Abstract
Infusions of cholinomimetics, either systemically in normal humans, or directly into the brain stem of unanesthetized cats and rats, readily induce rapid eye-movement (REM) sleep. In anesthetized cats and rats, infusions of cholinomimetics have been shown to produce both increases and decreases in arterial blood pressure. We determined the relation of these blood pressure changes to REM sleep, by examining both blood pressure and sleep states after injecting carbachol at midbrain, pontine, and medullary sites in unanesthetized cats. In the pons, carbachol infusions produced an early decrease in blood pressure followed by a sustained hypertensive effect. The early blood pressure decrease was associated with the occurrence of REM sleep; however, higher values were associated with later REM sleep episodes. In other brain stem sites carbachol did not produce REM sleep or its associated reductions in blood pressure. Instead it produced a hypertensive response that increased throughout the 1-h observation period. We hypothesize that pontine muscarinic mechanisms trigger REM sleep and a REM sleep-associated decrease in blood pressure. Thereafter, nicotinic receptors mediating the blood pressure increase override the muscarinic-induced decrease.
INTRODUCTION
A number of studies have shown that administration of cholinergic compounds either systemically in normal humans (22) or locally into the pontine tegmentum of unanesthetized rats (18) and cats (1–3, 9–11, 16, 19–21) promotes rapid eye-movement (REM) sleep. In anesthetized rats and cats such infusions produce marked alterations in blood pressure (BP). For example, in anesthetized rats, infusions of carbachol, a mixed cholinergic receptor agonist, into the fourth ventricle produces an initial decrease in BP followed by a prolonged pressor response (5, 12). Carbachol infusions into the medullary region between the medial vestibular nucleus and the solitary tract nucleus produce a marked hypertensive response (5). Push–pull infusions of acetylcholine or carbachol in the medulla of anesthetized cats produce a pressor response (4). Muscarinic mechanisms have been hypothesized to contribute to the initial hypotensive response whereas nicotinic receptors mediate the longer lasting pressor response (5). Muscarinic activation is also implicated in generation of REM sleep (19, 20) and, indeed, BP values in the cat are lowest during REM sleep (15).
The relation of cholinergic mechanisms regulating BP and REM sleep remains unclear, however, as no study has examined the BP effects due to brain stem cholinergic infusion in unanesthetized animals and related the BP change to sleep states. Accordingly, we studied in freely behaving cats, the arterial blood pressure and sleep effects after infusions of carbachol at midbrain, pontine, and medullary sites. The results indicated that pontine carbachol infusions produced an initial hypotensive effect linked to REM sleep.
METHODS
Under Nembutal anesthesia (35 mg/kg) four cats were chronically implanted with a standard array of sleep-recording electrodes including bilateral, tripolar depth electrodes in the lateral geniculate nucleus to record pontogeniculooccipital (PGO) waves, and electrodes to record the dorsal neck electromyogram (EMG), cruciate electroencephalogram (EEG), and eye movements. From one to three stainless-steel cannulas targeted bilaterally into various brain stem sites were also implanted. The cannulas consisted of a 45-mm length of 23-gauge stainless-steel tubing. Stylettes of equal length were inserted into the cannulas to prevent occlusion. An Intramedic PE-100 catheter was inserted into the femoral artery so that its tip rested in the abdominal aorta. The catheter was placed subcutaneously to terminate at the headplug. A continuous slow drip of heparinized Ringer’s solution administered at 3 ml/h preserved the patency of the catheter. Arterial blood pressure (BP) was monitored using a Statham pressure transducer.
One week after recovery from surgery the experimental protocol commenced. All infusions were made with a 33-gauge stainless-steel cannula attached to PE tubing and a Hamilton microliter syringe. The cannula, PE tubing, and syringe were prefilled with Ringer’s or carbachol solution before the cannula was lowered into the brain.
Ringer’s infusions commenced when the animals displayed an EEG pattern indicative of drowsiness, i.e., long trains of 4- to 16-Hz sensorimotor rhythms with well sustained, regular EMG. A total volume of 0.5 μl Ringer’s was injected during 5 min (0.1 μl/min), and sleep and BP recordings were obtained until cessation of at least one REM sleep episode.
Subsequently, the Ringer’s cannula was replaced with one containing carbachol solution and infusions commenced when the animals showed signs of drowsiness. A total volume of 0.5 μl carbachol (8 μg/μl) solution was injected (0.1 μl/min) and sleep recordings were obtained for at least 1 h. Both Ringer’s and carbachol were sequentially infused during each recording session.
In subsequent experimental sessions, infusions were made using the other implanted cannulas. At least 48 h elapsed between sessions. After exploring all implanted cannulas, a lethal dose of Nembutal was administered and the animals perfused through the heart with 0.9% saline followed by 10% Formalin. The brains were removed and placed in 30% sucrose–Formalin solution. Histological verification of infusion sites was made by examining 40-μm-thick frozen sections stained with cresyl violet.
The sleep data were scored in 60-s blocks for the number of seconds of EEG desynchrony, atonia (lack of EMG tone), and rapid eye movements. An index of PGO activity was obtained by counting the number of PGO spikes during 10 s in the middle of each 1-min block. Mean BP during the 1-min block was determined using an Apple–Isaac (Cyborg Corp.) computer system programmed to take a 10-s sample of the BP at 1-min intervals. The two BP samples spanning each 1-min block were then averaged and nonparametric statistics were used to compare BP averages between groups.
RESULTS
Four groups were formed after histological verification of the cannula locus: (A) midbrain, (B) pontine, (C) pontomedullary, and (D) medullary. Figure 1 summarizes the locations of the infusion sites and the number of infusions made at each site.
FIG. 1.
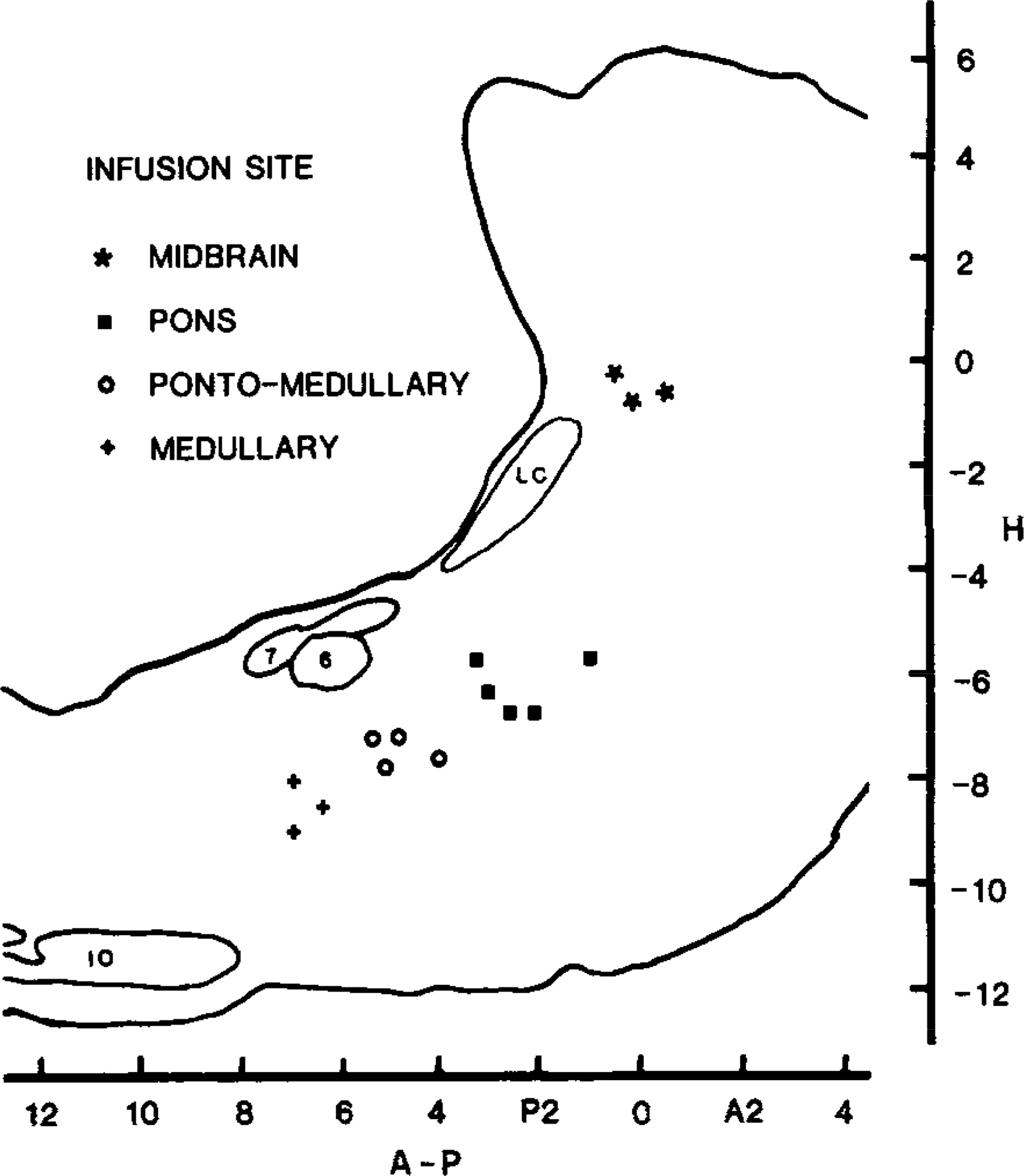
Composite brain stem sagittal sections summarize the loci of the carbachol infusion sites for four cats. In the pons, pontomedullary, and medullary sites the infusions were made at L 1.6, and in the midbrain infusions were at L 3.2. Abbreviations: IO—inferior olive, LC—locus ceruleus, 6—nucleus of the sixth nerve, 7—genu of the seventh nerve.
Figure 2 summarizes the changes in BP after brain stem carbachol microinfusions. During control recordings the BP was significantly lower in all groups during REM sleep compared with non-REM sleep (Wilcoxon matched-pairs test, T = −1; P < 0.01). Carbachol produced a rapid increase in BP in all groups except the pontine group. During the first 15 min after carbachol infusion, BP values in the pontine group were significantly lower than the other groups (Mann–Whitney U test, P < 0.05). In this group BP was on average at or below the value seen in control REM sleep periods. This response occurred in conjunction with REM sleep identified by atonia, PGO spikes, EEG desynchrony, and rapid eye movements. REM sleep occurred after all pontine infusions with a mean latency of 6.8 ± 2.8 min. Midbrain, pontomedullary, or medullary carbachol infusions were not followed by any tonic or phasic component of REM sleep within the first 20 min.
FIG. 2.
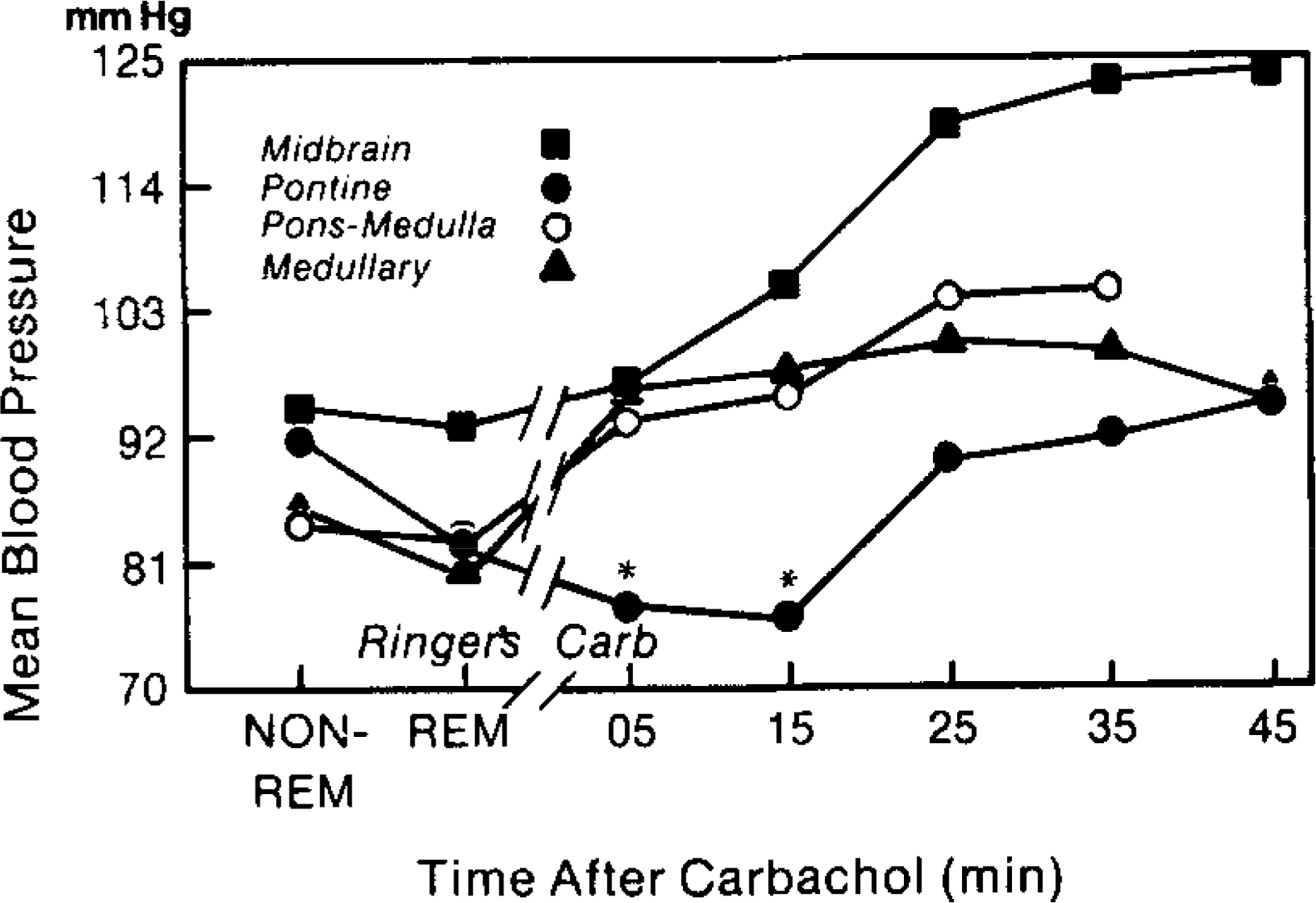
Alterations in blood pressure (BP) after carbachol infusions into midbrain, pons, pontomedullary, and medullary regions. The figure summarizes mean BP during Ringer’s-REM and non-REM and during the 5-min period of carbachol infusion and at 10-min intervals following infusion. BP levels during Ringer’s-REM sleep were lower than non-REM values. Pontine carbachol infusions significantly (P < 0.05) decreased BP during the 5- and 15-min intervals (asterisks). In the pontine group the BP decrease occurred in conjunction with REM sleep.
Figure 3 illustrates the time-dependent changes in BP and REM sleep after pontine carbachol infusion. Figure 3A presents results of an experiment in which carbachol infusions evoked all the tonic and phasic components of REM sleep for 20 min following the infusion. Initially, BP decreased after carbachol infusion, then progressively increased and 37 min later, when another REM sleep episode occurred, was higher than in previous REM sleep episodes. Figure 3B presents results of an experiment in which REM sleep was present for the first 10 min following carbachol infusion. Thereafter, the animal demonstrated atonia, rapid eye movements, and desynchronized EEG, but PGO activity was not observed. Note that the BP progressively increased during the carbachol-induced atonia state.
FIG. 3.
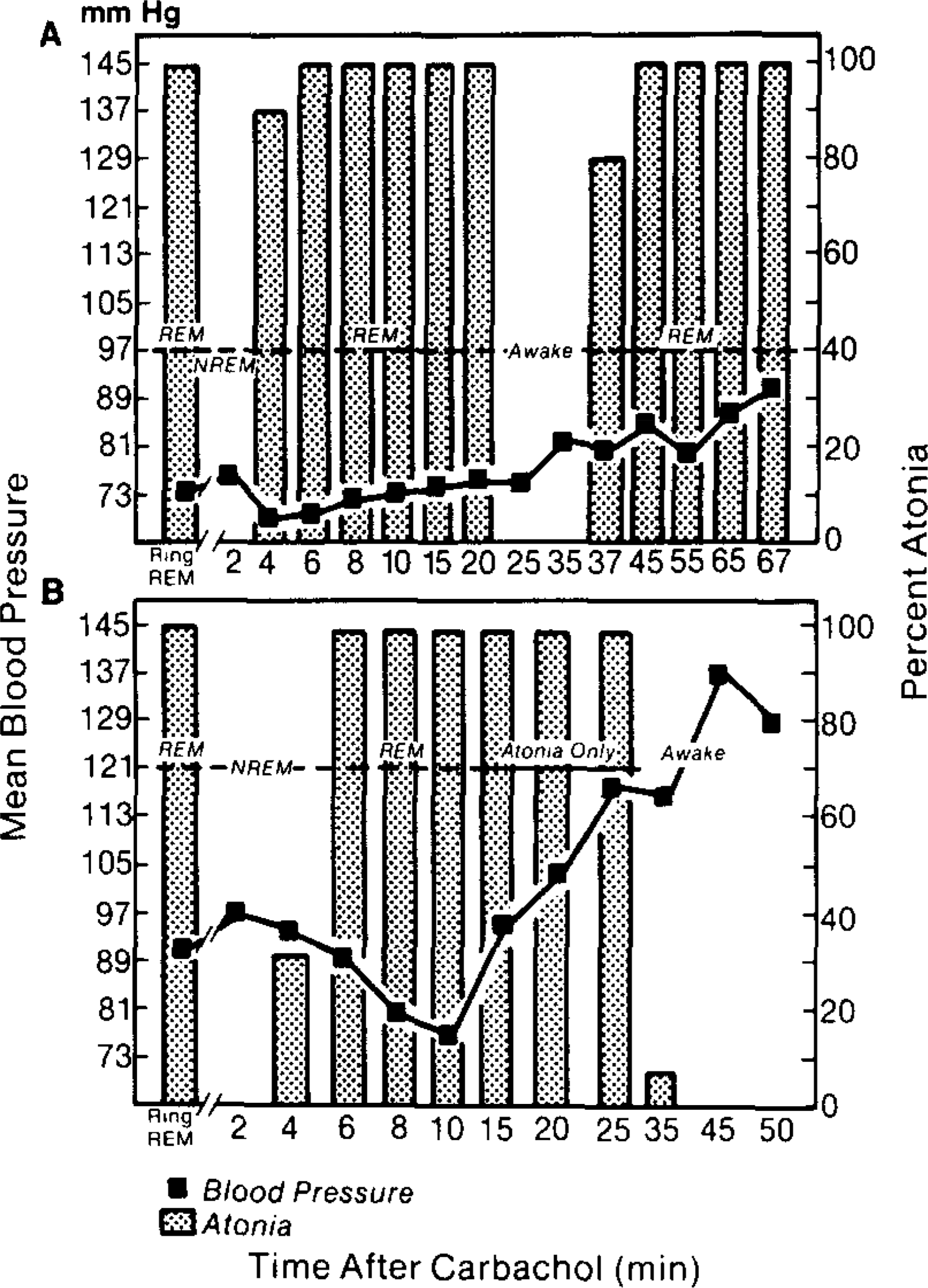
Mean BP and atonia (shaded bars) in two experimental sessions in which carbachol was infused into the pons. BP and atonia during control REM sleep are indicated by Ring REM. In A carbachol produced a REM sleep episode lasting 20 min. During this period the BP initially decreased and then was similar to control REM episodes. There was a progressive increase in BP when carbachol-induced REM ended. Thirty-seven minutes later when muscle tone decreased in conjunction with another REM episode, the BP was higher than during control REM episodes. In B infusion of carbachol in another cat produced a REM episode lasting 10 min (dotted line). During this period the BP was similar to or lower than control REM episodes. Subsequently, the animal demonstrated atonia, rapid eye movements, and desynchronized EEG but no PGO waves (solid line). Note the progressively increasing pressor response during atonia. BP continued to increase during subsequent waking behavior.
In the pontomedullary region, REM sleep episodes were observed in two of four infusions. These episodes were not seen until approximately 25 min after termination of the infusions. Figure 4 illustrates BP and muscle tone values following a typical Ringer’s and carbachol infusion session in the pontomedullary region. In contrast to pontine infusions, medullary carbachol injections produced a continuous increase in BP. Twenty-five minutes after the infusions, high BP values were noted in conjunction with REM sleep.
FIG. 4.
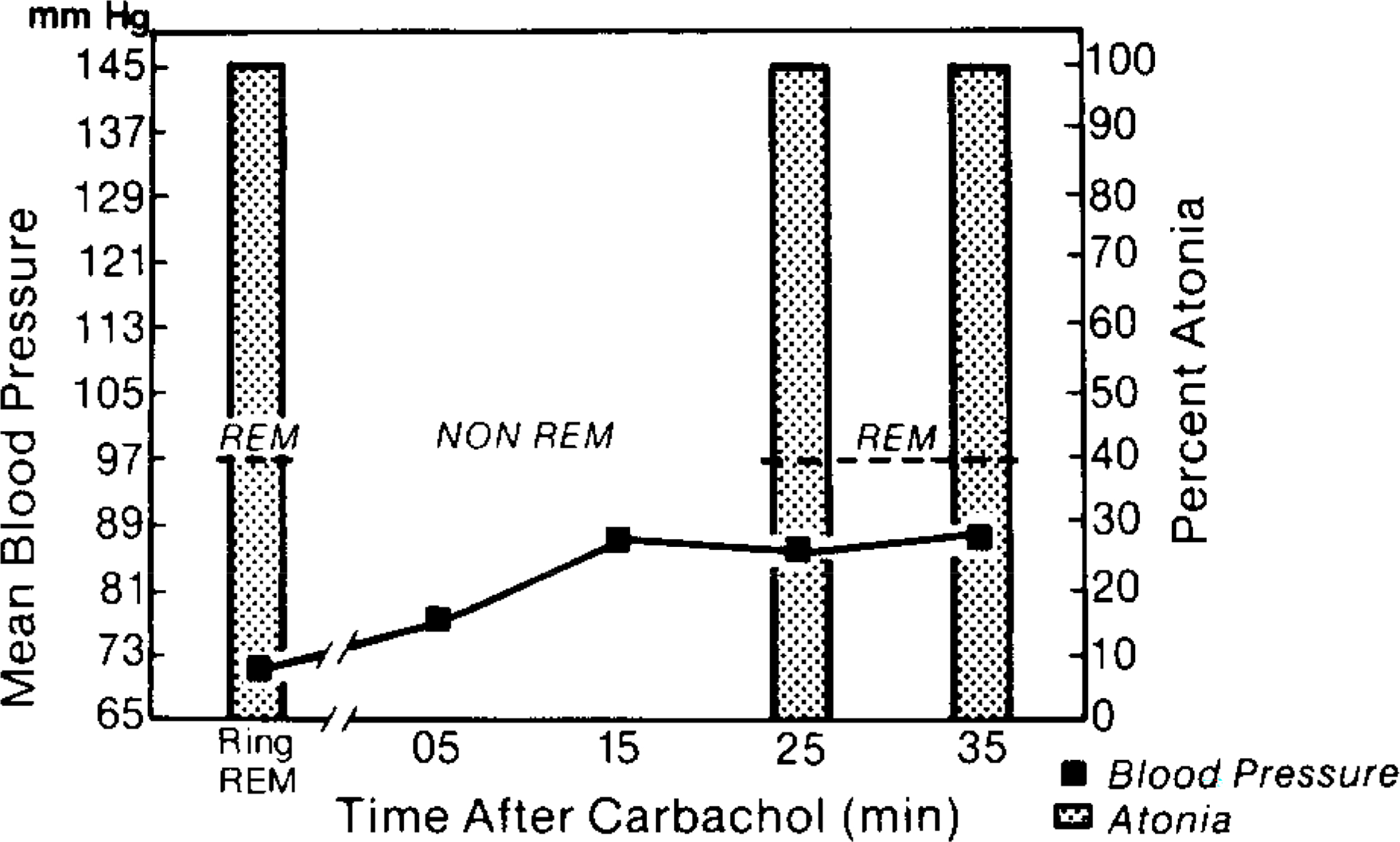
Mean BP and atonia in one experimental session where carbachol was infused into the pontomedullary junction at the level of the abducens nucleus. Note that REM sleep occurred 25 min after the infusion. During this period muscle tone was absent yet the BP was higher than control REM episodes when comparable muscle tone was present. In this respect the BP response at later REM episodes was similar to that in the pons (see Fig. 3). Note that the marked hypertension did not compromise REM sleep induction.
DISCUSSION
The purpose of this study was to determine the relation between BP changes and REM sleep after carbachol infusion in the unanesthetized cat. We find that carbachol infusion into the pons produced a BP decrease lasting approximately 20 min followed by a BP increase lasting about 1 h. The early decrease was linked to the occurrence of REM sleep. Approximately 15 to 20 min after infusion at pontine sites, a BP increase occurred. This increase appeared to be independent of behavioral state. A similar increase commencing at the time of infusion was seen in midbrain, pontomedullary, and medullary sites. The lack of an early decrease at these sites was correlated with the absence of early REM sleep periods after carbachol infusions. Thus, the early depressor response seen in these unanesthetized animals is linked to the evocation of REM sleep. Like carbachol-induced REM sleep, spontaneous REM sleep episodes are also accompanied by a BP decrease (15).
Whereas the initial BP reduction was restricted to pontine sites, carbachol infusions at all medial brain stem sites explored produced a progressively increasing pressor response lasting throughout the 1-h observation period. This increase was not reversed by later REM sleep periods. Therefore, the REM sleep state and its atonia may be dissociated from BP reduction. Conversely, REM sleep occurrence did not block the long-latency carbachol-induced BP elevation (see Figs. 3 and 4). Whereas these slow BP increases were compatible with REM sleep, it has been reported that phasic BP elevation tends to disrupt REM sleep (8, 17).
Some studies in anesthetized rats (5, 12) and cats (6, 7, 11) have shown that cholinomimetics produce an initial hypotensive effect followed by progressively rising BP values. The hypotensive response has been shown to be atropine-sensitive whereas the pressor response can be blocked by nicotinic blockers such as hexamethonium or mecamylamine. Infusions of nicotine into the area postrema of the anesthetized rat produce a predominantly hypertensive response which was blocked by hexamethonium but not atropine (13, 14). In anesthetized cats (6, 7), physostigmine infusions into the left vertebral artery produce hypotension without a subsequent pressor response, Muscarinic blockers eliminated the depressor response. Local infusions of carbachol into the pontine tegmentum of anesthetized cats have been shown to produce a depressor response which was blocked by atropine (11).
We determined that only pontine carbachol infusions produced a BP response which was initially linked to carbachol-triggered REM sleep episodes. It has also been shown that only pontine carbachol infusions produce REM sleep (2). Since pontine muscarinic receptors are implicated in the generation of REM sleep (19, 20) we hypothesize that muscarinic mechanisms mediate both the BP and REM sleep response during the first 10 to 20 min following pontine carbachol infusion. Thereafter, nicotinic mechanisms throughout the brain stem begin to override the muscarinic BP response while pontine muscarinic mechanisms continue to trigger the REM-sleep-generating system.
Acknowledgments
This work was supported by the Medical Research Service of The Veterans Administration, National Science Foundation grant BNS-8200023, and National Institutes of Health grant NS14610. Dr. Shiromani’s present address is Department of Psychiatry, University of California San Diego, CA 92093.
Abbreviations:
- BP
blood pressure
- PGO
pontogeniculooccipital
- REM
rapid eye-movement
REFERENCES
- 1.AMATRUDA TT, BLACK DA, MCKENNA TM, MCCARLEY RW, AND HOBSON JA. 1975. Sleep cycle control and cholinergic mechanisms: differential effects of carbachol at pontine brainstem sites. Brain Res. 98: 501–515. [DOI] [PubMed] [Google Scholar]
- 2.BAGHDOYAN HA, RODRIGO-ANGULA ML, MCCARLEY RW, AND HOBSON JA. 1984. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 306: 39–52. [DOI] [PubMed] [Google Scholar]
- 3.BAXTER BL 1969. Induction of both emotional behavior and a novel form of REM sleep by chemical stimulation applied to cat mesencephalon. Exp. Neurol. 23: 220–230. [DOI] [PubMed] [Google Scholar]
- 4.BHARGAVA KP, JAIN IP, SAXENA JN, AND TANGRI KK. 1978. Central adrenoceptors and cholinoceptors in cardiovascular control. Br. J. Pharmacol. 63: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BREZENOFF HE, AND JENDEN DJ. 1970. Changes in arterial blood pressure after microinjections of carbachol into the medulla and IVth ventricle of the rat brain. Neuropharmacology 9: 341–348. [DOI] [PubMed] [Google Scholar]
- 6.DEWILDT DJ, AND PORSIUS AJ. 1981. Central cardiovascular effects of physostigmine in the cat; possible cholinergic aspects of blood pressure regulation. Arch. Int. Pharmacodyn. 253: 22–39. [PubMed] [Google Scholar]
- 7.DEWILDT DJ, AND PORSIUS AJ. 1981. The influence of physostigmine on sympathetic outflow and haemodynamics by an action upon the pontomedullary region of the cat. Arch Int. Pharmacodyn. 253: 40–51. [PubMed] [Google Scholar]
- 8.FRYSINGER RC, MARKS JD, TRELEASE RB, SCHECHTMAN VL, AND HARPER RM. 1984. Sleep states attenuate the pressor response to central amygdala stimulation. Exp. Neurol. 83: 604–617. [DOI] [PubMed] [Google Scholar]
- 9.GEORGE R, HASLETT WL, AND JENDEN DJ. 1964. A cholinergic mechanism in the pontine reticular formation: induction of paradoxical sleep. Int. J. Neuropharmacol. 3: 541–552. [DOI] [PubMed] [Google Scholar]
- 10.HOBSON JA, GOLDBERG M, VIVALDI E, AND RIEW D. 1983. Enhancement of desynchronized sleep signs after pontine microinjection of the muscarinic agonist bethanechol. Brain Res. 275: 127–136. [DOI] [PubMed] [Google Scholar]
- 11.KATAYAMA Y, DEWITT DS, BECKER DP, AND HAYES RL. 1984. Behavioral evidence for a cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res. 296: 241–262. [DOI] [PubMed] [Google Scholar]
- 12.KRSTIC MK 1982. A further study of the cardiovascular responses to central administration of acetylcholine in rats. Neuropharmacology 21: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 13.KUBO T, AND MISU Y, 1981. Changes in arterial blood pressure after microinjections of nicotine into the dorsal area of the medulla oblongata of the rat. Neuropharmacology 20: 521–524. [DOI] [PubMed] [Google Scholar]
- 14.KUBO T, AND MISU Y. 1981. Cardiovascular response to microinjection of physostigmine and choline into the dorsal medullary site of the rat. Neuropharmacology 20: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 15.MANCIA G, AND ZANCHETTI A. 1980. Cardiovascular regulation during sleep. Pages 1–53 in OREM J AND BARNES CD, Eds„ Physiology in Sleep. Academic Press, New York. [Google Scholar]
- 16.MITLER MM, AND DEMENT W. 1974. Cataplectic-like behavior in cats aner microinjections of carbachol in pontine reticular formation. Brain Res. 68: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SCHNEIDER-HELMERT D 1983. Experimental elevation of blood pressure induced as an internal stimulas during sleep in man: effects on cortical vigilance and response threshold in different sleep stages. Sleep 6: 339–347. [DOI] [PubMed] [Google Scholar]
- 18.SHIROMANI P 1983. Long-term Cholinergic Stimulation of Pontine Nuclei: Effects on Paradoxical Sleep and Memory. Doctoral thesis, The City University of New York, New York. [Google Scholar]
- 19.SHIROMANI P, AND MCGINTY D. 1983. Pontine sites for cholinergic PGO waves and atonia: localization and blockade with scopolamine. Soc. Neurosci. Abstr. 9: 1203. [Google Scholar]
- 20.SHIROMANI P, AND MCGINTY D. 1986. Excitatory and inhibitory responses of pontine neurons to local cholinergic infusion: relation to REM sleep. Submitted for publication. [DOI] [PubMed]
- 21.SILBERMAN E, VIVALDI E, GARFIELD J, MCCARLEY RW, AND HOBSON JA. 1980. Carbachol triggering of desynchronized sleep phenomena: enhancement via small volume infusions. Brain Res. 191: 215–224. [DOI] [PubMed] [Google Scholar]
- 22.SITARAM N, AND GILLIN JC. 1979. Acetylcholine: possible involvement in sleep and analgesia. Pages 311–343 in DAVIS KL AND BERGER PA, Eds., Brain Acetylcholine and Neuropsychiatric Disease. Plenum, New York.<: /References> [Google Scholar]


