Summary
Behavioral state organization was studied in the caudal portion of chronically maintained cats with transections at the ponto-medullary junction or midpontine level. The cats spent most of their time in a ‘quiescent state.’ This state was periodically interrupted by ‘phasic activations.’ During quiescence, ECG and reticular unit activity rates were low and regular. EMG levels resembled those seen during non-REM sleep in intact cats. During phasic activations, unit activity in the nucleus gigantocellularis and neck EMG activity increased to levels seen in the intact cat during active waking. Gross postural changes, vestibular slow phase head nystagmus and head shake reflexes could be observed at these times. No periods of neck muscle atonia were observed in either state. No periods of brain-stem controlled rapid eye movements (REMs) occurred. Unit activity patterns similar to those seen in the intact cat during REM sleep were never observed. Physostigmine administration did not produce REM sleep signs, but rather, triggered an aroused state. Phasic activations occurred in a regular ultradian rhythm, with a period similar to that seen in the REM sleep cycle. We conclude that the chronic medullary cat retains primitive aroused and quiescent states, but does not have any of the local signs of REM sleep. However, the medulla does have the capability of generating ultradian rhythmicities which may contribute to the control of the basic rest activity cycle and the REM, non-REM sleep cycle.
Keywords: medulla, sleep, eve movements, atonia, REM sleep, physostigmine, ultradian rhythm, unit activity
Behavioral state organization, as defined by patterns of electrooculogram (EOG), electrocardiogram (ECG), respiration, electromyogram (EMG) and unit activity, has been investigated in chronically maintained animals with brain-stem transections at the midbrain level (Jouvet 1962; Hobson 1965; Villablanca 1966). The brain-stem of such animals exhibits quiescent and aroused states. It also generates the defining characteristics of REM sleep, including rapid eye movements, neck muscle atonia, extreme miosis and respiratory and cardiac irregularities.
The behavioral state organization of chronically maintained animals with transections at the medullary or midpontine level has not been extensively studied. Characterization of the state organization of these preparations would offer an insight into medullary and pontine contributions to state control. The localized lesion technique has not been successfully applied to the analysis of medullary contributions to state control, since most animals do not survive large medullary lesions. The transection approach has the additional advantage of allowing one to directly observe the states generated by medullary mechanisms, rather than requiring one to rely on inferred deficits in state control after lesions.
Analysis of state organization in the midpontine or medullary preparation presents several problems. The foremost is the maintenance of these preparations for long periods. Analysis of behavioral state in the caudal portion of midpontine preparations has been confined to the first few days after transection (Jouvet 1962). No studies of behavioral state organization in animals transected at the low pontine level or ponto-medullary junction have been reported. It has been shown that progressive normalization of physiological functions occurs over the first 2 weeks of survival time in midbrain decerebrate preparations (Villablanca 1966; Slosarska and Zernicki 1973). One would expect that a similar process would occur in medullary preparations. Therefore observations of such preparations over a short survival time would be likely to underestimate their physiological and behavioral capabilities. A second problem results from the finding that small pontine lesions can block the atonia of REM sleep without preventing its other manifestations (Jouvet and Delorme 1965). Therefore, the absence of atonia cannot be taken to demonstrate the absence of REM sleep in midpontine and medullary preparations. A more direct indication of neuronal activity patterns is necessary. A third problem is the use of eye movements as an indicator of behavioral state. Transections at the midpontine level leave only the output of the abducens nucleus as an expression of ponto-medullary oculomotor activity. It is necessary to distinguish abducens controlled eye movements from those elicited by activity in the oculomotor and trochlear nuclei rostral to the transection.
In the present study, we have attempted to deal with these difficulties. Medullary and midpontine preparations have been maintained in stable condition for long periods. We have continuously monitored muscle tone, ECG, respiration, and medullary unit activity, to enable description of behavioral state. We have also employed a surgical technique for restricting brain-stern controlled eye movements to a single eye to facilitate their description.
Methods
Subjects
Ten mongrel male cats weighing between 3.3 and 6.2 kg served as subjects. Two were controls for studies of ultradian rhythmicities. These animals received standard sleep scoring implants (Siegel et al. 1977). Two animals were controls for the effects of cerebellar lesions. The remaining 6 animals received brain-stem transections at the ponto-medullary junction.
Surgical procedures
Aseptic surgeries were performed in 2 stages. The first surgery employed sodium pentobarbital anesthesia. With the animal’s head held by a stereotaxic instrument, a lateral incision was made and the temporalis muscle retracted rostrally. The perimeter of the squamous portion of the temporal bone was drilled out and the bone removed. The dura was cut and the temporal lobe gently elevated to visualize the right third nerve. The nerve was severed by suction. The bone was reattached with dental cement and the skin sutured. A midline incision was then made and electrodes were implanted for recording EMG and EOG bilaterally (Siegel et al. 1977).
After a minimum of 1 week, baseline recordings were taken in 4 of the 6 experimental animals (nos. 25, 27, 31 and 46). After a minimum of 2 weeks the second surgery was performed. Halothane/oxygen anesthesia was employed. The anesthetic was administered through an endotracheal tube which remained in place throughout surgery and for the first few post-surgical hours. The occipital and interparietal bones were removed with rongeurs and the dura over the cerebellum cut in the midline. The medial cerebellum was aspirated to expose the aqueduct and rostral portion of the floor of the fourth ventricle. In 2 control animals (nos. 19 and 20) cerebellar aspiration was the only procedure performed. The trochlear nerves were severed at their decussation. A spatula was then lowered along a calibrated Plexiglas plate to transect the brain-stem. Pressure was applied at the base of the skull at 3–5 mm lateral to midline on the left side to completely sever the abducens nerve. Care was taken to employ reduced pressure on the right side and on the midline to spare the right abducens nerve and basilar artery. Movement of the transected brain-stem within the posterior fossa renders conventional rigid microelectrode recordings unstable. Therefore we implanted bundles of flexible 32 μm microwires attached to microdrives for unit recording in the nucleus gigantocellularis of the medulla (Siegel et al. 1979). After controlling bleeding with bovine thrombin, the skull defect was sealed with dental cement. The cat was then removed from the stereotaxic and placed on a heating pad in the recording chamber.
Maintenance of preparations
Respiratory assistance was required for up to 15 min after transection. However, some preparations, including the most caudal transection (cat 46) breathed spontaneously immediately after transection. Several cats had periods of shallow regular respiration alternating with periods of deep breaths or clusters of deep breaths (Biot’s breathing) separated by apneic periods. When the endotracheal tube was in place, expired CO2 levels were continuously monitored with a Beckman LB2 gas analyzer. After removal of the endotracheal tube, CO2 levels were checked twice daily throughout the survival period. End-tidal CO2 levels were elevated in cat 25 up to a maximum 6.7% during the first week after transection. After this period, CO2 levels were maintained, without assistance, in the 4–5.5% range throughout survival in cat 25 and all other cats with the exception of cat 46. Cat 46, after maintaining his expired CO2 level in the normal range for 7 days, became hypercapnic and was placed on a respirator for 2 days. After this period he was again able to regulate CO2 level without assistance. Except where noted, all data to be described below were taken from animals after the initial 1 week post-transection period, at a time when CO2 levels were in the normal range.
After removal of the endotracheal tube, respiration was continuously monitored with a thoracic strain gauge. Temperature was recorded with a rectal probe. The output of the probe was used to control a water circulating heating pad, maintaining core temperature between 37 and 38.5°C. During the first post-transection week, the cats were injected subcutaneously with 50 ml of 5% dextrose solution 3 times a day at 8:00, 14:00 and 20:00 h, and 15 ml of an amino acid solution, Freamine (McGaw), once a day at 8:00 h. During the remainder of the survival period only the 8:00 Freamine injections were given and 50 ml of KMR liquid food (Borden Inc.) was administered by gavage at 8:00, 14:00 and 20:00 h. The cats were turned once a day at 8:00 h. Regular application of petroleum jelly to exposed mucous membranes prevented drying and infection. Mucous was aspirated from the pharynx twice daily at 8:00 and 20:00 h. Lights were turned on at 8:00 and off at 20:00. With the exception of cat 46, all cats were sacrificed in stable condition at the end of the planned observation period. Cat 46 died as a result of the obstruction of its endotracheal tube, which was reinserted on day 15.
Recording
Continuous polygraphic recordings of ECG, EOG and respiration were made throughout the survival period. The polygraph ran at 25 mm/min with automatic speed switching to 10 mm/sec for 1 min every 45 min. At the slower speed, ECG rate was displayed as a tachograph output and unit activity as vertically integrated pulse counts with resets every second. At higher speeds, ECG and pulse outputs triggered by unit potentials were displayed. Unit recordings were made daily after the first survival week and were tape recorded. Unit discharge frequency counts were printed out at 1 or 10 sec intervals on an Anadex printer. Twelve hour tape recordings of ECG, EOG, EMG and respiration were made between 20:00 and 8:00 h at weekly intervals throughout the survival period.
Histology
All cats, with the exception of cat 46, were killed with an overdose of Nembutal after passing a 15 μA 15 sec current through unit microwires. After perfusing with saline and 10% formalin, brains were removed and sectioned in the sagittal plane. Transection levels and unit locations were plotted with reference to the sagittal plates of the Berman (1968) atlas.
Data analysis
Unit activity rates are based on 10 consecutive 10 sec intervals during quiescence. Peak unit rates are based on the mean of 1 sec samples for each unit during 2 phasic arousals. Our comparison of pre- and post-transection EMG levels is based on 10 samples taken in each state during the baseline and in the post-transection period. Behavioral state periodicities were calculated on 24 h samples taken during the 20:00 to 8:00 h period. Phasic arousals clustered within 1 min of each other were scored as a single event for the purpose of determining the interval between arousal clusters. Arousal periodicities, ECG rates and respiration rates are based on 5 cats (nos. 23, 24, 25, 27 and 31) for days 7–8 and 4 surviving cats (nos. 24, 25, 27 and 31) for days 23–24. EMG levels are based on data from 4 cats that had both baseline and post-transection recordings (nos. 25, 27, 31 and 46). Cat 46’s second post-transection EMG data points were derived from days 14–15.
Periodicities were analyzed as follows. Each record was scored for time of onset of phasic arousal or, in intact controls, for time of REM sleep onset. Sustained waking episodes obscure ultradian rhythmicities in intact animals (Sterman et al. 1965). Therefore, in our control animals, any cycle interrupted by waking periods of greater than 10 rain was excluded from the analysis. No such exclusion was performed in the transected animals. Spectral analyses were performed on animals 24, 25, 27 and 31 on data collected 1, 2 and 3 weeks after transection. REM sleep onset or phasic arousal times were entered in a binary format into a large array. Each element of the array represented 1 min, so that a ‘one’ represented an onset of a phasic arousal or REM sleep period and a ‘zero’ represented all other points. Since sharp onset impulse data will produce harmonics, we performed a sine wave transform. The period of each sine wave represented the interval between arousals. Intervals less than 4 min were set to zero to allow specification of at least 4 points on each sine wave and to eliminate very high frequency periodicities from the data. Twenty-four hour data samples were divided into 3 segments to facilitate analysis. Segments slightly shorter than 8 h were padded with zeroes so that all spectral estimates were performed on data segments of exactly the same length. Each segment was cosine tapered 10% at each end. The data were then analyzed with the BMDP spectral analysis program P1T. After fast Fourier transforms were performed, the resulting periodograms were smoothed by averaging adjacent values with a cosine weighing function. Each peak of the resulting smoothed spectral plot has approximately 8 df (Dixon et ai. 1983). The 3 segments were averaged into a single periodogram that represented the full 24 h of data. An equivalent noise bandwidth was calculated for each 24 h periodogram, by dividing the area under each curve by the amplitude of the peak of the curve. This provides an estimate of the relative amount of energy concentrated in the peak.
Results
Three transected animals were maintained in a stable neurological condition for periods of 28–31 days (nos. 25, 27 and 31), a fourth for 23 days (no. 24), a fifth for 16 days (no. 46) and a sixth for 10 days (no. 23). Continuous recordings of ECG, EMG, EOG and respiration were made throughout the survival periods. Control animals were allowed at least 2 weeks to recover from electrode implantation. They were then habituated to the recording chamber for 1 week and recorded from for 48 h.
Histology
The medial portion of the anterior lobe of the cerebellum was removed in all cats. The medial cerebellar nuclei suffered varying amounts of damage. The rostral transections, the ‘midpontine’ preparations (nos. 23, 24, 25 and 27), passed just caudal to the locus coeruleus complex (Figs. 1 and 2). The abducens nucleus and nerve were intact caudal to the cut. The motor nucleus of the trigeminal and trigeminal nerve roots suffered varying amounts of damage. The most caudal transections, the ‘medullary preparations’ (nos. 31 and 46), passed through the abducens nucleus. The transections were complete in 4 cats (nos. 24, 25, 27 and 46). In these animals the rostral and caudal portions of the brain-stem were found to be completely separated when the brain was removed from the skull. The basilar artery was the only structure connecting the two sections. A few filaments (less than 1 mm) of the lateral portions of the brachium pontis or ventral portion of the trapezoid body were spared in two preparations (nos. 23 and 31). The results in these animals did not differ from those in cats with complete mechanical separation of the brain-stem at the transection.
Fig. 1.
Levels of transection of all cats employed in the study. RN, red nucleus; LC, locus coeruleus; 7, genu of facial nerve; 6, abducens nucleus; IO, inferior olive. Stereotaxic coordinates derived from sagittal plates of Berman (1968) atlas at L1.2.
Fig. 2.
Top: sagittal section at 1.2 mm lateral to midline through the transection in cat 24. Abducens nucleus is prominent caudal to transection. Cresyl violet stain. Bottom: sagittal section at 1.2 mm lateral to midline through the transection in cat 25.
Behavioral states
The behavioral states and periodicities observed in regions controlled by brain areas caudal to the transection were similar in the midpontine and medullary preparations. The dominant state throughout the survival period was one that can be described as a quiescent state (Fig. 3). At this time, heart rate, respiration and unit activity were slow and regular and muscle tone was low, although not completely absent (Fig. 4).
Fig. 3.
States seen in the chronic medullary cat. EMG, dorsal neck electromyogram; EKG, electrocardiogram; Resp, thoracic strain gauge. Samples were taken from day 7 in cat 27. EMG calibration, 50 μV.
Fig. 4.
Nuchal EMG amplitude in intact cats during active waking (A-WA), quiet waking (Q-WA), light and deep slow wave sleep (SWS-1, SWS-2) and REM sleep, compared to EMG levels seen during arousals and in quiescent state after transection. Amplitude levels based on 10 samples taken in each state in both baseline and post-transection recordings in cats 25, 27, 31 and 46.
A second state can be described as phasic activation. It occurred periodically in the undisturbed cat throughout the survival period and consisted of a brief, 1–3 min activation of EMG, ECG and respiration (Fig. 3). During this period, 1–3 bursts of nuchal muscle activity occurred. Phasic activation could also be triggered by light stimulation of the skin and was always induced by noxious stimulation such as aspiration of mucus from the pharynx.
At times, particularly in the first week after transection, increased neck muscle tone persisted for several hours after a phasic arousal (Fig. 3). Medullary RF unit activity often showed a correlated activity increase in discharge rate during these ‘tonic activations’ (see below).
A low level of EMG activity was present during quiescent periods. Amplitude of the EMG at these times was comparable to that seen on the same leads in non-REM sleep during baseline recordings (Fig. 4). No periods of total EMG suppression resembling that seen in REM sleep were observed, despite continuous recordings throughout the survival periods in all animals.
Animals remained on their sides with limbs and head extended in an opisthotonic posture. After the third day, rigidity diminished during quiescent periods. Phasic arousals produced a return to extensor rigidity. Lateral flexion of the spinal column could also occur in the animals with more rostral transections (nos. 23, 24 and 25) and these movements were sometimes successful in rolling the cat from the side on which it had been placed to the opposite side. A series of such spontaneous movements often displaced the animals across the 60 cm length of the cage floor over a 12 h period. EMG amplitude during phasic arousals was indistinguishable from that seen in control recordings taken in the same cats during active waking (Fig. 4).
All transected animals spontaneously defecated and urinated while lying on their sides. Vomiting was frequently observed after the first few gavage feedings. Oral stimulation produced tongue movements in all animals. Irritation of the outer canthus of the pinna produced a vigorous torsional head shake response.
Cerebellar lesions
In 2 animals (nos. 19 and 21) the medial cerebellum was aspirated as in the transected animals, but no further brain-stem lesion was made. These animals exhibited waking, non-REM and REM sleep states, including complete muscle atonia, as others have reported (Jouvet 1962; Guglielmino and Strata 1971; Raffaele et al. 1971; De Andres and Reinoso-Suarez 1979; Paz et al. 1982).
Head movements eliciated by vestibular stimulation
Three transected animals (nos. 24, 25 and 27) were tested for rotatory and post-rotatory head nystagmus between days 21 and 31 after transection. With the body supported and head unrestrained they were accelerated to a speed of 0.3 or 0.9 rev/sec, maintained at that speed for 1 min and then rapidly braked to a stop. Clockwise acceleration produced a compensatory slow head movement to the left side while braking produced a slow movement to the right side. The cat remained with head turned 90° to the sagittal plane for several seconds after the termination of stimulation. No anticompensatory head movements, which prevent such large head displacements during vestibular stimulation in the intact cat, were seen during vestibular stimulation in the transected cats.
Eye movements
Cutting the third nerve on the right side, the sixth nerve on the left side and the fourth nerve bilaterally, produced a preparation in which forebrain controlled eye movements were restricted to the left eye and brain-stem controlled eye movements to the right eye in cats 24, 25 and 27. The more caudal transections (nos. 31 and 46) passed through the abducens nucleus and nerve, and therefore caudal brain-stem commanded eye movements could not be observed.
Brain-stem commanded eye movements were unrelated to phasic arousals and did not change in rate during tonic arousal periods. They did not exhibit any marked periodicity or clustering. Instead they occurred in an apparently random pattern (Fig. 5). Most activity was recorded as a stereotyped biphasic deflection of the EOG. Visual observation of the eye revealed that this activity corresponded to brief eyeball retractions, with correlated nictitating membrane displacement over the pupil. The retraction could not be triggered by irritation of the cornea as in the intact animal, since the trigeminal receptors for this stimulus were separated by the transection from the retractor bulbi motoneurons. No change in medullary reticular unit activity accompanied eye retractions.
Fig. 5.
Abducens controlled eye movements prior to and after brain-stem transection. In baseline recording, periods of rapid eye movement can be observed during REM sleep. After transection only isolated nictitating membrane blinks are observed. Calibration, 100 μV.
Unit activity
A total of 50 medullary RF units were recorded in 3 animals (nos. 23, 24 and 25). Each unit was observed for a minimum of 2 h. Units were located in the nucleus gigantocellularis between P4 and P10, L0.8 and L1.6 (Fig. 6). Units discharged regularly during quiescent periods. Nineteen of the 50 units had noise-free records during phasic arousal. All but one of these units increased discharge rate at these times (Fig. 7 and Table II). The exceptional unit ceased discharging during phasic activations. The increase in unit discharge rate during phasic arousal over baseline values averaged 129% (Fig. 8). Peak rates of medullary units were comparable to rates seen in the intact cat in active waking (Siegel et al. 1979). We never saw units which increased activity substantially (i.e., more than 20% over the 1 min rate in a 10 sec period) without a correlated phasic activation.
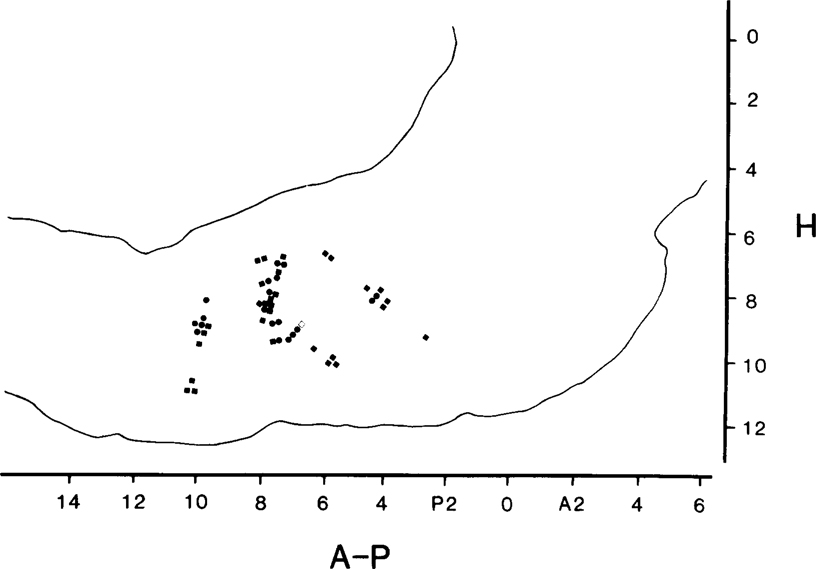
Fig. 6.
Locations of medullary units recorded after transection. Squares, units with rates > 4.0/sec in quiescence; circles, units with rates < 4.0/sec. Empty square, unit decreasing rate during phasic arousal.
Fig. 7.
Compressed display of medullary reticular formation (RF) unit discharge rate during sleep-waking cycle in intact cat, and after brain-stem transection. Tracing is output of a digital counter indicating number of action potentials and resetting at 1 sec intervals. Note the long periods of accelerated and irregular unit discharge rate during waking and REM sleep in the intact cat. RF cells in the transected cats have extremely regular discharge rates with 1 sec counts virtually identical for long time periods. Short periods of increased unit activity occur in conjunction with phasic arousals.
TABLE II.
Unit activity in medullary reticular formation. Nineteen units were observed during both phasic activation and quiescence. A total of 49 were observed in quiescence. First column presents data from all units observed in quiescent state. Second column presents data for quiescent state for units recorded during both quiescent and activated state. Third column presents data from activated state.
| Quiescence | Phasic activation | ||
|---|---|---|---|
|
| |||
| Mean (spikes/sec) | 14.2 | 16.2 | 37.1 |
| Number of units observed | 49 | 19 | 19 |
| S.D. | 22.9 | 16.6 | 38.5 |
| Minimum rate | 0.4 | 0.7 | 0.0 |
| Maximum rate | 129.5 | 71.3 | 150.0 |
Fig. 8.
Increases in discharge rates of medullary units with phasic arousal from quiescent state baseline. Rate is in spikes/sec. Unit activity rates are based on 10 consecutive 10 sec intervals during quiescence and on the mean of 1 sec samples during 2 phasic arousals.
Physostigmine administration
Physostigmine salicylate was administered intravenously in doses ranging from 0.05 to 0.2 mg/kg to 2 cats. In one animal (no. 25) the injections were performed on post-transection day 18 while in the other (no. 31) they were performed on day 31. No periods of atonia, hypotonia or rapid eye movements were seen in conjunction with physostigmine administration. High doses of physostigmine produced increases in muscle tone.
Cyclicity
Table III presents data on the intervals between phasic arousals. These data were drawn from the 20:00 to 8:00 h periods, during which time the cats were not fed or otherwise disturbed. Arousals were spaced very regularly during some periods, while at other times clusters of 3 or 4 arousals separated by long quiescent periods occurred. At 7 days post transection, activations recurred at approximately 9.4 min intervals, while at 23 days post transection, the mean interval was 22.6 min. Two cats (nos. 25 and 46) were observed while on a respirator to determine if changes in respiratory rate or CO2 level triggered phasic arousals. Arousals continued to occur during assisted respiration. In the spontaneously respiring animals, expired CO2 levels increased during phasic arousals, presumably due to the deep breaths clearing alveolar CO2. CO2 levels returned to normal within 1–2 min.
TABLE III.
Intervals between phasic arousals in minutes.
| X | S.D. | Cats |
|||||
|---|---|---|---|---|---|---|---|
| 23 | 24 | 25 | 27 | 31 | |||
|
| |||||||
| Days 7–8 | 9.4 | 6.3 | 19.8 | 8.6 | 9.1 | 6.5 | 3.1 |
| Days 23–24 | 22.6 | 6.9 | – | 22.8 | 31.0 | 22.6 | 14.1 |
Fig. 9 presents the results of the spectral analysis of the periodicity data. In control animals, ultradian periodicities produced prominent peaks between 30 and 60 min. ENB values were 11.6 and 13.9. In the first week after transection there was little evidence of any prominent ultradian rhythmicity in the occurrence of phasic arousals, with equivalent noise bandwidth (ENB) values ranging from 21.1 to 25.1. By the third post-transection week, all transected animals showed marked periodicities in the 30–60 rain range. ENB values ranged from 12.2 to 14.8, not significantly different from control values.
Fig. 9.
Periodicities in intact control and in transected animals. The left column of the figure presents spectral power density plots of REM sleep onset periodicities in intact control animals. The right columns of the figure present spectral density plots of phasic arousals in 2 representative animals taken 1, 2 and 3 weeks after transection. In control animals, the spectral power density plot has its peaks between 30 and 60 min. One week after transection, no prominent peak is visible in the spectral power density of either animal and the equivalent noise bandwidth (ENB) is correspondingly high. By the third week, prominent peaks are present at between 30 and 60 rain in both transected animals. ENB values are reduced and are comparable to values in intact animals.
Discussion
Is REM sleep present?
We saw no pattern of medullary activity resembling that seen in REM sleep despite long survival times which would have allowed recovery from the acute effects of the transection. The medullary signs of REM sleep in the intact animal include rapid eye movements, accelerated and irregular reticular unit activity, irregularities in respiration and heart rate, and muscle atonia (Jouvet 1967). Rapid eye movements controlled from the abdutens nucleus, visible in control recordings after 3rd nerve section, were absent after transection. Only isolated nictitating membrane blinks, bearing no obvious relation to other state parameters, were present after the transection. Therefore the caudal pons and medulla did not generate periods of rapid eye movement, despite the presence of the vestibular nuclei and peri-abducens eye movement related interneurons behind the cut (Hikosaka and Kawakami 1977; Nakao et al. 1980). Rostral pontine and midbrain structures are apparently required for the generation of spontaneous horizontal eye movements.
RF unit activity varied only during phasic arousal periods. At other times, unit rates were remarkably constant. There were no periods of irregularity resembling those seen in REM sleep. Moreover, there was no dissociation between unit activity and other signs of behavioral state so characteristic of the ‘paradoxical’ sleep phase. Increases in unit activity were associated with increased, not decreased muscle tone. Respiratory and ECG rate changes occurred in conjunction with phasic activations. However, apart from these episodes, only gradual shifts in these parameters occurred throughout the survival period.
Physostigmine evokes a REM sleep-like state in midbrain decerebrate preparations. This state consists of neck muscle atonia, rapid eye movements and irregular bursts of activity in medial reticular regions (Matsuzaki 1969; Hoshino and Pompeiano 1976). Physostigmine might be expected to trigger a similar REM sleep state or fragments of the REM sleep state in midpontine or medullary preparations, even if none occurred spontaneously. However, physostigmine injections at and beyond the levels effective in midbrain decerebrate cats were ineffective in the medullary cat. Therefore physostigmine must act by affecting midbrain or pontine neurons rostral to our transection levels, or require effector mechanisms in these rostral regions in order to produce REM sleep signs.
What behavioral states are present?
Medullary cats retain primitive aroused and quiescent states which, in some respects, resemble states seen in the intact cat. The ‘quiescent state’ occupied most of the recording time. During this state ECG rate is comparable to that seen in the intact animal in non-REM sleep (Sieck and Harper 1980). Muscle tone is also comparable to that seen in non-REM sleep. Unit activity rates are remarkably regular, again most closely resembling the low variability of medullary unit discharge rates seen in non-REM sleep (Siegel et al. 1979).
The presence of sustained quiescent periods in the medullary preparation indicates that no active pons-forebrain mechanism is needed to generate this state. Rather, the withdrawal of descending influences from the pons and other rostral structures is sufficient to produce the observed regularization of physiological control. The loss of these descending influences may act by leaving medullary hypnogenic regions (Magnes et al. 1961; Bonvallet and Bloch 1976) unopposed. One may hypothesize that a similar withdrawal is responsible for the regularization of respiration, heart rate, muscle activity and unit activity seen in non-REM sleep in the intact animal. An alternate hypothesis is that the regularization of function is a passive result of the withdrawal of excitatory input from rostral structures, not requiring medullary hypnogenic areas.
Phasic activations occurred spontaneously and after noxious stimuli. They were invariably associated with changes in posture, enhanced nuchal EMG, and increased activity in all but one of the medullary RF units recorded. This most closely resembled the pattern of activity seen upon arousal from sleep, a time at which virtually all RF cells become briefly active (Siegel and McGinty 1977; Siegel et al. 1979). Cats with ‘midpontine’ transections often made righting attempts during phasic activations. Thus the phasic arousals can be seen as a crude, short duration, rudiment of waking behavioral activity.
Atonia and medullary unit activity
The increased rate of most medullary RF units during the muscle tone increase of phasic activation periods also indicates that generalized medullary RF activation is not sufficient to produce muscle atonia and in fact is correlated with increased muscle tone in the medullary cat. In the intact cat, most medullary units are active during both waking movement and REM sleep, both periods of intense activation of brain motor systems (Siegel et al. 1979). The unit activity in the transected preparations during phasic activation can therefore be seen as related to the coincident motor activation.
No periods of neck muscle atonia were seen in any of the transected cats. Thus the medullary inhibitory area is not sufficient to produce atonia. This is presumably due to the loss of descending excitatory influences from the pons (Sakai et al. 1981) which normally increase activity in a subgroup of medullary cells during REM sleep. Direct electrical excitation of this area in the midbrain decerebrate cat produces bilateral inhibition of muscle tone (Magoun and Rhines 1946). However, a recent study has shown that direct stimulation of the medullary inhibitory region in cats transected at the ponto-medullary level produces little inhibition (Siegel et al. 1983). Therefore, even when directly excited, the medullary inhibitory region must interact with changes produced by rostral brain-stem regions in order to generate muscle atonia.
Reflexes
Sherrington (1917) first analyzed the head shake reflex seen in the midbrain decerebrate cat. This reflex could be readily induced by stimulation of the external canthus of the pinna in all of our transected animals, even those with transections behind the trigeminal nerve. It was indistinguishable in vigor from the reflex seen in the intact cat. Therefore it is clear that the medullary portion of the brain-stem and spinal cord are sufficient to mediate this behavior.
The presence of the slow phase of head nystagmus in these preparations demonstrates that pontine and rostral brain-stem regions are not required for this reflex response to vestibular stimulation. However, the absence of the anticompensatory head movements that would counteract these movements and produce head nystagmus demonstrates that rostral brain areas are needed for this reflex. Recent work has also shown that pontine reticular formation lesions can block anticompensatory head movements in the rat (Sirkin et al. 1980).
Cyclicity
The basic rest activity cycle (BRAC) underlies the timing of REM/non-REM sleep cycle as well as periodicities in waking behaviors (Kripke 1972; Sterman et al. 1972; Lavie et al. 1974; Orr and Hoffman 1974). The BRAC seen in the REM, non-REM sleep alternation can be very regular during long rest periods, with an average cycle length of 24–27 min (Delorme et al. 1964; Sterman et al. 1965; Ursin 1968). In the undisturbed chronic medullary cat we see periods of regular cyclicity with durations strikingly similar to those seen in the intact cat (Table III). Spectral density plots reveal peaks in the 30–60 min range, similar to those seen in the intact cat. Thus the medulla, while lacking the REM sleep state, can generate ultradian periodicities similar to those which underlie the REM, non-REM alternation. It may be the interaction of this medullary generator with pontine mechanisms that controls the triggering of the REM sleep state.
Résumé
Etat comportemental du chat médullaire ou médiopontique chronique
Ľorganisation du comportement a été étudiée dans la partie cérébrale caudale de chats porteurs de transections au niveau de la jonction ponto-bulbaire ou au niveau medio-pontin et maintenus chroniques. Les chats étaient généralement ‘inactifs’. Cet état était périodiquement interrompu par des ‘éveils phasiques’. Pendant l’inactivité, I’ECG et la fréquence de l’activité unitaire de la réticulée étaient lents et réguliers. Les niveaux de I’EMG ressemblaient á celui du chat intact en sommeil non-REM. Au cours des éveils phasiques, l’activité unitaire du noyau gigantocellulaire et l’activité EMG du cou augmentaient jusqu’aux niveaux de l’éveil actif chez le chat intact. Pendant ces épisodes, on pouvait observer des ajustements posturaux grossiers, des phases lentes vestibulaires du nystagmus de la tête, et des réflexes d’ébrouement de la tête. 11 n’a jamais été observé, de période d’atonie des muscles du cou quel que soit l’état considéré, ni de mouvements rapides des yeux contrô1és par le tronc cérébral, ni de patterns d’activité unitaire semblables á ceux vus chez le chat intact au cours du sommeil REM. Ľadministration de physostigmine n’a pas entraîné de signe de sommeil REM mais a plutôt déclenché un état d’éveil. Les éveils phasiques apparaissaient avec un rythme ultradien régulier, et une période similaire á celle du cycle du sommeil REM. En conclusion, le chat médullaire chronique conserve des états primitifs de sommeil et d’éveil, mais ne présente aucun des signes locaux de sommeil REM. Toutefois, la moelle posséde la capacité de produire des rythmicités ultradiennes qui pourraient contribuer au contrô1e de l’activité cyclique de repos de base et au cycle sommeil REM/nonREM.
TABLE I.
Respiration and ECG rates, quiescent state.
| X | S.D. | Cats |
|||||
|---|---|---|---|---|---|---|---|
| 23 | 24 | 25 | 27 | 31 | |||
|
| |||||||
| Respiration rate (breaths/min) | |||||||
| Day 7 | 12.2 | 6.6 | 5.0 | 15.4 | 5.0 | 18.0 | 17.6 |
| Day 23 | 10.6 | 3.5 | – | 8.6 | 8.0 | 10.0 | 15.6 |
| ECG rate (beats/min) | |||||||
| Day 7 | 139.2 | 18.8 | 130 | 130 | 172 | 126 | 138 |
| Day 23 | 120.0 | 37.2 | – | 106 | 162 | 76 | 136 |
Acknowledgments
This work was supported by the Medical Research Service of the Veterans Administration, NIH Grant NS14610 and NSF Grant BNS-82-00023.
We thank Dr. D.O. Walter for helpful advice on statistical analysis procedures.
References
- Berman AL, The Brain Stem of the Cat. University of Wisconsin Press, Madison, WI, 1968. [Google Scholar]
- Bonvallet M and Bloch V Bulbar control of cortical arousal. Science, 1976, 133: 1133–1134. [DOI] [PubMed] [Google Scholar]
- De Andres I and Reinoso-Suarez F Participation of the cerebellum in the regulation of the sleep-wakefulness cycle through the superior cerebellum peduncle. Arch. ital. Biol, 1979, 117: 140–163. [PubMed] [Google Scholar]
- Delorme F, Vimont P, et Jouvet D. Etude statistique du cycles veille-sommeils chez le chat. C.R. Soc. Biol. (Paris), 1964, 158: 2128–2130. [PubMed] [Google Scholar]
- Dixon WJ, Brown MB, Engelman L, Frane JW, Hill MA, Jennrich RI and Toporek JD BMDP Statistical Software. University of California Press, Berkeley, CA, 1983. [Google Scholar]
- Guglielmino S and Strata P Cerebellum and atonia of the desynchronized phase of sleep. Arch. ital. Biol, 1971, 109: 210–217. [PubMed] [Google Scholar]
- Hikosaka O and Kawakami T Inhibitory reticular neurons related to the quick phase of vestibular nystagmus — their location and projection. Exp. Brain Res, 1977, 27: 377–396. [DOI] [PubMed] [Google Scholar]
- Hobson JA The effects of chronic brain-stem lesions on cortical and muscular activity during sleep and waking in the cat. Electroenceph. clin. Neurophysiol, 1965, 19: 41–62. [DOI] [PubMed] [Google Scholar]
- Hoshino K and Pompeiano O Selective discharge of pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat. Arch. ital. Biol, 1976, 144: 244–277. [PubMed] [Google Scholar]
- Jouvet M Recherches sur les structures nerveuses el les mécanismes responsables des différentes phases du sommeil physiologique. Arch. ital. Biol, 1962, 100: 125–206. [PubMed] [Google Scholar]
- Jouvet M Neurophysiology of the states of sleep. Physiol. Rev, 1967, 47: 117–177. [DOI] [PubMed] [Google Scholar]
- Jouvet M, et Delorme F Locus coeruleus et sommeil paradoxal, C.R. Soc. Biol. (Paris), 1965, 159: 895–899. [Google Scholar]
- Kripke D An ultradian biologic rhythm associated with perceptual deprivation and REM sleep. Psychosom. Med, 1972, 34: 221–234. [DOI] [PubMed] [Google Scholar]
- Lavie P, Lord JW and Frank RA Basic rest-activity cycle in the perception of the spiral after-effect: a sensitive detector of a basic biological rhythm. Behav. Biol, 1974, 11: 373–379. [DOI] [PubMed] [Google Scholar]
- Magnes J, Moruzzi G and Pompeiano O Synchronization of the EEG produced by low-frequency electrical stimulation of the region of the solitary tract. Arch. ital. Biol, 1961, 99: 33–67. [Google Scholar]
- Magoun HW and Rhines R An inhibitory mechanism in the bulbar reticular formation. J. Neurophysiol, 1946, 9: 165–171. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Differential effects of sodium butyrate and physostigmine upon the activities of para-sleep in acute brain stem preparations. Brain Res, 1969, 13; 247–265. [DOI] [PubMed] [Google Scholar]
- Nakao S, Curthoys IS and Markham CH Direct inhibitory projection of pause neurons to nystagmus-related ponto-medullary reticular burst neurons in the cat. Exp. Brain Res, 1980, 40: 283–293. [DOI] [PubMed] [Google Scholar]
- Orr WC and Hoffman HJ A 90-min cardiac biorhythm: methodology and data analysis using modified periodogram and complex demodulation. I.E.E.E. Trans. bio-med. Engng, 1974, 21: 130–143. [DOI] [PubMed] [Google Scholar]
- Paz C, Reygadas E and Fernandez-Guardiola A Sleep alterations following total cerebellectomy in cats. Sleep, 1982, 5: 218–226. [DOI] [PubMed] [Google Scholar]
- Raffaele R, Sapienza S, Urbano A and Ventura M Changes in the sleep-wakefulness rhythm after chronic bilateral interruption of the middle cerebellar peduncles in the cat. Brain Res, 1971, 26: 195–199. [Google Scholar]
- Sakai K, Sastre JP, Kanamori N and Jouvet M Statespecific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Pompeiano O and Ajmone Marsan C (Eds.), Brain Mechanisms and Perceptual Awareness. Raven Press, New York, 1981: 405–429. [Google Scholar]
- Sherrington CS Reflexes elicitable in the cat from pinna vibrissae and jaws. J. Physiol. (Lond.), 1917. 51:404–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC and Harper RM Discharge of neurons in the parabrachial pons related to the cardiac cycle: changes during different sleep-waking states. Brain Res, 1980, 199: 385–389. [DOI] [PubMed] [Google Scholar]
- Siegel JM and McGinty DJ Pontine reticular formation neurons: relationship of discharge to motor activity. Science, 1977, 196: 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, McGinty DJ and Breedlove SM Sleep and waking activity of pontine gigantocellular field neurons. Exp. Neurol, 1977, 56: 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL and McGinty DJ Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res, 1979, 179: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Tomaszewski KS and Wheeler R Behavioral states after brainstem transection at the medullary level. Soc. Neurosci. Abstr, 1981, 7: 233. [Google Scholar]
- Siegel JM, Nienhuis R and TomaszewskL KS Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res, 1983, 268: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkin DW, Schallert T and Teitelbaum P Involvement of the pontine reticular formation in head movements and labyrinthine righting in the rat. Exp. Neurol, 1980, 69: 435–457. [DOI] [PubMed] [Google Scholar]
- Slosarska M and Zernicki B Sleep-waking cycle in the cerveau isolé cat. Arch. ital. Biol, 1973, 111: 138–155. [PubMed] [Google Scholar]
- Sterman MB, Knauss T, Lehmann D and Clemente CD Circadian sleep and waking patterns in the laboratory cat. Electroenceph. clin. Neurophysiol, 1965, 19: 509–517. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Lucas EA and Macdonald LR Periodicity within sleep and operant performance in the cat. Brain Res, 1972, 38: 327–341. [DOI] [PubMed] [Google Scholar]
- Ursin R The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res, 1968, 11: 347–356. [DOI] [PubMed] [Google Scholar]
- Villablanca J Behavioral and polygraphic study of ‘sleep’ and ‘wakefulness’ in chronic decerebrate cats. Electroenceph. clin. Neurophysiol, 1966, 21: 562–577. [DOI] [PubMed] [Google Scholar]