Abstract
The relationship between first-trimester GWG (T1GWG) and risk of hypertensive disorders of pregnancy (HDP) remained uncertain. This study aimed to investigate the association between T1GWG and risk of de novo HDP. Meanwhile, we explored the mediated effect and constructed an early GWG category to evaluate the predictive capacity for HDP. T1GWG was defined as the weight difference between 13 ± 1 gestational weeks and pre-conception. HDP group was defined as having diagnosis of de novo HDP, including gestational hypertension or de novo pre-eclampsia (PE) during the current pregnancy. Early GWG category was constructed according to the risk of HDP within each pre-pregnancy body mass index (BMI) group. Cox regression model was utilized to check the association between the T1GWG and HDP. Serial mediation model was adopted to evaluate the potential mediators including mean arterial pressure (MAP) at 13th and 20th week. The logistic regression model with bootstrap was performed to assess the predictive capacity of Early GWG category and MAP for the risk of HDP. A total of 17,901 pregnant women (mean age, 29.0 years) were recruited from 2013 to 2017 at the Tongzhou Maternal and Child Health Hospital in Beijing, China. Compared to women in Class 1 of early GWG category, women in the Class 2, 3, 4 have increased risks of HDP by 1.42, 4.27, and 4.62 times, respectively (hazard ratio [HR] = 2.42, 95% CI: 2.11–2.77; HR = 5.27, 95% CI: 4.05–6.86; HR = 5.62, 95% CI: 4.05–7.79). The MAP measured at 13th and 20th week totally mediated 33.1 and 26.7% of association between T1GWG GWG and HDP in total participants and overweight/obesity pregnancies, respectively. The area under receiver operator characteristic curve for predictive model utilizing early GWG category and MAP measured at 13th and 20th week for the risk of HDP is 0.760 (95% CI: 0.739–0.777). The T1GWG was associated with de novo HDP, which was partially mediated by MAP measured at 13th and 20th week. Early GWG category showed a better predictive capacity for the risk of HDP compared to the National Academy of Medicine criteria for T1GWG.
Keywords: de novo hypertensive disorders of pregnancy, gestational weight gain, mean arterial pressure, overweight, obesity, national academy of medicine criteria
Introduction
Hypertensive disorders of pregnancy (HDP) are characterized by the abnormal elevation of blood pressure during pregnancy. It can be classified into four categories: chronic hypertension, gestational hypertension (GH), de novo and superimposed pre-eclampsia (PE), and eclampsia (1). GH and de novo PE are regarded as de novo HDP. Mean arterial pressure (MAP) was consisted of systolic blood pressure (SBP) and diastolic blood pressure (DBP) as a composite index to assess the blood pressure in clinical practice. Previous studies have inducted that the blood pressure was an important predictive factor to evaluate the risk of HDP before diagnosis (2, 3). The HDP are the leading causes of maternal and fetal morbidity and mortality globally. HDP is a dominant cause of maternal death in developing countries, accounting for one fifth of maternal deaths worldwide (4). The etiology of de novo HDP is still unknown. A recent review summarized the main modifiable risk factors, such as body mass index, anemia, lower education level; and non-modifiable risk factors, such as maternal age, primiparous, multiple pregnancy, HDP history, gestational diabetes mellitus, pre-existing type 2 diabetes mellitus, pre-existing urinary tract infection, single nucleotide polymorphism in the angiotensinogen gene, and a family history of HDP or type 2 diabetes mellitus (5).
Gestational weight gain (GWG) is a natural process during pregnancy. The change in maternal lifestyle and fetus, such as increasing nutrient intake, decreasing physical activity, and fetal growth, will both lead pregnant women to increase their body weight. Deviations from proper weight gain in either direction during pregnancy have been found to be associated with adverse pregnancy outcomes, such as small for gestational age (SGA), large for gestational age (LGA), macrosomia, cesarean delivery, gestational diabetes mellitus, PE, postpartum weight retention, and obesity of the offspring (6). The guideline for GWG from the National Academy of Medicine (NAM) have been applied to guide pregnancy weight management for over 30 years in the United States (7). The updated NAM guideline in 2009 provided a body mass index (BMI)-specific guideline (8), which provides a major set of recommendations for optimal total GWG during the entire pregnancy. HDP, usually diagnosed in the second and third trimester, was found to associate with the total GWG by several studies (9–11). Since the total GWG is calculated based on the subtraction between the pre-pregnancy and antepartum weights, the association between total GWG and HDP published previously could not provide evidence for a temporal relationship.
Gestational weight gain as a non-intrusive and easily attainable clinical indicator has been applied to estimate the risk of adverse complications, such as GDM, SGA, and LGA. Due to the absence of temporal relationship, previous studies focusing on total GWG and HDP cannot provide risk assessment of HDP in terms of GWG. This study aimed to assess the association between first-trimester GWG (T1GWG) and de novo HDP. Meanwhile we explored the mediators and evaluated the predictive capacity of T1GWG and mediators for HDP.
Materials and Methods
Study Design and Participants
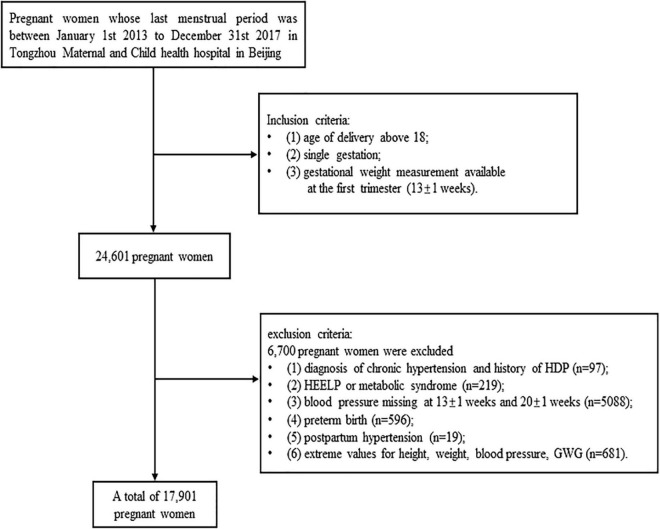
This study analyzed the data of a birth cohort, which were collected from 2013 to 2017 at Tongzhou Maternal and Child health hospital in Beijing, China. There are 17,901 pregnant women enrolled in the current study. The inclusion criteria of this cohort study were: (1) age of delivery above 18 years, (2) single gestation, (3) T1GWG available at the first trimester (13 ± 1 weeks). The exclusive criteria were: (1) diagnosis of chronic hypertension and history of HDP, (2) HEELP or metabolic syndrome, (3) blood pressure missing at 13 ± 1 and 20 ± 1 weeks, (4) preterm birth, (5) postpartum hypertension, (6) extreme values for height, weight, and blood pressure (height < 1 m or height > 2 m, weight < 30 kg or weight > 150 kg, SBP < 70 mmHg or SBP > 270 mmHg, DBP < 50 mmHg or DBP > 140 mmHg, T1GWG < −5.9 kg or T1GWG > 11 kg). The flow chart was presented as Figure 1.
FIGURE 1.
Study flowchart. Flowchart illustrating the selection of the study population in current study.
Data Collection
The sociodemographic data of pregnant women were collected from the first antenatal clinical record, such as race, age, level of education, employment condition, elder gestation, gestational season, elder gestation, and pre-pregnant weight and height. The pre-pregnant weight was obtained from the pregnant women’s self-reports. The gestational weight and blood pressure were measured by trained nurses at regular antenatal clinic. The GWG was defined as the difference between gestational weight and pre-pregnant weight. The MAP equaled to SBP plus 2/3 DBP. The pre-pregnant BMI was calculated using formula: BMI = (weight in kg) divided by (square of height in m). The pre-pregnant BMI class was identified according to the BMI criteria for Asian women (the BMI criteria of underweight, normal weight, overweight, and obesity were defined as < 18.5, 18.5–23, 23–27.5, ≥27.5) (12). De novo HDP that includes GH and de novo PE was the primary outcome of this study. The diagnosis of GH or de novo PE was made by obstetricians according to the latest Chinese clinical practice guideline which was consistent with the diagnostic guidelines in the developed countries (13). International Classification of Diseases 10 (ICD-10) codes were used to define GH and de novo PE (such as O13.01, O13.02 for GH; O11.01, O14.001, O14.101, O14.102, O14.901 for de novo PE). The text containing information related to GH and de novo PE in medical records was extracted to double confirm the disease and identify the week of disease diagnosis. The normotensive group was defined as free from any diagnosis of GH, PE, and without a history of hypertensive disorders.
Ethics
The study was approved by the Institutional Review Board of Peking University Health Science Center (No. IRB00001052-21023).
Statistics
Missing values of demographic variables, we imputed the missing data by the k-nearest neighbor (KNN) algorithm [Beretta and Santaniello (14)]. The Shapiro–Wilk test was applied to check the normality of the distribution for each variable. Normally distributed variables were presented as means with SD and analyzed with t-test for group comparisons between HDP and Normotensive groups. Non-normally distributed variables were shown as medians with interquartile range and analyzed with chi-square test or Mann–Whitney U-test.
An early GWG category (EwtGCat) in terms of the risk of HDP was constructed. The risk ratios (RR) for HDP were calculated using the multivariable Poisson regression model for each T1GWG interval within the particular pre-pregnant BMI class vs. all other women within that BMI class, borrowing the LifeCycle Project method (6). Considering the T1GWG was in a relatively small scale, we choose the 25th, 50th, 75th percentiles as the cut-off points for the category. The Class 1 of the EwtGCat included the women with underweight and normal weight pre-pregnant BMI. The women with overweight or obese during pregnancy were further grouped into class 2, 3, and 4. Class 2 was defined as the weight gain with significant negative association (RR < 1). Class 3 was the weight gain interval without statistical difference. Class 4 was the weight gain interval that showed remarkable positive association (RR > 1). The continuous T1GWG was also transformed into categorical variables using quartile method and NAM criteria. Multivariable Cox hazards regression model was utilized (15) to access the association between T1GWG (continuous, quartile, EwtGCat, and NAM criteria) and HDP after adjusting the covariables. The covariables include race, age, level of education, employment condition, elder gestation, and gestational season.
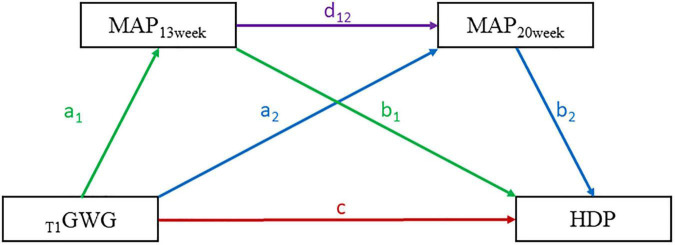
We constructed a serial mediation model to assess the effect of T1GWG on HDP with MAP at 13th and 20th weeks (MAP13 week and MAP20 week) as mediators by adjusting covariables. The direct effect (DE) defined as T1GWG to HDP. The indirect effects (IE) included three aspects: (1) IE1 was defined as the pathway from T1GWG to MAP13 week to HDP, (2) IE2 was the pathway from T1GWG to MAP20 week to HDP, (3) IE3 was the pathway from T1GWG to MAP13 week to MAP14 week to HDP. All the estimated effects were validated by bootstrapping method for 5,000 loops. The serial mediation model pattern was presented as Figure 2. The bruceR package (version 0.7.0) was adopted in this part of analyses. The stratified analyses were conducted to explore the effect of EwtGCat on the risk of HDP across different subgroups, such as parity (unipara vs. multipara), maternal age (>35 age vs. ≤35 age), conceptional season (spring/winter vs. summer/autumn), education level (high vs. low). The sensitivity analyses were further performed to assess the association between T1GWG and onset time of HDP.
FIGURE 2.
The serial mediation pattern of the association between first-trimester GWG (T1GWG) and hypertensive disorders of pregnancy (HDP). The respective colorful lines represent the different pathways of the T1GWG effects on HDP. The direct effect (DI) of T1GWG on HDP was presented by red line. The indirect effect (IE) of T1GWG to MAP13 week to HDP was presented by green lines. The IE from T1GWG to MAP13 week to MAP20 week to HDP was presented by purple lines. The IE of T1GWG to MAP20 week to HDP was presented by blue lines. The serial mediation equation included three parts: (1). MAP13 week ∼ a1*T1GWG + covariables. (2) MAP20 week ∼ a2*T1GWG + d12*MAP13 week + covariables. (3) HDP ∼ c* T1GWG + b1* MAP13 week + b2* MAP20 week + covariables. And the c was defined as the DI from T1GWG to HDP, the mediation effects were expressed as follows: (1) IE1 (T1GWG to MAP13 week to HDP) = a1*b1. (2) IE2 (T1GWG to MAP20 week to HDP) = a2*b2. (3) IE3 (T1GWG to MAP13 week to MAP20 week to HDP) = a1*d12*b2.
The adjusted logistic regression model was adopted to assess the prediction performance for HDP with 1,000 bootstrapping replications (16). Three different models were constructed and compared with each other by the Area Under Receiver Operator Characteristic Curve (AUC), sensitivity, and specificity. The models were as follow: modelNAM includes NAM criteria and covariables; modelEwtGCat included EwtGCat and covariables; modelEwtGCat&MAP included EwtGCat, MAP13 week, MAP20 week and covariables. All the statistical analyses were performed on the R software (version 4.0.0). The p-value < 0.05 was considered as significant difference.
Results
Characteristics of the Birth Cohort
Table 1 shows the maternal socio-demographical and first-trimester weight gain characteristics between de novo HDP and normotensive groups. The percentage of de novo HDP in our study was 5.31%. The 17,901 pregnant women included in this study had the mean maternal age of 29.0 years (SD = 3.8). Women in the following strata had a significantly higher percentage of HDP diagnosis: educational level with high school or lower, overweight or obesity with pre-pregnant BMI class and unipara. The T1GWG was significantly higher in HDP group compared with normotensive women (p = 0.001). The detailed characteristics of the participants are listed in Table 1.
TABLE 1.
Maternal socio-demographical and first-trimester weight gain characteristics.
| Characteristics | No HDP (N = 16950) |
HDP (N = 951) |
P-value |
| T1GWG, mean(SD) | 1.2 (2.4) | 1.5 (2.5) | 0.001 |
| MAP13 week, mean (SD) | 83.2 (8.2) | 88.9 (8.2) | <0.001 |
| MAP20 week, mean (SD) | 80.6 (8.0) | 86.9 (8.3) | <0.001 |
| Age, mean(SD) | 29.0 (3.8) | 29.1 (3.8) | 0.800 |
| Education level, N(%) | |||
| High school or lower | 4269 (25.2) | 286 (30.1) | |
| Vocational college | 5440 (32.1) | 322 (33.9) | <0.001 |
| University or above | 7241 (42.7) | 343 (36.1) | |
| Employment condition, N(%) | |||
| Unemployment | 2220 (13.1) | 128 (13.5) | 0.700 |
| Employment | 14730 (86.9) | 823 (86.5) | |
| Race, N(%) | |||
| Minority | 1031 (6.1) | 44 (4.6) | 0.066 |
| Ethnic Han | 15919 (93.9) | 803(95.4) | |
| Maternal age (>35 age), N(%) | |||
| No | 15432 (91.0) | 664 (89.5) | 0.100 |
| Yes | 1518 (9.0) | 189 (10.5) | |
| Parity, N(%) | |||
| Unipara | 12447 (73.4) | 745 (78.3) | <0.001 |
| Multipara | 4503 (26.6) | 206 (21.7) | |
| Pre-pregnant BMI class, N(%) | |||
| Underweight | 1927 (11.4) | 45 (4.7) | <0.001 |
| Normal weight | 9596 (56.6) | 370 (38.9) | |
| Overweight | 4490 (26.5) | 391 (41.1) | |
| Obesity | 937 (5.5) | 145 (15.2) | |
| Conceptional season, N(%) | |||
| Spring | 4044 (23.9) | 311 (32.7) | <0.001 |
| Summer | 3696 (21.8) | 189 (19.9) | |
| Autumn | 4427 (26.1) | 161 (16.9) | |
| Winter | 4783 (28.2) | 290 (30.5) |
T1GWG, first trimester gestational weight gain; HDP, hypertensive disorders of pregnancy; BMI, body mass index; N, number; MAP, mean arterial pressure.
The Construction of Early Gestational Weight Gain Category
The Early GWG Category (EwtGCat) was constructed according to the Supplementary Table 1. The Class 1 of EwtGCat was identified as the women with underweight and normal weight pre-pregnant BMI classes, and there was no association between T1GWG and HDP. As for underweight and obesity, the significant negative association was obtained in Class 2 which the interval of T1GWG was below 0 kg. Class 3 was the interval of T1GWG from 0 to 2.5 kg which we failed to observe any statistical association. Class 4 was the interval of T1GWG above 2.5 kg which presented a significant positive association between T1GWG and HDP.
The Association Between T1GWG and de novo Hypertensive Disorders of Pregnancy
As the Table 2 shown, T1GWG was significantly higher in the HDP pregnancies compared with normotensive women (HR = 1.07, 95% CI: 1.04–1.09, p < 0.001). In comparison with women in the lowest quartile of T1GWG, the hazards of HDP elevated in the women with Q3, Q4 of T1GWG (HR = 1.29, 95% CI: 1.08–1.55, p = 0.005; HR = 1.43, 95% CI: 1.21–1.69, p < 0.001). There were significant associations between T1GWG and HDP in the Class 2, 3, 4 compared with Class 1 under EGC criteria (HR = 2.42, 95% CI: 2.11–2.77, p < 0.001; HR = 5.27, 95% CI: 4.05–6.86, p < 0.001; HR = 5.62, 95% CI: 4.05–7.79, p < 0.001). However, there is no significant hazard difference between normal and abnormal groups under NAM criteria (HR = 1.04, 95% CI: 0.91–1.20, p = 0.551).
TABLE 2.
Hazard ratios for the association between the first trimester gestational weight gain (T1GWG) and hypertensive disorders of pregnancy (HDP).
| No HDP (N) | HDP (N) | HR | 95% CI | P-value | |
| T1GWG (continuous) | 16950 | 951 | 1.07 | 1.04–1.09 | <0.001 |
| T1GWG quartile | |||||
| Q1 | 6055 | 298 | ref | ||
| Q2 | 3271 | 181 | 1.19 | 0.99–1.43 | 0.065 |
| Q3 | 3510 | 208 | 1.29 | 1.08–1.55 | 0.005 |
| Q4 | 4114 | 264 | 1.43 | 1.21–1.69 | <0.001 |
| EwtGCat (category, N) | |||||
| Class 1 | 11523 | 415 | ref | ||
| Class 2 | 4916 | 432 | 2.42 | 2.11–2.77 | <0.001 |
| Class 3 | 308 | 64 | 5.27 | 4.05–6.86 | <0.001 |
| Class 4 | 203 | 40 | 5.62 | 4.05–7.79 | <0.001 |
| NAM recommendation (category, N) | |||||
| Normal | 11983 | 666 | ref | ||
| Abnormal | 4967 | 285 | 1.04 | 0.91–1.20 | 0.551 |
T1GWG, the first trimester gestational weight gain; EwtGCat, early gestational weight gain categories; HDP, hypertensive disorders of pregnancy; NAM, national academy of medicine; HR, hazard ratios; CI, confidence interval. The HR was adjusted by race, age, education level, elderly maternal employment condition, parity. The cutoff points of Q1, Q2, Q3, Q4 were defined by the 25th, 50th, 75th quartiles of the distribution of T1GWG.
The Serial Mediation Effect of Mean Arterial Pressure on the Association Between T1GWG and Hypertensive Disorders of Pregnancy
The Table 3 indicated that MAP13 week and MAP20 week totally mediated 37.7% of association between T1GWG and HDP in all participants (IEtotal = 1.001 × 10–3, p < 0.001; DE = 2.654 × 10–3, p < 0.001). The indirect mediation effect was consisted in 3 pathways, such as (1) T1GWG to MAP13 week to HDP (IE1 = 3.330 × 10–4, mediate proportion: 12.5%, p < 0.001); (2) T1GWG to MAP20 week to HDP (IE2 = 3.970 × 10–4, mediate proportion: 15.0%, p < 0.001); (3) T1GWG to MAP13 week to MAP20 week to HDP (IE3 = 2.710 × 10–4, mediate proportion: 10.2%, p < 0.001). We reran the serial mediation model to test the IE of MAP in overweight/obesity women, which is similar to the results of all participants. The MAP13 week and MAP14 week mediated 26.7% of total effect of T1GWG on HDP. IE1, IE2, and IE3 explained 6.1, 13.7, and 6.9%, respectively, of the total effect through 3 mediated pathways (all p < 0.001). However, we could not detect meaningful mediation effect in the underweight and normal weight women.
TABLE 3.
Mediation effect of mean arterial pressure (MAP) to the association between the T1GWG and HDP.
| Participant | Mediation effect | Estimate | 95% CI | Proportion | P-value |
| Total participants | |||||
| Indirect effect | 1.001 × 10–3 | (7.020 × 10–4, 1.273 × 10 –3) | 37.7% | <0.001 | |
| T1GWG-MAP13 week-HDP | 3.330 × 10–4 | (2.060 × 10 –4, 4.880 × 10 –4) | 12.5% | <0.001 | |
| T1GWG -MAP20 week-HDP | 3.970 × 10–4 | (2.260 × 10 –4, 5.670 × 10 –4) | 15.0% | <0.001 | |
| T1GWG -MAP13 week-MAP20 week-HDP | 2.710 × 10–4 | (1.700 × 10 –4, 3.700 × 10 –4) | 10.2% | <0.001 | |
| Direct effect | |||||
| T1GWG -HDP | 1.652 × 10–3 | (1.860 × 10 –4, 3.105 × 10 –3) | 62.3% | 0.026 | |
| Total effect | 2.654 × 10–3 | (1.157 × 10 –3, 4.113 × 10 –3) | 100.0% | <0.001 | |
| Overweight/obesity participants | |||||
| Indirect effect | 1.853 × 10–3 | (1.233 × 10–3, 2.563 × 10–3) | 26.7% | <0.001 | |
| T1GWG -MAP13 week-HDP | 4.200 × 10–4 | (1.780 × 10–4, 7.120 × 10–4) | 6.1% | <0.001 | |
| T1GWG -MAP20 week-HDP | 9.500 × 10–4 | (5.630 × 10–4, 1.386 × 10–3) | 13.7% | <0.001 | |
| T1GWG -MAP13 week-MAP20 week-HDP | 4.810 × 10–4 | (2.240 × 10–4, 7.680 × 10–4) | 6.9% | <0.001 | |
| Direct effect | |||||
| T1GWG -HDP | 5.088 × 10–3 | (2.126 × 10–3, 7.987 × 10–3) | 73.3% | <0.001 | |
| Total effect | 6.941 × 10–3 | (4.035 × 10–3, 9.835 × 10–3) | 100.0% | <0.001 |
T1GWG, the first trimester gestational weight gain; MAP, mean arterial pressure; HDP, hypertensive disorders of pregnancy. The serial mediation model was adjusted by race, age, level of education, employment condition, maternal age (>35 age), conceptional season. The pattern of serial mediation model was presented in Figure 2.
The Subgroup Analyses for the Early Gestational Weight Gain Category
The stratified analyses were conducted to assess the effect of EwtGCat on HDP under different variables. The Class 1 and Class 2 of EwtGCat were defined as low EwtGCat and the Class 3 and Class 4 were defined as high EwtGCat. The Figure 3 showed that high EwtGCat was elevated the risk of HDP especially in the women with multipara, maternal age above 35, spring/winter conception and low education level (HR = 3.338, 95% CI: 1.607–6.936; HR = 3.004, 95% CI: 1.119–8.064; HR = 2.313, 95% CI: 1.447–3.696; HR = 2.704, 95% CI: 1.431–5.016, respectively). In the sensitivity analyses, we failed to observe a significant association between T1GWG and onset time of HDP (p = 0.677).
FIGURE 3.
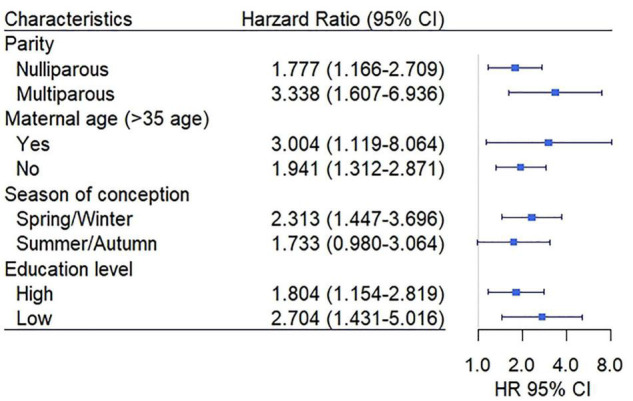
The association between an early GWG category (EwtGCat) and HDP under different subgroup. The Class 1 and Class 2 were defined as low EwtGCat, and Class 3 and Class 4 were grouped into high EwtGCat. The low education level represents high school or below and the high education level includes vocational college and university or above.
Predictive Performance of Early Gestational Weight Gain Category for Hypertensive Disorders of Pregnancy
We assessed the predictive capacity for HDP by modelNAM, modeleEwtGCat, and modelEwtGCat&MAP. As shown in Figure 4, the AUC, sensitivity, and specificity of the modelNAM, which included NAM criteria and covariables, were 0.587 (95% CI: 0.561–0.611), 0.544 (95% CI: 0.463–0.606), and 0.578 (95% CI: 0.492–0.667). In terms of modelEwtGCat, the AUC was improved to 0.668 (95% CI: 0.649–0.688). The modelEwtGCat&MAP demonstrated the best predictive performance for HDP comparing to the other two model (AUC = 0.760, 95% CI: 0.739–0.777; sensitivity = 0.703, 95% CI: 0.677–0.719; specificity = 0.686, 95% CI: 0.635–0.726). The detail information of the predictive models was listed in Supplementary Table 2.
FIGURE 4.
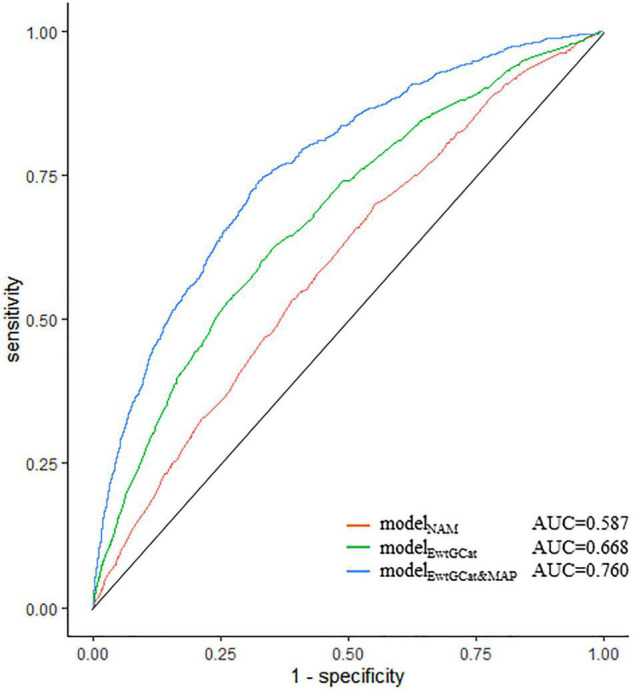
The predictive capacity of prediction models for HDP. The ROC plot demonstrated the predictive capacity of prediction models. The modelNAM, modelEwtGCat, and modelEwtGCat&MAP were presented in red, green, and blue curves, respectively. The modelNAM included NAM criteria; modelEwtGCat included EwtGCat, modelEwtGCat&MAP was consisted of EwtGCat, MAP13 week, MAP2 week. All the models were adjusted by race, age, level of education, employment condition, maternal age (>35 age), gestational season.
Discussion
In this study, we observed a significant association between T1GWG and HDP. The MAP13 week and MAP20 week mediated the association between T1GWG and HDP. Meanwhile, we established a risk-specific EwtGCat to assess the risk of HDP according to the pre-pregnant BMI class and T1GWG of pregnant women. The combination of EwtGCat and MAP showed remarkable greater predictive capacity for HDP in comparison with NAM criteria only.
The previous studies mainly focus on the relationship between total GWG and adverse outcome during the total gestation (17–19). There are few studies investigating the effect of GWG during the first trimester on HDP. And the current tools such as NAM criteria did not access the GWG elevation during the first trimester in terms of HDP (20, 21). The GWG recommendation of NAM criteria provided a different recommended range of GWG per each pre-pregnant BMI class. According to the cut-off points from the NAM criteria, several studies had demonstrated an association between total GWG and HDP (22–24). However, edema and fluid retention that commonly occurred during later trimesters can potentially confound the accurate effect of GWG on HDP (25). The NAM guideline was conducted to reduce the risk of multiple adverse outcomes (26), which failed to distinguish the risk of HDP during this period. In contrast, EGC were constructed by evaluating the risk of HDP per pre-pregnant BMI class. Our study constructed a novel specific-risk category to assess the effect of GWG on the risk of de novo HDP and discovered that excessive GWG elevated the risk of HDP in the first trimester, which filled the research gap in the early gestation period.
Abnormal elevated blood pressure was the core symptom of HDP. Numerous studies had demonstrated that the elevated blood pressure associated with the risk of HDP during pregnancy (27–29). However, there were few studies to explore the effect of T1GWG on blood pressure before the HDP diagnosis. To our knowledge, our study is the first study to assess the serial mediation effects of blood pressure on the association between T1GWG and HDP before 20 weeks. Our mediation analyses indicated that the serial mediation effects were significant in the total participants and overweight/obesity pregnancies. Thus, the blood pressure before 20 weeks was an independent mediator involving in the association between T1GWG and HDP. Furthermore, we compared the capacities of EwtGCat and NAM criteria to predict the risk of HDP. The combination of EwtGCat, MAP13 week, and MAP20 week showed significantly higher capacity to predict HDP, compared to the NAM during the first trimester.
Hypertensive disorders of pregnancy is one of the most common gynecological diseases that affects 10% of pregnancies (30). An epidemiological study showed that 10–16% of maternal mortality worldwide was attributed to HDP and this disease was also associated with both a short- and long-term substantial disease burden (31, 32). Increasing number of studies supported that endothelial damage, vascular inflammation, and metabolic dysfunction participate the development of HDP, such as endothelial pathway, NF-κb signaling pathway, abnormal glucose-metabolism, and dyslipidemia (33–36). However, the early identification of HDP remains significantly limited in the clinical practice. GWG is a potential clinical indicator for the risk of HDP, which is non-intrusive and routinely measured in clinical practice. During the first trimester, weight gain came mainly from fat accumulation, while weight of the fetus, extravascular fluid, and maternal fat contributed to weight gain in the later trimesters. Our study focused on the weight gain during the first trimester, which was less likely to be affected by the above concern.
The potential mechanism underlying the association between T1GWG and HDP remained elusive. In the previous studies, maternal obesity was considered as an important risk factor for HDP. A multicenter Chinese retrospective study showed that overweight and obesity were a risk factor for HDP (37). Another Japanese study obtained a similar result that obese pregnant women were significantly associated with an increased risk of HDP (38). Current theory believes that obesity is a chronic inflammation and accumulating studies have found abnormal immune cells and cytokines in pregnant women with obesity such as CD4 + T cells, macrophages, IL-6, and TNF-α (39–42). Endothelial damage and vascular inflammation are the underlining pathological modifications at every stage of HDP development.
Some strengths were presented in this study. First, the gestational weight gain by the end of first trimester was used to study the temporal relationship between GWG and de novo HDP. Second, we constructed a risk-specific EwtGCat which showed greater capacity to identify the risk of de novo HDP. Third, this is the first study to explore the mediated mechanism underlying the association between T1GWG and HDP. Fourth, we illustrated a great potential for using EwtGCat and MAP for the prediction of HDP risk. Meanwhile, there are some limitations to our present study. First, the study subjects were all from the Tongzhou Maternal and Child Health Hospital (Beijing, China), which represent the northern Chinese population. Second, we did not collect the information of maternal lifestyle, such as the physical exercise and stress condition. These factors may be the potential confounding bias on the present study.
Conclusion
The GWG during the first trimester was associated with the risk of de novo HDP. MAP13 week and MAP20 week partially mediated the association between T1GWG and HDP. Early GWG category showed a better predictive capacity for the risk of HDP compared to the NAM criteria for first-trimester GWG. Therefore, the pregnancies were supposed to keep the gestational weight gain in an appropriated range to avoid the hazards of HDP. The overweight and obese women especially need to pay more attention on their blood pressure during pregnancy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Peking University Health Science Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YJ and ZY: conceptualization, formal analysis, and methodology. ZY, JC, YP, YJ, SZ, HB, HW, SL, JY, and JL: data curation. H-JW: funding acquisition and supervision. ZY, JC, YP, YJ, SZ, HB, HW, and SL: investigation. H-JW, NH, and TS: project administration. ZY: visualization and writing – original draft. YJ and H-JW: writing – review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the study members for their valuable contribution in this study.
Funding
This study was funded by the National Natural Science Foundation of China (92046019) and the Beijing Natural Science Foundation (7212144).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.862323/full#supplementary-material
References
- 1.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. 10.1161/hypertensionaha.117.10803 [DOI] [PubMed] [Google Scholar]
- 2.Gasse C, Boutin A, Cote M, Chaillet N, Bujold E, Demers S. First-trimester mean arterial blood pressure and the risk of preeclampsia: the Great Obstetrical Syndromes (GOS) study. Pregnancy Hypertens. (2018) 12:178–82. 10.1016/j.preghy.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Wang X, Hu W, Chen Y, Ning W, Lu S, et al. A risk model that combines MAP, PlGF, and PAPP-A in the first trimester of pregnancy to predict hypertensive disorders of pregnancy. J Hum Hypertens. (2022) 36:184–91. [DOI] [PubMed] [Google Scholar]
- 4.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. (2006) 367:1066–74. 10.1016/S0140-6736(06)68397-9 [DOI] [PubMed] [Google Scholar]
- 5.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. (2017) 40:213–20. 10.1038/hr.2016.126 [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen KM, Yaktine AL. editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; (2009). [PubMed] [Google Scholar]
- 8.World Health Organization [WHO]. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization; (1995). p. 1–452. [PubMed] [Google Scholar]
- 9.Zhou A, Xiong C, Hu R, Zhang Y, Bassig BA, Triche E, et al. Pre-pregnancy BMI, gestational weight gain, and the risk of hypertensive disorders of pregnancy: a cohort study in Wuhan, China. PLoS One. (2015) 10:e0136291. 10.1371/journal.pone.0136291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan P, Tang F, Sun G, Ren W. Effect of maternal weight gain according to the Institute of Medicine recommendations on pregnancy outcomes in a Chinese population. J Int Med Res. (2019) 47:4397–412. 10.1177/0300060519861463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Premru-Srsen T, Kocic Z, Fabjan Vodusek V, Gersak K, Verdenik I. Total gestational weight gain and the risk of preeclampsia by pre-pregnancy body mass index categories: a population-based cohort study from 2013 to 2017. J Perinat Med. (2019) 47:585–91. 10.1515/jpm-2019-0008 [DOI] [PubMed] [Google Scholar]
- 12.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 13.Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. [Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China2020]. Zhonghua Fu Chan Ke Za Zhi. (2020) 55:227–38. 10.3760/cma.j.cn112141-20200114-00039 [DOI] [PubMed] [Google Scholar]
- 14.Beretta L, Santaniello A. Nearest neighbor imputation algorithms: a critical evaluation. BMC Med Inform Decis Mak. (2016) 16:74. 10.1186/s12911-016-0318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Li J, Qin G, Liew Z, Hu J, Laszlo KD, et al. Maternal hypertensive disorder of pregnancy and offspring early-onset cardiovascular disease in childhood, adolescence, and young adulthood: a national population-based cohort study. PLoS Med. (2021) 18:e1003805. 10.1371/journal.pmed.1003805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. (2001) 54:774–81. 10.1016/s0895-4356(01)00341-9 [DOI] [PubMed] [Google Scholar]
- 17.Garnaes KK, Morkved S, Salvesen O, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP Trial). PLoS Med. (2016) 13:e1002079. 10.1371/journal.pmed.1002079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth. (2020) 20:390. 10.1186/s12884-020-03071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Wan S, Gu S, Mou Z, Dong L, Luo Z, et al. Gestational weight gain and adverse pregnancy outcomes: a prospective cohort study. BMJ Open. (2020) 10:e038187. 10.1136/bmjopen-2020-038187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhstaller KE, Bastek JA, Thomas A, McElrath TF, Parry SI, Durnwald CP. The effect of early excessive weight gain on the development of hypertension in pregnancy. Am J Perinatol. (2016) 33:1205–10. 10.1055/s-0036-1585581 [DOI] [PubMed] [Google Scholar]
- 21.Hutcheon JA, Stephansson O, Cnattingius S, Bodnar LM, Wikstrom AK, Johansson K. Pregnancy weight gain before diagnosis and risk of preeclampsia: a population-based cohort study in nulliparous women. Hypertension. (2018) 72:433–41. 10.1161/HYPERTENSIONAHA.118.10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. (2013) 209:e1–17. 10.1016/j.ajog.2013.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren M, Li H, Cai W, Niu X, Ji W, Zhang Z, et al. Excessive gestational weight gain in accordance with the IOM criteria and the risk of hypertensive disorders of pregnancy: a meta-analysis. BMC Pregnancy Childbirth. (2018) 18:281. 10.1186/s12884-018-1922-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrasekaran S, Levine LD, Durnwald CP, Elovitz MA, Srinivas SK. Excessive weight gain and hypertensive disorders of pregnancy in the obese patient. J Matern Fetal Neonatal Med. (2015) 28:964–8. 10.3109/14767058.2014.939624 [DOI] [PubMed] [Google Scholar]
- 25.Lewandowska M, Wieckowska B, Sajdak S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. J Clin Med. (2020) 9:1980. 10.3390/jcm9061980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badon SE, Quesenberry CP, Xu F, Avalos LA, Hedderson MM. Gestational weight gain, birthweight and early-childhood obesity: between- and within-family comparisons. Int J Epidemiol. (2020) 49:1682–90. 10.1093/ije/dyaa110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao W, Dong M, Sun X, Liu X, Xiao J, Feng B, et al. Associations of maternal ozone exposures during pregnancy with maternal blood pressure and risk of hypertensive disorders of pregnancy: a birth cohort study in Guangzhou, China. Environ Res. (2020) 183:109207. 10.1016/j.envres.2020.109207 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Wang X, Hu W, Chen Y, Ning W, Lu S, et al. A risk model that combines MAP, PlGF, and PAPP-A in the first trimester of pregnancy to predict hypertensive disorders of pregnancy. J Hum Hypertens. (2021) 36:184–91. 10.1038/s41371-021-00488-6 [DOI] [PubMed] [Google Scholar]
- 29.Vermunt JV, Kennedy SH, Garovic VD. Blood pressure variability in pregnancy: an opportunity to develop improved prognostic and risk assessment tools. Curr Hypertens Rep. (2020) 22:10. 10.1007/s11906-019-1014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. (2009) 33:130–7. 10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 31.Creanga AA. Maternal mortality in the United States: a review of contemporary data and their limitations. Clin Obstet Gynecol. (2018) 61:296–306. 10.1097/GRF.0000000000000362 [DOI] [PubMed] [Google Scholar]
- 32.Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. (2016) 4:1025–36. 10.1016/S2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- 33.Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta. (2005) 26:53–8. 10.1016/j.placenta.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 34.Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. (2004) 164:1049–61. 10.1016/s0002-9440(10)63192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. (2009) 30:S43–8. 10.1016/j.placenta.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramlakhan KP, Johnson MR, Roos-Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol. (2020) 17:718–31. [DOI] [PubMed] [Google Scholar]
- 37.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. (2014) 9:e100180. 10.1371/journal.pone.0100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimura R, Kohmura-Kobayashi Y, Narumi M, Furuta-Isomura N, Oda T, Tamura N, et al. Comparison of three classification systems of Prepregnancy Body Mass Index with Perinatal Outcomes in Japanese Obese Pregnant Women: a retrospective study at a single center. Int J Med Sci. (2020) 17:2002–12. 10.7150/ijms.47076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. (2010) 54:281–94. 10.1387/ijdb.082763jb [DOI] [PubMed] [Google Scholar]
- 40.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. (2008) 51:982–8. 10.1161/HYPERTENSIONAHA.107.108837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. (2013) 6:650–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. (2007) 30:S120–6. 10.2337/dc07-s203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.