials are evaluating the safety/efficiency of mesenchymal cells and their derived vesicles in alleviating COVID-19 induced cytokine storm.
Although attractive, these applications need to bypass several challenges before clinical translation. For improvement of the interpretation efficiency of preclinical findings, appropriate controls should be used. Remarkably, several works evaluating the efficacy of cell-derived DDS didn’t use empty vectors as controls in their in vivo efficacy studies. More improvements in the study design are necessary to provide confidence in their data. Some of the reviewed reports didn’t provide enough information about the vector properties such as size, charge, and shape, which are important factors influencing its in vivo fate. Also, the delivery route largely affects the trafficking, and thereby, the drug efficiency. Most studies utilized the intravenous route whereas the local pulmonary delivery is another potential route against COVID-19 pneumonia. Besides efficiency, safety is another issue that should be thoroughly examined, especially after long-term usage. Interactions between the vectors and the host cells, particularly the immune cells, should be fully investigated. Further, repeated administrations may result in the production of alloantibodies that may alter the carrier trafficking, reduce the efficacy and lead to adverse immunologic reactions. Also, the potential toxicity towards non-diseased organs should be discarded. Doubtfully, the possible tumorigenic effects of cell-based therapies and extracellular vesicles should be highly considered. The stability, essential to assure efficiency and safety, is another significant challenge to surmount. For instance, stem cells or other source cells may suffer from poor survival after isolation, limited expansion in vitro, and the possibility of changing and losing their potential after multiple passages. Also, stem cells or cell-derived DDS are unstable and suffer from storage and transportation problems. Further, the production techniques, such as collection, isolation, purification, and manipulation, are still in their early stages stage which makes large-scale manufacturing more difficult. Therefore, effective standardized and reproducible techniques are needed to obtain stable vectors with high purity, high yields, high drug loading, targeted delivery, and acceptable cost. Standardized protocols to control the quality and identify the specific cell markers are also required.
Declaration of Competing Interest
The authors declare that they are no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81872823, and 82073782), the Shanghai Science and Technology Committee (No. 19430741500), the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (zdsys-202103, China).
References
- 1.Subramanian B., Adolfo P., Ponmalai K. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021;39:3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Yin W., Jiang Y., Xu H.E. Structure genomics of SARS-CoV-2 and its Omicron variant : drug design templates for COVID-19. Acta Pharmacol. Sin. 2022;1–13 doi: 10.1038/s41401-021-00851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik P.E., Iglesias J., Varon J., Kory P. A scoping review of the pathophysiology of COVID-19. Int. J. Immunopathol. Pharmacol. 2021;35:1–16. doi: 10.1177/20587384211048026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology : A review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loke X.Y., Imran S.A.M., Tye G.J., Zaman W.S. Wan Kamarul, Nordin F. Immunomodulation and regenerative capacity of MSCs for long-COVID. Int. J. Mol. Sci. 2021;22:12421. doi: 10.3390/ijms222212421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Toshio H. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parasher A. COVID-19 : Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021;97:312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. Cytokine and growth factor reviews the cytokine storm in COVID-19 : An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y., Chan K.H., Hung I.F.N. Safety and Efficacy of COVID-19 Vaccines: A systematic review and meta-analysis of different vaccines at phase 3. Vaccines. 2021;9:989. doi: 10.3390/vaccines9090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasmi A., Srinath S., Dadar M., Pivina L., Menzel A., Benahmed A.G., Chirumbolo S., Bjørklund G. A global survey in the developmental landscape of possible vaccination strategies for COVID-19. Clin. Immunol. 2022;108958 doi: 10.1016/j.clim.2022.108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19 : The current evidence and treatment strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou H., Yang Y., Dai H., Xiong Y., Wang J. Vol. 12. 2021. Recent Updates in Experimental Research and Clinical Evaluation on Drugs for COVID-19 Treatment; pp. 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echeverría-esnal D., Martin-ontiyuelo C., Navarrete M.E., Cuscó M.D., Ferrández O., Horcajada J.P., Martin-ontiyuelo C., Navarrete M.E., Cuscó M.D., Ferrández O., Horcajada J.P., Grau S., Echeverría-esnal D., Martin-ontiyuelo C., Navarrete-rouco M.E., Cuscó M.D. Expert Review of Anti-infective Therapy Azithromycin in the treatment of COVID-19 : a review. Expert Rev. Anti-Infect. Ther. 2021;19:147–164. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 15.Haji M., Moradi-kalbolandi S., Zarei M., Bose D., Majidzadeh-A K., Farahmand L. Potential role of interferons in treating COVID-19 patients. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soy M., Tabak F., Kayhan S. Cytokine storm in COVID-19 : pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iovino L., Thur L.A., Gnjatic S., Chapuis A., Milano F., Hill J.A. Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lariccia V., Magi S., Serfilippi T., Toujani M., Gratteri S., Amoroso S. Challenges and Opportunities from Targeting Inflammatory Responses to SARS-CoV-2 Infection : A Narrative Review. J. Clin. Med. 2020;9:4021. doi: 10.3390/jcm9124021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat S., Kumari M., Jayabaskaran C. Nano-engineered tools in the diagnosis, therapeutics, prevention, and mitigation of SARS-CoV-2. J. Control. Release. 2021;338:813–836. doi: 10.1016/j.jconrel.2021.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikoba U., Peng H., Li H., Miller C., Yu C., Wang Q. Nanocarriers in therapy of infectious and inflammatory diseases. Nanoscale. 2015;7:4291–4305. doi: 10.1039/c4nr07682f. [DOI] [PubMed] [Google Scholar]
- 21.Su Y., Gao J., Kaur P., Wang Z. Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics. 2020;12:1222. doi: 10.3390/pharmaceutics12121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Placha D., Jampilek J. Chronic inflammatory diseases, anti-Inflammatory agents and their delivery nanosystems. Pharmaceutics. 2021;13:64. doi: 10.3390/pharmaceutics13010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Mohammad I.S., Fan L., Zhao Z., Nurunnabi M., Sallam M.A., Wu J., Chen Z., Yin L., He W. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm. Sin. B. 2021;11:2585–2604. doi: 10.1016/j.apsb.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoulikha M., Xiao Q., Boafo G.F., Sallam M.A., Chen Z., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W., Kapate N., Shields C.W., Mitragotri S. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2020;165–166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Eljarrah A., Gergues M., Pobiarzyn P.W., Sandiford O.A., Rameshwar P. Therapeutic Potential of Mesenchymal Stem Cells in Immune-Mediated Diseases. Adv. Exp. Med. Biol. 2019;1201:93–108. doi: 10.1007/978-3-030-31206-0_5. [DOI] [PubMed] [Google Scholar]
- 27.Bonsack B., Corey S., Shear A., Heyck M., Cozene B., Sadanandan N., Zhang H., Gonzales-Portillo B., Sheyner M., Borlongan C.V. Mesenchymal stem cell therapy alleviates the neuroinflammation associated with acquired brain injury. CNS Neurosci. Ther. 2020;26:603. doi: 10.1111/cns.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dash P., Piras A.M., Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J. Control. Release. 2020;327:546–570. doi: 10.1016/j.jconrel.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Benmansour N.C., Carvelli J., Vivier E. Complement cascade in severe forms of COVID-19 :Recent advances in therapy. Eur. J. Immunol. 2021;00:1–8. doi: 10.1002/eji.202048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zirui Tay M., Meng Poh C., Ramp L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20 doi: 10.1038/s41577-020-0311-8. 363–374. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landete P., Loaiza C.A.Q., Aldave-Orzaiz B., Muñiz S.H., Maldonado A., Zamora E., Cerna A.C.S., del Cerro E., Alonso R.C., Couñago F. Clinical features and radiological manifestations of COVID-19 disease. World J. Radiol. 2020;12:247. doi: 10.4329/wjr.v12.i11.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauretani F., Ravazzoni G., Federica Roberti M., Longobucco Y., Adorni E., Grossi M., De Iorio A., La Porta U., Fazio C., Gallini E., Federici R., Salvi M., Ciarrocchi E., Rossi F., Bergamin M., Bussolati G., Grieco I., Broccoli F., Zucchini I., Ielo G., Morganti S., Artoni A., Arisi A., Tagliaferri S., Maggio M. Assessment and treatment of older individuals with COVID-19 multi-system disease: clinical and ethical implications. Acta Bio Medica Atenei Parm. 2020;91:150. doi: 10.23750/abm.v91i2.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C., He Q., Qian H., Liu J. Overview of the pathogenesis of COVID-19 (Review) Exp. Ther. Med. 2021;22 doi: 10.3892/etm.2021.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong R.S. Inflammation in COVID-19: from pathogenesis to treatment. Int. J. Clin. Exp. Pathol. 2021;14:831. [PMC free article] [PubMed] [Google Scholar]
- 35.Romagnoli S., Peris A., De Gaudio A.R., Geppetti P. SARS-CoV-2 and COVID-19: From the bench to the bedside. Physiol. Rev. 2020;100:1455–1466. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripathi N., Tripathi N., Goshisht M.K. COVID-19: inflammatory responses, structure-based drug design and potential therapeutics. Mol. Divers. 2021;2021(1):1–17. doi: 10.1007/s11030-020-10176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustine J.N., Jones D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvelli J., Demaria O., Vély F., Batista L., Chouaki Benmansour N., Fares J., Carpentier S., Thibult M.L., Morel A., Remark R., André P., Represa A., Piperoglou C., Assante Miranda L., Baron W., Belaid N., Caillet C., Caraguel F., Carrette B., Carrette F., Chanuc F., Courtois R., Fenis A., Giordano M., Girard-Madoux M., Giraudon-Paoli M., Gourdin N., Grondin G., Guillot F., Habif G., Jaubert S., Lopez J., Le Van M., Lovera N., Mansuy M., Bonnet E., Sansaloni A., Reboul A., Mitry E., Nekkar-Constant C., Péri V., Ricaut P., Simon L., Vallier J.B., Vétizou M., Zerbib R., Ugolini S., Etiennot M., Galluso J., Lyonnet L., Forel J.M., Papazian L., Velly L., André B., Briantais A., Faucher B., Jean E., Seguier J., Veit V., Harlé J.R., Pastorino B., Delteil C., Daniel L., Boudsocq J.P., Clerc A., Delmond E., Vidal P.O., Savini H., Coutard B., Cordier P.Y., Le Dault E., Guervilly C., Simeone P., Gainnier M., Morel Y., Ebbo M., Schleinitz N., Vivier E. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bime C., Casanova N.G., Nikolich-Zugich J., Knox K.S., Camp S.M., Garcia J.G.N. Strategies to DAMPen COVID-19-mediated lung and systemic inflammation and vascular injury. Transl. Res. 2021;232:37–48. doi: 10.1016/j.trsl.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021;6 doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed-Hassan H., Sisson B., Shukla R.K., Wijewantha Y., Funderburg N., Li Z., Jr D.H., Demberg T., M L.N.P. Innate immune responses to highly pathogenic coronaviruses and other significant respiratory viral infections. Front. Immunol. 2020;11:1979. doi: 10.3389/fimmu.2020.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acharya D., Liu G.Q., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20:397. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su C.M., Wang L., Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021;11:13464. doi: 10.1038/s41598-021-92941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh L., Bajaj S., Gadewar M., Verma N., Ansari M.N., Saeedan A.S., Kaithwas G., Singh M. Modulation of Host Immune Response Is an Alternative Strategy to Combat SARS-CoV-2 Pathogenesis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.660632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Hong W., Ren W., Xu T., Qian Z., He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021;61(6):1–25. doi: 10.1038/s41392-021-00631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin C., Zhao Q., Li W., Zhao Z., Wang J., Deng T., Zhang P., Shen K., Li Z., Zhang Y. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020;102:416–426. doi: 10.1016/j.actbio.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Kohan H.G., Jamali F. Anti-Inflammatory Properties of Drugs Used to Control COVID-19 and their Effects on the Renin-Angiotensin System and Angiotensin-Converting Enzyme-2. J. Pharm. Pharm. Sci. 2020;23:259–277. doi: 10.18433/jpps31346. [DOI] [PubMed] [Google Scholar]
- 50.Cosar B., Yagmur Z., Unal S., Turan A. SARS-CoV-2 mutations and their viral variants. Cytokine Growth Factor Rev. 2020;S1359-6101:00053–00058. doi: 10.1016/j.cytogfr.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgulchik N., Athanasopoulou F., Chu E., Lam Y. Potential therapeutic approaches for targeted inhibition of inflammatory cytokines following COVID-19 infection-induced cytokine storm. Interface Focus. 2021;12:20210006. doi: 10.1098/rsfs.2021.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pane A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annane D. Corticosteroids for COVID-19. J. Intensive Med. 2021;1:14–25. doi: 10.1016/j.jointm.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C. Xiang, Wang J. Jiao, Li H., Yuan L. Tao, Gale R.P., Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geng J., Wang F., Huang Z., Chen X., Wang Y. Perspectives on anti-IL-1 inhibitors as potential therapeutic interventions for severe COVID-19. Cytokine. 2020;143 doi: 10.1016/j.cyto.2021.155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rattis B.A., Ramos S.G., Celes M.R. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.675287. 675287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drosos A.A., Pelechas E., Drossou V., P. V Voulgari, Colchicine Against SARS-CoV-2 Infection: What is the Evidence? Rheumatol. Ther. 2022:1–11. doi: 10.1007/s40744-022-00425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thapa Magar K., Boafo G.F., Li X., Chen Z., He W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022;33:587–596. [Google Scholar]
- 59.Teng C., Li B., Lin C., Xing X., Huang F., Yang Y., Li Y., Azevedo H.S., He W. Targeted delivery of baicalein-p53 complex to smooth muscle cells reverses pulmonary hypertension. J. Control. Release. 2022;341:591–604. doi: 10.1016/j.jconrel.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Xiao Q., Li X., Li Y., Wu Z., Xu C., Chen Z., He W. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm. Sin. B. 2021;11:941. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisani A., Pompa P.P., Bardi G. Potential Applications of Nanomaterials to Quench the Cytokine Storm in Coronavirus Disease 19. Front. Bioeng. Biotechnol. 2020;8:906. doi: 10.3389/fbioe.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sajid M.I., Moazzam M., Cho Y., Kato S., Xu A., Way J.J., Lohan S., Tiwari R.K. siRNA Therapeutics for the Therapy of COVID-19 and Other Coronaviruses. Mol. Pharm. 2021;18:2105–2121. doi: 10.1021/acs.molpharmaceut.0c01239. [DOI] [PubMed] [Google Scholar]
- 63.Gedefaw L., Ullah S., Lee T.M.H., Yip S.P., Huang C.-L. Targeting Inflammasome Activation in COVID-19: Delivery of RNA Interference-Based Therapeutic Molecules. Biomedicines. 2021;9:1823. doi: 10.3390/biomedicines9121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tai T.T., Wu T.J., Wu H.D., Tsai Y.C., Wang H.T., Wang A.M., Shih S.F., Chen Y.C. A Strategy to Treat COVID-19 Disease With Targeted Delivery of Inhalable Liposomal Hydroxychloroquine: A Preclinical Pharmacokinetic Study. Clin. Transl. Sci. 2021;14:132. doi: 10.1111/cts.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H., Lee H.S., Ahn J.H., Hong K.S., Jang J.G., An J., Mun Y.H., Yoo S.Y., Choi Y.J., Yun M.Y., Song G.Y., Joo J., Na D.H., Kim H.N., Park H.H., Lee J.Y., Lee W. Lung-selective 25-hydroxycholesterol nanotherapeutics as a suppressor of COVID-19-associated cytokine storm. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., Li B., Zhu T., Tan Q., Tang L., Zhou H., Huang X., Shan H. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-infl ammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces. 2021;13:20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- 67.Lee Y.Y., Park H.H., Park W., Kim H., Jang J.G., Hong K.S., Lee J.Y., Seo H.S., Na D.H., Kim T.H., Bin Choy Y., Ahn J.H., Lee W., Park C.G. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2021;267 doi: 10.1016/j.biomaterials.2020.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dormont F., Brusini R., Cailleau C., Reynaud F., Peramo A., Gendron A., Mougin J., Gaudin F., Varna M., Couvreur P. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 2020;6:eaaz5466. doi: 10.1126/sciadv.aaz5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee W., Ahn J.H., Park H.H., Kim H.N., Kim H., Yoo Y., Shin H., Hong K.S., Jang J.G., Park C.G., Choi E.Y., Bae J.S., Seo Y.K. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct. Target. Ther. 2020;51(5):1–11. doi: 10.1038/s41392-020-00292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., Cyster J.G. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valizadeh H., Abdolmohammadi-vahid S., Danshina S., Ziya Gencer M., Ammari A., Sadeghi A., Roshangar L., Aslani S., Esmaeilzadeh A., Ghaebi M., Valizadeh S., Ahmadi M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tahmasebi S., El-Esawi M.A., Mahmoud Z.H., Timoshin A., Valizadeh H., Roshangar L., Varshoch M., Vaez A., Aslani S., Navashenaq J.G., Aghebati-Maleki L., Ahmadi M. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J. Cell. Physiol. 2021;236:5325–5338. doi: 10.1002/jcp.30233. [DOI] [PubMed] [Google Scholar]
- 73.Hatamipour M., Sahebkar A., Alavizadeh S.H., Dorri M., Jaafari M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic Med. Sci. 2019;22:282–289. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Z., He G., Huang N., Thilakavathy K., Lim J.C.W., Kumar S.S., Xiong C. Glycyrrhizic Acid : A natural plant ingredient as a drug candidate to treat COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.707205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selyutina O.Y., Polyakov N.E. Glycyrrhizic acid as a multifunctional drug carrier- from physicochemical properties to biomedical applications : A modern insight on the ancient drug. Int. J. Pharm. 2019;559:271–279. doi: 10.1016/j.ijpharm.2019.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao Q., Li X., Liu C., Yang Y., Hou Y., Wang Y., Su M., He W. Liposome-based anchoring and core-encapsulation for combinatorial cancer therapy. Chin. Chem. Lett. 2022 doi: 10.1016/J.CCLET.2022.01.083. [DOI] [Google Scholar]
- 77.Rao L., Tian R., Chen X. Cell-Membrane-Mimicking Nanodecoys against Infectious Diseases. ACS Nano. 2020;14:2569–2574. doi: 10.1021/acsnano.0c01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H., Liu Y., He R., Xu D., Zang J., Weeranoppanant N., Dong H., Li Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020;8:552–568. doi: 10.1039/c9bm01392j. [DOI] [PubMed] [Google Scholar]
- 79.Zhou J., Krishnan N., Jiang Y., Fang R.H., Zhang L. Nanotechnology for virus treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018;30 doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Q., Lee J., Lee H., Shim G., Oh Y. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B. 2021;11:2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narain A., Asawa S., Chhabria V., Patil-Sen Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine. 2017;12:2677–2692. doi: 10.2217/nnm-2017-0225. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y., Deng Y., Liu Z., Yin M., Hou M., Zhao Z. Vol. 6432. 2021. Cytokine-scavenging nanodecoys reconstruct osteoclast / osteoblast balance toward the treatment of postmenopausal osteoporosis; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y., Wang K., Pan Y., Rao L., Luo G. Engineered Cell Membrane-Derived Nanoparticles in Immune Modulation. Adv. Sci. 2021;8:2102330. doi: 10.1002/advs.202102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X., Li D., Liu G. Cell membrane-derived biomimetic nanodecoys for viruses. Sci. China Life Sci. 2020;638(63):1254–1256. doi: 10.1007/s11427-020-1669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Zhang Y., Zhang Y., Lv P., Zhang P., Chu C., Mao J., Wang X., Li W., Liu G. Bio-engineered cell membrane nanovesicles as precision theranostics for perihilar cholangiocarcinoma. Biomater. Sci. 2020;8:1575–1579. doi: 10.1039/c9bm02088h. [DOI] [PubMed] [Google Scholar]
- 87.Zhang R., Wu S., Ding Q., Fan Q., Dai Y., Guo S., Ye Y., Li C. Recent advances in cell membrane-camouflaged nanoparticles for inflammation therapy. Drug Deliv. 2021;28:1109–1119. doi: 10.1080/10717544.2021.1934188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z., Wang Z., Dinh P.C., Zhu D., Popowski K.D., Lutz H., Hu S., Lewis M.G., Cook A., Andersen H., Greenhouse J., Pessaint L., Lobo L.J., Cheng K. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat. Nanotechnol. 2021;16:942–951. doi: 10.1038/s41565-021-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Gri A., Zhang L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thamphiwatana S., Angsantikul P., Escajadillo T., Zhang Q., Olson J., Luk B.T., Zhang S., Fang R.H. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. U. S. A. 2017;114:11488–11493. doi: 10.1073/pnas.1714267114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi Y., Xie F., Rao P., Qian H., Chen R., Chen H., Li D., Mu D., Zhang L., Lv P., Shi G., Zheng L., Liu G. TRAIL-expressing cell membrane nanovesicles as an anti-inflammatory platform for rheumatoid arthritis therapy. J. Control. Release. 2020;320:304–313. doi: 10.1016/j.jconrel.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 92.Rao L., Xia S., Xu W., Tian R., Yu G., Gu C., Pan P., Meng Q.F., Cai X., Qu D., Lu L., Xie Y., Jiang S., Chen X. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2020;117:27141–27147. doi: 10.1073/pnas.2014352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ai X., Wang D., Honko A., Duan Y., Gavrish I., Fang R.H., Griffiths A., Gao W., Zhang L. Surface Glycan Modification of Cellular Nanosponges to Promote SARS-CoV-2 inhibition. J. Am. Chem. Soc. 2021;143:17615–17621. doi: 10.1021/jacs.1c07798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z., Xiang L., Lin F., Cai Z., Ruan H., Wang J., Liang J., Wang F., Lu M., Cui W. Inhaled ACE2-engineered microfluidic microsphere for intratracheal neutralization of COVID-19 and calming of the cytokine storm. Matter. 2022;5:336–362. doi: 10.1016/j.matt.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dou Y., Li C., Li L., Guo J., Zhang J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release. 2020;327:641–666. doi: 10.1016/j.jconrel.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang C.Y., Lin W., Gao J., Shi X., Davaritouchaee M., Nielsen A.E., Mancini R.J., Wang Z. pH-responsive nanoparticles targeted to lungs for improved therapy of acute lung inflammation/injury. ACS Appl. Mater. Interfaces. 2019;11:16380–16390. doi: 10.1021/acsami.9b04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su M., Yang B., Xi M., Qiang C., Yin Z. Therapeutic effect of pH-responsive dexamethasone prodrug nanoparticles on acute lung injury. J. Drug Deliv. Sci. Technol. 2021;66 doi: 10.1016/j.jddst.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhai Z., Ouyang W., Yao Y., Zhang Y., Zhang H., Xu F., Gao C. Bioactive Materials Dexamethasone-loaded ROS-responsive poly (thioketal) nanoparticles suppress inflammation and oxidative stress of acute lung injury. Bioact. Mater. 2020;232 doi: 10.1016/j.bioactmat.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L., Guo J., Wang Y., Xiong X., Tao H., Li J., Jia Y., Hu H., Zhang J. A broad-spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ Ttoxicity. Adv. Sci. 2018;5:1800781. doi: 10.1002/advs.201800781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deng Z., Liu S. Inflammation-responsive delivery systems for the treatment of chronic inflammatory diseases, Drug Deliv. Transl. Res. 2021;11:1475–1497. doi: 10.1007/s13346-021-00977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li C., Wu P., Dou Y., Li Q., Zhang J. Bioresponsive nanoplatforms for imaging and therapy of cardiovascular diseases. View. 2022;3:20200137. [Google Scholar]
- 102.Jermy B.R., Ravinayagam V., Almohazey D., Alamoudi W.A., Dafalla H., Akhtar S., Tanimu G. PEGylated green halloysite/spinel ferrite nanocomposites for pH sensitive delivery of dexamethasone : A potential pulmonary drug delivery treatment option for COVID-19. Appl. Clay Sci. 2022;216 doi: 10.1016/j.clay.2021.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang D., Wang Y., Wijaya A., Liu B., Maruf A., Wang J., Xu J., Liao X., Wu W., Wang G. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis, Regen. Biomater. 2021;8:rbab033. doi: 10.1093/rb/rbab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathi K., Rosenberg-Hasson Y., Maecker H., Carlo D.J., Moss R.B. Brief report: Tempol, a novel antioxidant, inhibits both activated T cell and antigen presenting cell derived cytokines in-vitro from COVID-19 patients. Clin. Immunol. 2021;231 doi: 10.1016/j.clim.2021.108828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brusini R., Varna M., Couvreur P. Advanced nanomedicines for the treatment of in fl ammatory diseases. Adv. Drug Deliv. Rev. 2020;157:161–178. doi: 10.1016/j.addr.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He W., Xing X., Wang X., Wu D., Wu W., Guo J., Mitragotri S. Nanocarrier-Mediated Cytosolic Delivery of Biopharmaceuticals. Adv. Funct. Mater. 2020;30:1910566. [Google Scholar]
- 108.Jin K., Luo Z., Zhang B., Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B. 2018;8:23–33. doi: 10.1016/j.apsb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bush L.M., Healy C.P., Javdan S.B., Emmons J.C., Deans T.L. Biological cells as therapeutic delivery vehicles. Trends Pharmacol. Sci. 2021;42:106–118. doi: 10.1016/j.tips.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jo J., Emi T., Tabata Y. Design of a Platelet-Mediated Delivery System for Drug-Incorporated Nanospheres to Enhance Anti-Tumor Therapeutic Effect. Pharmaceutics. 2021;13:1724. doi: 10.3390/pharmaceutics13101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jahromi L.P., Shahbazi M., Maleki A., Azadi A., Santos H.A. Chemically engineered immune cell-derived microrobots and biomimetic nanoparticles : emerging biodiagnostic and therapeutic tools. Adv. Sci. 2021;8:2002499. doi: 10.1002/advs.202002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S., Dong X., Gao J., Wang Z. Targeting inflammatory vasculature by extracellular vesicles. AAPS J. 2018;20:37. doi: 10.1208/s12248-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karn V., Ahmed S., Tsai L., Dubey R., Ojha S., Singh H.N., Kumar M., Gupta P.K., Sadhu S., Jha N.K., Kumar A., Pandit S., Kumar S. Extracellular vesicle-based therapy for COVID-19 : Promises, challenges and future prospects. Biomedicines. 2021;9:1373. doi: 10.3390/biomedicines9101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim J.-K., Youn Y.J., Bin Lee Y., Kim S.H., Song D.K., Shin M., Jin H.K., Bae J., Shrestha S., Hong C. Extracellular vesicles from dHL-60 cells as delivery vehicles for diverse therapeutics. Sci. Rep. 2021;11:8289. doi: 10.1038/s41598-021-87891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Claridge B., Lozano J., Poh Q.H., Greening D.W. Development of extracellular vesicle therapeutics : Challenges, considerations, and opportunities. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.734720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sushnitha M., Evangelopoulos M., Tasciotti E., Taraballi F. Cell Membrane-Based Biomimetic Nanoparticles and the Immune System : Immunomodulatory Interactions to Therapeutic Applications. Front. Bioeng. Biotechnol. 2020;8:627. doi: 10.3389/fbioe.2020.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mingliang F., Minxing J. Core-shell nanotherapeutics with leukocyte membrane camouflage for biomedical applications. J. Drug Target. 2020;28:873–881. doi: 10.1080/1061186X.2020.1757102. [DOI] [PubMed] [Google Scholar]
- 118.Sun Y., Su J., Liu G., Chen J., Zhang X., Zhang R., Jiang M., Qiu M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017;96:115–128. doi: 10.1016/j.ejps.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 119.Molinaro R., Pasto A., Taraballi F., Giordano F., Azzi J.A., Tasciotti E., Corbo C. Biomimetic nanoparticles potentiate the anti-inflammatory properties of dexamethasone and reduce the cytokine storm syndrome : An additional weapon against COVID-19 ? Nanomaterials. 2020;10:2301. doi: 10.3390/nano10112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan Q., He L., Meng X., Wang W., Pan H., Yin W., Zhu T., Huang X., Shan H. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J. Nanobiotechnology. 2021;19:173. doi: 10.1186/s12951-021-00926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu C., Zheng J., Ding Y., Meng Y., Tan F., Gong W., Chu X., Kong X., Gao C. Cepharanthine loaded nanoparticles coated with macrophage membranes for lung inflammation therapy. Drug Deliv. 2021;28:2582–2593. doi: 10.1080/10717544.2021.2009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao J., Chu D., Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Control. Release. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao J., Wang S., Wang Z. High Yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park J.H., Jiang Y., Zhou J., Gong H., Mohapatra A., Heo J., Gao W., Fang R.H., Zhang L. Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021;7:eabf7820. doi: 10.1126/sciadv.abf7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corbo C., Molinaro R., Taraballi F., Furman N.E.T., Hartman K.A., Sherman M.B., De Rosa E., Kirui D.K., Salvatore F., Tasciotti E. Unveiling the in vivo protein corona of circulating leukocyte-like carriers. ACS Nano. 2017;11:3262–3273. doi: 10.1021/acsnano.7b00376. [DOI] [PubMed] [Google Scholar]
- 126.Martinez J.O., Molinaro R., Hartman K.A., Boada C., De Rosa E., Kirui D., Zhang S., Evangelopoulos M., Angela M., Bibb J.A., Cooke J.P., Tasciotti E. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics. 2018;8:1131–1145. doi: 10.7150/thno.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Molinaro R., Corbo C., Martinez J.O., Taraballi F., Evangelopoulos M., Minardi S., Yazdi I.K., Zhao P., De Rosa E., Sherman M.B., De Vita A., Furman N.E.T., Wang X., Parodi A. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krishnamurthy S., Gnanasammandhan M., Xie C., Huang K., Cui M.Y., Chan J.M. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8:6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J., Teng C., Li C., He W. Deliver Anti-inflammatory Drug Baicalein to Macrophages by Using a Crystallization Strategy. Front. Chem. 2020;8:787. doi: 10.3389/fchem.2020.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teng C., Lin C., Huang F., Xing X., Chen S., Ye L., Azevedo H.S., Xu C., Wu Z., Chen Z., He W. Intracellular codelivery of anti-inflammatory drug and anti-miR 155 to treat inflammatory disease. Acta Pharm. Sin. B. 2020;10:1521–1533. doi: 10.1016/j.apsb.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zinger A., Sushnitha M., Naoi T., Baudo G., De Rosa E., Chang J., Tasciotti E., Taraballi F. Enhancing Inflammation targeting using tunable leukocyte-based biomimetic nanoparticles. ACS Nano. 2021;15:6326–6339. doi: 10.1021/acsnano.0c05792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molinaro R., Pasto A., Corbo C., Taraballi F., Giordano F., Martinez J.O., Zhao P., Wang X., Zinger A., Boada C., Hartman K.A., Tasciotti E. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11:13576–13586. doi: 10.1039/c9nr04253a. [DOI] [PubMed] [Google Scholar]
- 133.Molinaro R., Evangelopoulos M., Hoffman J.R., Corbo C., Taraballi F., Martinez J.O., Hartman K.A., Cosco D., Costa G., Romeo I., Sherman M., Paolino D., Alcaro S., Tasciotti E. Design and development of biomimetic nanovesicles using a microfluidic approach. Adv. Mater. 2018;30 doi: 10.1002/adma.201702749. [DOI] [PubMed] [Google Scholar]
- 134.Boada C., Zinger A., Tsao C., Zhao P., Martinez J.O., Naoi T., Sukhoveshin R., Sushnitha M., Molinaro R. Rapamycin-loaded leukosomes reverse vascular inflammation. Circ. Res. 2020;126:25–37. doi: 10.1161/CIRCRESAHA.119.315185. [DOI] [PubMed] [Google Scholar]
- 135.Fan H., Wang L., Liu W., An X., Liu Z., He X., Song L., Tong Y. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bailly C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan H., Shao D., Lao Y., Li M., Hu H., Leong K.W. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv. Sci. 2019;6:1900605. doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang H., Zang J., Zhao Z., Zhang Q., Chen S. The Advances of neutrophil-derived effective drug delivery systems : A key review of managing tumors and inflammation. Int. J. Nanomedicine. 2021;16:7663–7681. doi: 10.2147/IJN.S328705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dong X., Gao J., Zhang C.Y., Hayworth C., Frank M., Wang Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation to Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano. 2019;13:1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao J., Dong X., Su Y., Wang Z. Acta Biomaterialia Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021;123:354–363. doi: 10.1016/j.actbio.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Recchiuti A., Patruno S., Mattoscio D., Isopi E., Pomilio A., Lamolinara A., Iezzi M., Pecce R., Romano M. Resolvin D1 and D2 reduce SARS-Cov-2-induced inflammatory responses in cystic fibrosis macrophages. FASEB J. 2021;36 doi: 10.1096/fj.202001952R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li C., Wu X., Liu S., Shen D., Zhu J., Liu K. Role of resolvins in the inflammatory resolution of neurological diseases. Front. Pharmacol. 2020;11:612. doi: 10.3389/fphar.2020.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang M., Hong J., Jung M., Kwon S.P., Song S.Y., Kim H.Y., Lee J., Kang S., Han J., Koo J., Ryu J.H., Lim S., Sohn H.S., Choi J., Doh J., Kim B. T-cell-mimicking nanoparticles for cancer immunotherapy. Adv. Mater. 2020;32 doi: 10.1002/adma.202003368. [DOI] [PubMed] [Google Scholar]
- 144.Wang Q., Ren Y., Mu J., Egilmez N., Zhuang X., Deng Z., Zhang L., Yan J., Miller D., Zhang H. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75:2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Combes F., Meyer E., Sanders N.N. Immune cells as tumor drug delivery vehicles. J. Control. Release. 2020;327:70–87. doi: 10.1016/j.jconrel.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 146.Li Z., Hu S., Cheng K. Platelets and their biomimetics for regenerative medicine and cancer therapies. J. Mater. Chem. B. 2018;6:7354–7365. doi: 10.1039/C8TB02301H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu Y., Hu Q., Jiang C., Gu Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 2019;58:81–91. doi: 10.1016/j.copbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 148.Han H., Raquel B., Santos A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. 2022;172:1–15. doi: 10.1016/j.ejpb.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 149.Johnson J., Wu Y., Blyth C., Goubran H., Burnouf T., Lichtfuss G., Goubran H., Burnouf T. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39:598–612. doi: 10.1016/j.tibtech.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Wang H., Wu J., Williams G.R., Fan Q., Niu S., Wu J., Xie X., Zhu L. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnology. 2019;17:60. doi: 10.1186/s12951-019-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y., Li R., Liang J., Zhu Y., Zhang S., Zheng Z., Qin J., Pang Z., Wang J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018;11:6086–6101. [Google Scholar]
- 152.Xia Y., Ning P., Wang Z., Chen X. Calming the cytokine storm in pneumonia by biomimetic nanoparticles. Matter. 2020;3:18–20. doi: 10.1016/j.matt.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tao S., Guo S., Zhang C. Platelet-derived Extracellular Vesicles : An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017;13:828–834. doi: 10.7150/ijbs.19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soleymani S., Yari F., Bolhassani A., Bakhshandeh H. Platelet microparticles : An effective delivery system for anti-viral drugs. J. Drug Deliv. Sci. Technol. 2019;51:290–296. [Google Scholar]
- 155.Spakova T., Janockova J., Rosocha J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int. J. Mol. Sci. 2021;22:7901. doi: 10.3390/ijms22189701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma Q., Fan Q., Xu J., Bai J., Han X., Dong Z., Zhou X., Liu Z., Gu Z., Wang C. Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter. 2020;3:287–301. doi: 10.1016/j.matt.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma Q., Fan Q., Han X., Dong Z., Xu J., Bai J., Tao W., Sun D., Wang C. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Release. 2021;329:445–453. doi: 10.1016/j.jconrel.2020.11.064. [DOI] [PubMed] [Google Scholar]
- 158.Vora S.M., Lieberman J., Wu H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021;21:694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Freeman T.L., Swartz T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li J., Zhao M., Xiang X., He Q., Gui R. A novel biomimetic nanomedicine system with anti-inflammatory and anti- osteoporosis effects improves the therapy efficacy of steroid-resistant nephrotic syndrome. J. Nanobiotechnology. 2021;19:417. doi: 10.1186/s12951-021-01165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jin H., Li J., Zhang M., Luo R., Lu P., Zhang W., Pi J., Zheng W. Berberine-loaded biomimetic nanoparticles attenuate inflammation of experimental allergic asthma via enhancing IL-12 expression. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.724525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Z., Li K., Maskey A.R., Huang W., Toutov A.A., Yang N., Srivastava K., Geliebter J., Tiwari R., Miao M., Li X. A small molecule compound berberine as an orally active therapeutic candidate against COVID-19 and SARS : A computational and mechanistic study. FASEB J. 2021;35 doi: 10.1096/fj.202001792R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bhalerao A., Raut S., Noorani B., Mancuso S., Cucullo L. Molecular mechanisms of multi-organ failure in COVID-19 and potential of stem cell therapy. Cells. 2021;10:2878. doi: 10.3390/cells10112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rao D., Huang D., Sang C., Zhong T., Zhang Z., Tang Z. Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front. Bioeng. Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.797359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mazini L., Rochette L., Malka G. Exosomes contribution in COVID-19 patients’ treatment. J. Transl. Med. 2021;19:234. doi: 10.1186/s12967-021-02884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jamshidi E., Babajani A., Soltani P., Niknejad H. Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Rev. Rep. 2021;17:176–192. doi: 10.1007/s12015-020-10109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Z., Huang Y., Zhou J., Deng X., He W., Liu X., Li Y., Zhong N., Sang L. Current Status of Cell-Based Therapies for COVID-19 : Evidence From Mesenchymal Stromal Cells in Sepsis and ARDS. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.738697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Raghav A., Khan Z.A., Upadhayay V.K., Tripathi P., Gautam K.A., Mishra B.K., Ahmad J., Jeong G. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells. 2021;2:587. doi: 10.3390/cells10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen X., Wang F., Huang Z., Wu Y., Geng J., Wang Y. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases : An update and concise review. Int. J. Med. Sci. 2021;18:2849–2870. doi: 10.7150/ijms.59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alzahrani F.A., Saadeldin I.M., Ahmad A., Kumar D., Azhar E.I., Siddiqui A.J., Kurdi B., Sajini A., Alrefaei A.F., Jahan S. The potential use of mesenchymal stem cells and their derived exosomes as immunomodulatory agents for COVID-19 patients. Stem Cells Int. 2020;2020:8835986. doi: 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akbari A., Rezaie J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Res Ther. 2020;11:356. doi: 10.1186/s13287-020-01866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dauletova M., Hafsan H., Mahhengam N., Olegovna A., Ahmadi M., Siahmansouri H. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19 : A review study. Clin. Immunol. 2021;226 doi: 10.1016/j.clim.2021.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jamalkhah M., Asaadi Y., Khyavy M.A., Khanali J., Soleimani M., Kiani J., Arefian E. MSC-derived exosomes carrying a cocktail of exogenous interfering RNAs an unprecedented therapy in era of COVID-19 outbreak. J. Transl. Med. 2021;19:164. doi: 10.1186/s12967-021-02840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dehbidi M.Y., Goodarzi N., Azhdari M.H., Doroudian M. Mesenchymal stem cells and their derived exosomes to combat Covid-19. Rev. Med. Virol. 2022;32 doi: 10.1002/rmv.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abdelgawad M., Bakry N.S., Farghali A.A., Latif A.A., Lotfy A. Mesenchymal stem cell - based therapy and exosomes in COVID - 19 : current trends and prospects. Stem Cell Res Ther. 2021;12:469. doi: 10.1186/s13287-021-02542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yan Y., Zhou W., Wang Y., Guo Q., Zhao F., Zhu Z., Xing Y., Zhang H., Aljofan M., Jarrah A.M., Makabel B., Zhang J. The Potential role of extracellular vesicles in COVID-19 treatment : Opportunity and challenge. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.699929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shah T.G., Predescu D., Predescu S. Mesenchymal stem cells - derived extracellular vesicles in acute respiratory distress syndrome : a review of current literature and potential future treatment options. Clin. Transl. Med. 2019;8:25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pinky S., Gupta V., Krishnakumar Y., Sharma A.K., Dinda S. Mohanty, Mesenchymal stem cell derived exosomes : a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Rep. 2021;17:33–43. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee B., Kang I., Yu K. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J. Clin. Med. 2021;10:711. doi: 10.3390/jcm10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rezakhani L., Fatahian A., Soleimanizadeh A., Rahmati S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19 : Real opportunities and range of promises. Chem. Phys. Lipids. 2021;234 doi: 10.1016/j.chemphyslip.2020.105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun Y., Liu G., Zhang K., Cao Q., Liu T., Li J. Mesenchymal stem cells - derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. doi: 10.1186/s13287-021-02629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li J.W., Li W., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia / reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 184.Wang J., Huang R., Xu Q., Zheng G., Qiu G., Ge M., Shu Q., Xu J. Mesenchymal stem cell–derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit. Care Med. 2020;48:e599–e610. doi: 10.1097/CCM.0000000000004315. [DOI] [PubMed] [Google Scholar]
- 185.Li J., Deng X., Ji X., Shi X., Ying Z., Shen K., Xu D., Cheng Z. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf-2/ARE and NF-κB signaling pathways. PeerJ. 2020;8 doi: 10.7717/peerj.9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiao K., He W., Guan W., Hou F., Yan P., Xu J., Zhou T., Liu Y., Xie L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. doi: 10.1038/s41419-020-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]