Abstract
We have investigated the motor and ponto-geniculo-occipital (PGO) wave response to startle eliciting stimuli in the unanesthetized cat. We found that the amplitude of the PGO spike recorded in the lateral geniculate nucleus (LGN) increases monotonically with increasing intensities of auditory stimuli. In contrast, the motor response to low intensity (< 75 dB) stimuli is characterized by electromyographic (EMG) suppression, while at higher intensities an EMG excitation is superimposed on this suppression. Thus PGO elicitation is accompanied by EMG suppression at low intensities and by a net EMG excitation at high intensities. While the amplitude of the auditory elicited PGO response is a graded function of stimulus intensity, somatic stimuli tend to elicit the PGO response in an all-or-none fashion. Both the motor and PGO responses to sensory stimulation change with behavioral state. The EMG suppression by auditory stimulation increases in duration during the transition to rapid eye movement (REM) sleep. Elicited PGO amplitude is highest in transitional sleep, lower in quiet waking and REM sleep and lowest in active waking. Prepulse inhibition of PGO spikes is greatly attenuated during transitional and REM sleep. We hypothesize the existence of 3 phasic response systems, a motor suppression system, a motor excitation (startle) system and a PGO elicitation system. While these systems are triggered concurrently by intense phasic stimuli in waking, they are modulated independently by stimulus intensity and behavioral state, and have different rates of habituation. These systems act in concert to produce behavioral responses to sudden onset stimuli.
Keywords: Sleep, Rapid eye movement sleep, Startle, Arousal, Audition, Ponto-geniculo-occipital spike, Prepulse inhibition, Motor inhibition
INTRODUCTION
One of the most salient features of rapid eye movement (REM) sleep in many mammals is the ponto-geniculo-occipito (PGO) wave, which appears shortly before REM and continues to occur throughout the REM period. These spontaneously occurring waves can be recorded from the visual cortex, pontine tegmentum, lateral geniculate nucleus (LGN) and other thalamic and cortical regions. PGO waves, which have similar wave forms and amplitudes to those that occur during sleep, are also present during wakefulness9. These spontaneously occurring PGO-like waves have been described as ‘eye-movement-potentials’, because of the close association of some of these waves with eye movements24, or ‘waking PGO’11. PGO-like waves can also be elicited by auditory and somatic stimuli during wakefulness and non-REM sleep7,8,19,33,34.
The occurrence of spontaneous PGO waves during non-REM sleep and the transition period has been shown to be linked to phasic electromyographic (EMG) suppression in both humans35 and cats21,32,33,35,36 On the other hand, there is also a relationship between the occurrence of PGO waves and the phasic muscular twitches during waking, non-REM and the active phase of REM. Morrison and Bowker33 have found a one-to-one relationship between PGO waves and muscle twitches in cats with paravermal cerebellum lesions during waking and non-REM sleep. With appropriate stimuli, PGO-like waves can be elicited simultaneously with the startle response in the cat and the rat7,33,34, and it has been hypothesized by Morrison and his colleagues32,33 that the PGO spike represents an endogenous component of the startle reflex. Thus, both increased and decreased muscle activity have been associated with PGO spikes.
In many mammals, including humans, intense auditory stimulation elicits the startle response28. However, moderate to intense (80–114 dB SPL) auditory stimuli have been reported to produce EMG suppression, with or without excitatory response, and inhibition of H reflex in humans 16,30,37. Inhibition of the monosynaptic reflex by preceding auditory stimulation has also been reported in the cat 1,42. When the reflex eliciting stimulus is preceded by another auditory ‘prepulse’, the subsequent startle response is suppressed22.
While PGO spikes, motor suppression, and startle response are all produced by auditory stimulation, basic questions about their relationship remain unanswered. Are PGO spikes linked to muscle suppression or to the startle response? How are the muscle suppression effects of auditory stimulation related to their muscle excitation effects? Are these relations independent of behavioral state? How does the probability of PGO elicitation and PGO amplitude change as a function of behavioral state and stimulus intensity? Answers to these questions should shed light on the mechanisms generating the PGO spike and motor changes after sensory stimulation. Accordingly, in the present study, we have simultaneously investigated the relations between PGO spikes, startle, and muscle tone suppression as a function of state and stimulus intensity. A preliminary report of some of these findings has been made44.
MATERIALS AND METHODS
A total of 8 adult cats were used. They were chronically implanted, under pentobarbital anesthesia (35 mg/kg), with standard electroencephalogram (EEG), electro-oculogram (EOG), and LGN electrodes as has been previously described39. All of these cats had PGO waves with a signal-to-noise ratio of at least 7:1 during the transition from non-REM to REM sleep. Uninsulated stainless steel wires’ 10 mm in length were placed with 5 mm separation to summate EMG activity from dorsal neck musculature. Experiments were performed, after at least 2 weeks of recovery from surgery, in a recording chamber (58 cm × 56 cm × 86 cm internal dimension) which had an ambient noise level of 55 ± 2 dB (re 20 μN/cm2, SPL/A Scale). Auditory stimuli were either clicks or white noise bursts. Clicks were produced by 0.15 ms, 10 V rectangular electrical pulses delivered through a Bogen CT-100 amplifier and fed to 2 Mitsubishi SG-69TB 3-way midrange loudspeakers. The intensity of the stimulus was controlled with a Hewlett-Packard 3500 decade attenuator set. White noise bursts were 20 or 25 ms in duration with 1 ms rise–decay times and were generated by a noise generator, gated through electronic switches, amplified and fed to the same two speakers. These speakers were located on diagonally opposite ends of the top of the recording chamber about 50 cm from the animal’s head. For the study of the effect of sleep–waking state on evoked LGN spikes, an 115 dB click stimulus was delivered once every 30 s to 2 min for 4–7 h while the animal went through several complete sleep–waking cycles. Somatic stimuli, 1.0–1.5 mA constant current electric pulses (0.2 ms rectangular wave) delivered through the neck EMG electrodes, were also utilized to elicit LGN responses in some animals. The effect of a weak prestimulus (auditory or somatic) on the subsequently evoked LGN spike was also examined. In these experiments, a 70 dB click, delivered through a Soundolier C803 loudspeaker situated between the other two speakers, or a 0.5–0.9 mA somatic stimulus, was given 100 ms before the 115 dB eliciting stimulus. Prestimulus trials and control trials without the preceding stimulus were presented either interspersed or in blocks, counterbalanced in order, across sleep–waking states.
The intensity function of the amplitude of the evoked LGN spike was determined by presenting a series of clicks with intensities ranging from 65 dB to 115 dB (SPL/A) during quiet waking and non-REM sleep. Stimulus intensity was increased or decreased in 10 dB steps after 50 presentations of each intensity level delivered at 2 min apart. One hour elapsed between each session. The order of presentation for each intensity level was randomized in each animal. EEG, EOG, LGN spikes, and EMG signals were monitored and recorded continuously on a Grass polygraph. The responses to stimuli were averaged using a DEC Micro-ll (MINC-11) minicomputer or an IBM PC/XT equipped with CED 1401 signal averaging package (Cambridge Electronic Design Ltd., Cambridge, England). The EMG signal was full-wave rectified, and startle amplitude was scored either from the averaged EMG response as the integral of the amplitude within 100 ms of stimulus onset and adjusted for the baseline EMG amplitude of the equivalent period before stimulus, or from individual responses as the peak amplitude when trial by trial correlation between startle and LGN responses was examined. The magnitude of the spontaneous PGO waves and evoked LGN response was measured as the peak-to-peak amplitude. The amplitude of the largest spontaneous PGO wave was determined for each cat from recordings of 4 consecutive non-REM-REM sleep cycles. Individual LGN responses were scored either from the oscilloscope traces or from the chart record. Only discrete responses which had a voltage at least 2 times the background noise level, and which occurred between 20 and 150 ms following stimulus onset, were scored. Trials in which the evoked responses were obscured by movement artifact or spontaneous PGO waves were discarded.
The duration of the stimulus-evoked EMG suppression was scored from individual trials photographed from the oscilloscope. The duration of the silent period was measured from the point where the EMG amplitude became totally flat (< 50μV) to the point where EMG amplitude recovery began (> 50 μV) during the 10–80 ms period following stimulus onset.
The animal’s sleep–waking state was categorized into active waking (AW), quiet waking (QW), non-REM sleep, transition from non-REM to REM, and REM sleep41. ‘Post-REM’ was defined as the QW or drowsy state during the 30 s period immediately after REM. Analysis of variance (ANOVA) of the data was done with the multivariate general linear model using SYSTAT statistical package (SYSTAT, Inc., Evanston, IL). Post-hoc comparisons among means were done with the Newman–Keuls test (NK).
RESULTS
Evoked LGN and EMG amplitude as a function of stimulus intensity in QW and non-REM
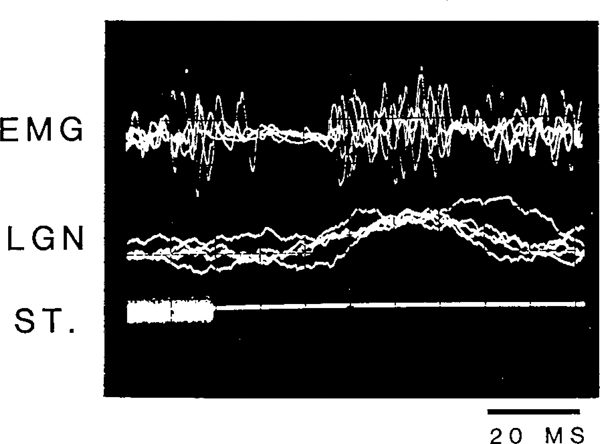
Fig. 1 shows a typical neck EMG and LGN response in the cat to an intense (105 dB) noise burst. Neck EMG response to intense auditory stimulation consisted of 3 components: an early, or primary, excitation and a late, or secondary, excitation separated by a period of EMG suppression which lasted between 10 and 30 ms in waking. The latency for the primary startle response was 6–20 ms and that for the secondary response was 40–60 ms. The evoked LGN response had a latency between 20 and 40 ms in QW and non-REM sleep. The amplitude and morphology of the startle response and the amplitude of the LGN response, however, changed as a function of the stimulus intensity.
Fig. 1.
Oscilloscope traces of neck EMG and LGN spike response to a 105 dB, 20 ms noise burst. Five traces were superimposed. ST, stimulus as recorded through a microphone inside the recording cage.
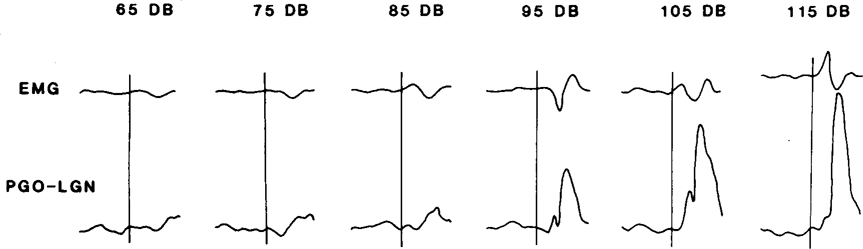
As shown in Fig. 2, during quiet waking and non-REM sleep the amplitude of the evoked LGN spike was a monotonic function of the intensity of the stimulus. An evoked response which had a wave form and time course similar to that of spontaneous PGO waves could be detected at an intensity as low as 65 dB. Response amplitude increased from 10 to 20% (of the largest amplitude for spontaneous PGO waves during REM) at 65 dB to 40–50% at 95 dB and 70–80% at 115 dB.
Fig. 2.
Averaged EMG and LGN spike responses to click stimuli of varying intensities. Fifty trials were run at each intensity level. The vertical bars represent stimulus onset. The total duration of the trace was 200 ms.
The EMG response to auditory stimulation during quiet waking and non-REM sleep was also dependent upon stimulus intensity. As also shown in Fig. 2, auditory stimuli of 65 and 75 dB mainly produced EMG suppression, in the absence of excitation. The latency for this silent period in EMG produced by weak stimuli is similar to that of the suppression elicited by more intense stimuli which separates the early and the late excitatory responses (Fig. 2). It ranged between 10 and 30 ms with a duration of 10–50 ms. The stimulus-evoked EMG suppression, when it occurred, always appeared bilaterally in the neck EMG. The pattern of EMG response changed from mainly suppression to a mixed pattern of excitation and suppression as the intensity increased (Fig. 2). At intermediate intensity levels between 85 and 105 dB late excitatory response which had a latency around 40–60 ms was often observed following a period of suppression. The early excitatory response was also observed at intermediate intensity levels, but it diminished after a few trials and only suppression or suppression followed by late excitation was observed afterwards at these intensity levels. The early excitation was more readily evoked by high intensity stimuli (Fig. 2) and on active muscles. EMG suppression was also longer and more complete at high intensity levels, although it was often obscured in the averaged data by the variation in latency and the larger magnitude of early and late excitations. The pattern of LGN and EMG change seen in Fig. 2 was seen in all 4 cats tested with varying stimulus intensities in waking and non-REM sleep.
Although not systematically studied in the present experiments, eye movements, which are closely associated with spontaneous PGO waves, could also be elicited concurrently with LGN spikes by auditory stimuli, and their amplitude was a function of stimulus intensity. The onset of the elicited eye movement usually followed the onset of the evoked LGN wave by 5–25 ms (average of 11 and 18 ms for 2 cats examined during QW and non-REM sleep).
In summary, the auditory-evoked LGN response during quiet waking and non-REM sleep is a graded process rather than an all-or-none phenomenon. At higher intensity levels the LGN response is associated with both muscle excitation and suppression (at different latencies). At lower intensities, LGN responses of lower amplitude are accompanied by EMG suppression.
State dependency of LGN spike elicitation to auditory and somatic stimulation
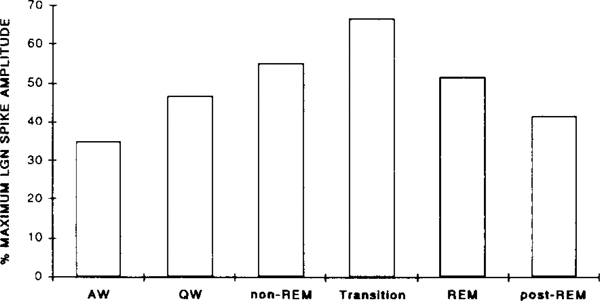
The change in evoked LGN spike amplitude as a function of the sleep–waking state of the animal was examined in 6 cats. Discrete PGO responses could be evoked by a 115 dB stimulus in all states, but the amplitude was a function of the state of the animal during stimulus presentation. As depicted in Fig. 3, the evoked LGN spike amplitude increased monotonically from AW (averaged 180 μV or 35% of the maximum amplitude) to transition state (averaged 380 μV or 67% of the maximum amplitude). The largest LGN spikes, up to 700 μV in some cases, were always elicited during the transition from non-REM to REM and early REM periods, and were comparable in amplitude to those of spontaneous PGO waves at this time. As REM progressed and clusters of spontaneous PGO spikes with smaller amplitudes appeared, the amplitude of the evoked LGN response also decreased and became variable. The evoked LGN spike amplitude decreased to waking levels as soon as the animal woke up from REM (post-REM). Repeated measures ANOVA revealed a significant quadratic trend of LGN spike amplitude as a function of state, F1,5 = 93.17, P < 0.001. Post-hoc comparisons revealed that the amplitude of elicited LGN spikes during transition was significantly greater than that during active waking, quiet waking, and post-REM period (all P values < 0.05). LGN spike amplitude during non-REM and REM was also significantly larger than that during AW. No difference in LGN spike amplitude was found between the post-REM period and that during AW or QW. The change in evoked LGN spike amplitude across states corresponded to the change in the amplitude of spontaneous LGN spikes. The correlation between the amplitude of LGN spikes occurring spontaneously before each trial and that of the subsequently evoked LGN spike, was 0.30, 0.36 and 0.40 (all P values < 0.001) for the 3 cats examined. (The coefficient of correlation for the amplitudes between adjacent spontaneous LGN spikes preceding the stimulus was 0.65, 0.49 and 0.64, all P values < 0.001 for these same cats.)
Fig. 3.
Changes in the amplitude of LGN spikes evoked by a 115 dB click stimulus as a function of state. Data from 6 cats were averaged. Amplitudes were normalized as percentage of the largest amplitude of the spontaneous PGO waves during transition and REM for each cat.
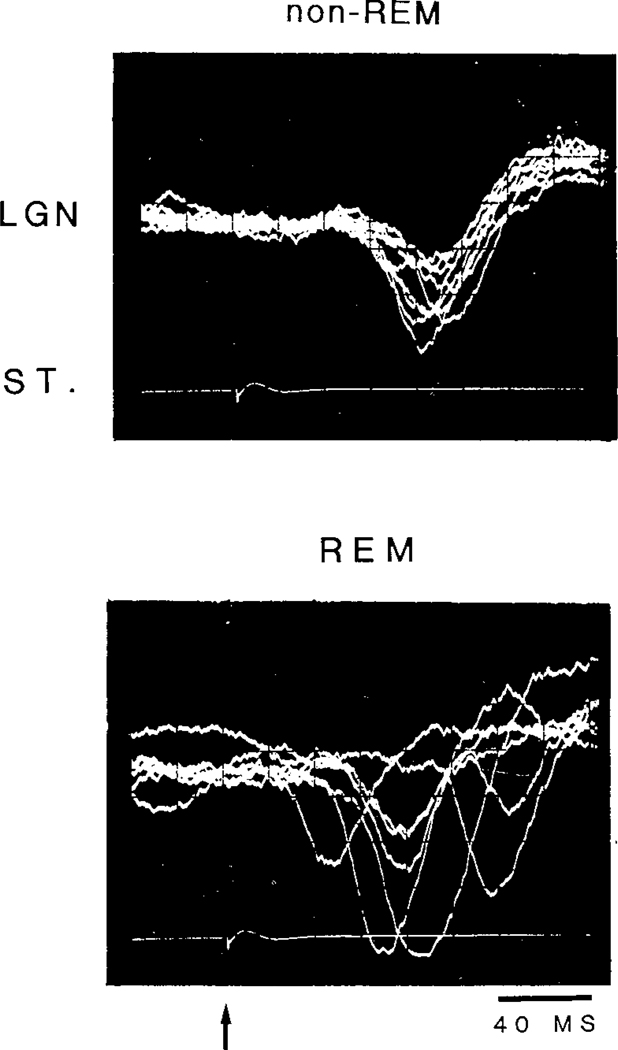
Amplitude variation of the evoked LGN spikes within a state was always smaller during QW and non-REM as compared to that during transition and REM (Fig. 4). Latency variation was also greater during transition and REM, ranging from 20 to over 150 ms, as compared to that during QW and non-REM, which was relatively constant around 40 msec (Fig. 4).
Fig. 4.
Oscilloscope traces of LGN spikes elicited by a 115 dB click stimulus during non-REM (upper) and REM (lower) states. Eight traces were superimposed. The arrow indicates stimulus onset.
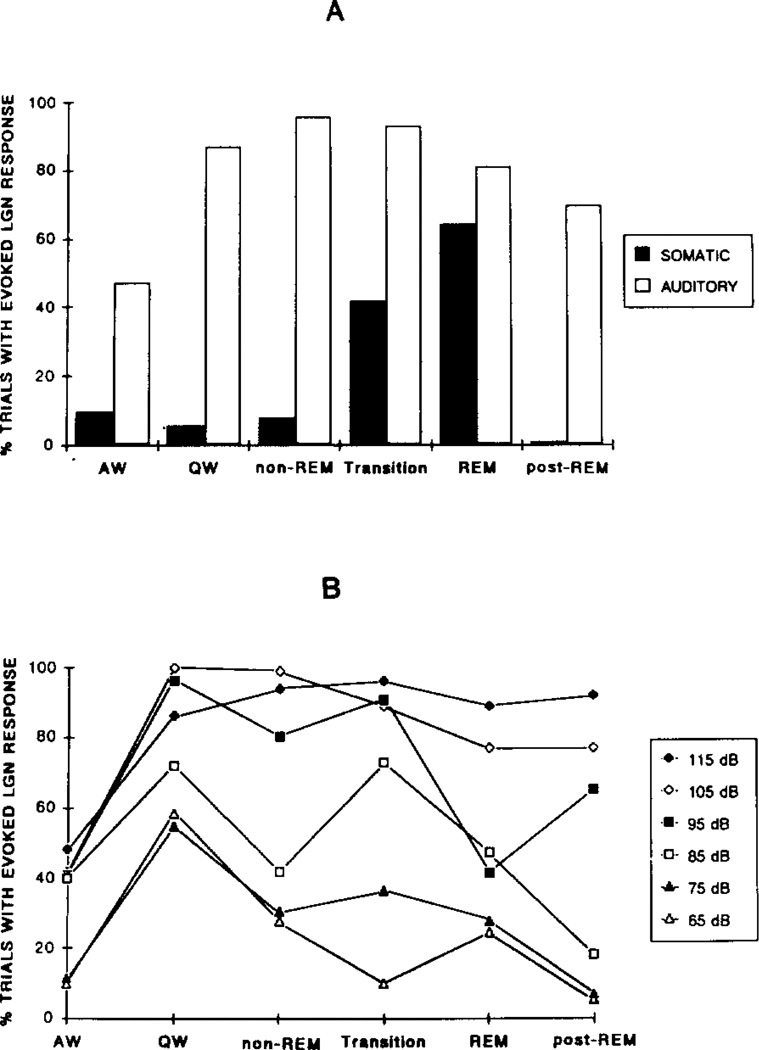
Somatic stimuli were used to elicit the PGO response in two animals. In contrast to the graded response evoked by auditory stimuli, LGN responses evoked by somatic stimuli were more of an ‘all-or-none’ phenomenon. The somatic stimuli used for eliciting LGN response, which had intensities between 1.0 and 1.5 mA, were able to invariably elicit muscle twitches around the dorsal neck and also to elicit facial reflexes. However, few discriminable evoked LGN responses were elicited by somatic stimulation during the waking and slow wave sleep states. On the other hand, responses with amplitudes comparable to that of the spontaneous waves were elicited during transition and REM. Fig. 5A shows the probability for evoking a discrete response by administration of an auditory stimulus or a somatic stimulus across states. Over 90% of the responses elicited by somatic stimuli occurred during the transition and REM periods. Repeated measures ANOVA showed a significant state factor, F5,5 = 36.2, P < 0.001. The probability of somatic stimuli eliciting an LGN response during transition and REM was significantly higher than that in other states (all P values < 0.05, NK). This is in contrast to the responses evoked by auditory stimuli for which the probability of evoking a response was not significantly different among QW, non-REM sleep, transition and REM sleep (all P values > 0.05 NK). This difference between auditory and somatic stimuli was not simply a function of stimulus intensity, since the auditory response pattern in Fig. 5A was also seen with 105 or 95 dB stimuli (Fig. 5B). Stimuli below 85 dB tended to be less effective in evoking LGN spikes during transition and REM than in QW but could evoke LGN spikes in QW, the opposite of the pattern seen with somatic stimulation. The latency for the LGN spike elicited by somatic stimulation during transition and REM ranged from 40 to 150 ms, similar to the latency range of spikes elicited by auditory stimuli in the same states.
Fig. 5.
A: probability for eliciting LGN spike response with a 115 dB click, or a 1.5 mA somatic stimulus delivered through neck EMG electrode pairs, in different states. Data from 6 cats (auditory) and 2 cats (somatic) were averaged. B: effect of varying auditory stimulus intensity on probability of LGN spike elicitation in a single cat. In both figures data are expressed as percentage of trials with discrete responses.
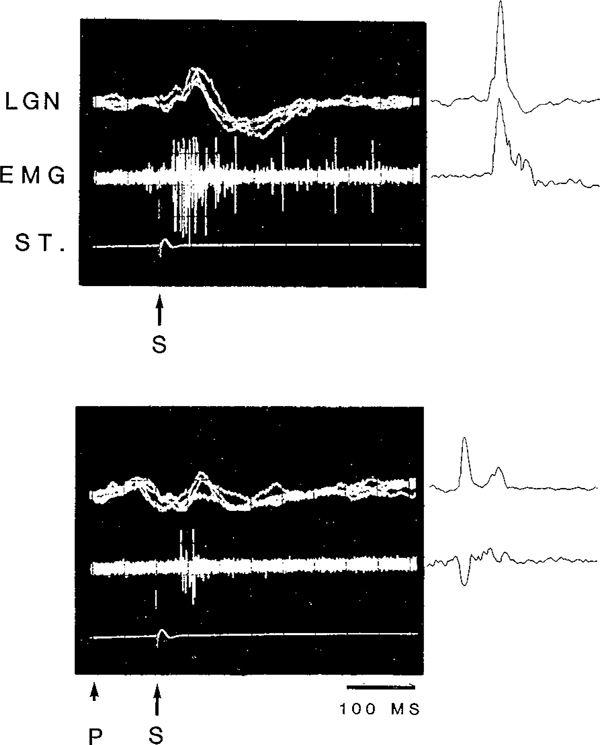
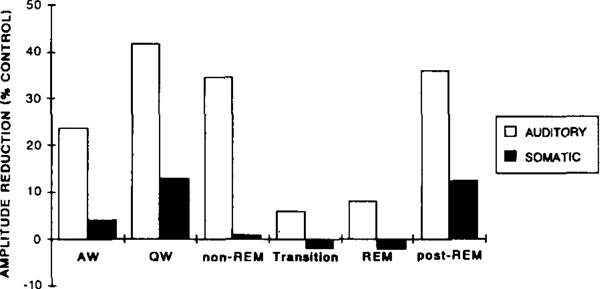
When a startle eliciting stimulus is preceded at short latency (50–400 msec) by another auditory stimuli, the amplitude of the subsequently evoked startle response is reduced22. We found that this was also true for the LGN response during waking and non-REM sleep. As demonstrated in Fig. 6, the amplitude of the startle response and that of the LGN response elicited simultaneously were both reduced when preceded at short latency (100 ms) by a 70 dB stimulus. The amount of amplitude reduction for the LGN wave was strongly affected by the state of the animal (Fig. 7). There was a significant cubic trend effect of state (F1,5 = 21.73, P < 0.01). While prepulse stimulation produced a great reduction in the amplitude of the elicited LGN wave during QW and non-REM, prepulse inhibition of the LGN wave was virtually absent during transition and REM. Post-hoc comparisons revealed significant differences in the amount of amplitude reduction during QW, non-REM and post-REM and that during transition and REM (all P values < 0.05, NK).
Fig. 6.
Oscilloscope traces (left) and computer averaged (right) LGN and EMG responses to a 115 dB click (S) with and without a preceding 80 dB click prestimulus (P) in quiet waking. The interval between the prestimulus and the startle-eliciting stimulus was 100 msec. The total durations of the oscilloscope and computer-averaged traces were both 500 ms.
Fig. 7.
Effects of an auditory (70 dB click) and a somatic (0.7 mA electrical pulse) prestimulus on the amplitude of the LGN spike elicited by a subsequent auditory stimulus (115 dB click) in different states. Data are amplitude reduction (control minus prepulse) and expressed as the percentage of the control amplitude to the eliciting stimulus alone during the same state.
Somatic prestimuli were also able to suppress subsequent auditory-evoked LGN spikes, and the effect was state dependent (F5,5 = 14.0, P < 0.01). However, this effect was much smaller than that produced by the auditory prestimulus (Fig. 7). The somatic prestimuli used were between 0.5 and 0.9 mA, an intensity sufficient to elicit muscle twitches, and full-size LGN spikes during transition and REM on 10% of the trials. The 70 dB auditory prestimulus also produced full sized LGN spikes on 10% of the trials in transition and REM. Somatic prestimuli were only effective during quiet wakefulness and not during sleep. The amount of amplitude reduction was significantly higher during quiet waking and post-REM as compared to that during non-REM, transition and REM (all P values < 0.05, NK).
State dependency of EMG responses to auditory stimulation
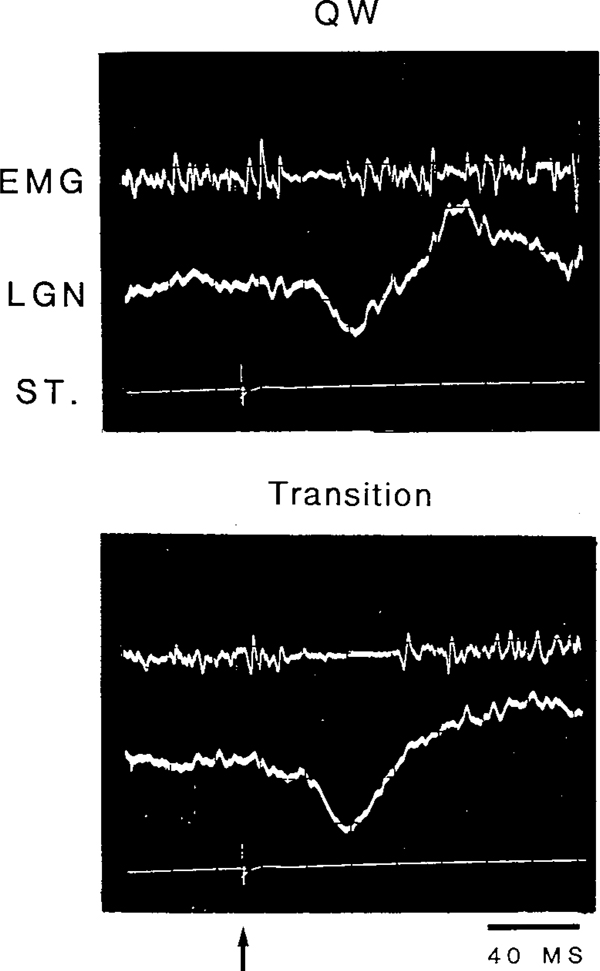
During QW, the short latency excitation, which is a manifestation of the primary startle reflex, could be elicited with the 115 dB stimulus. The primary response was followed by a silent period lasting 10–30 msec in QW. The late excitation, when present, always followed the silent period and had a latency of 40–60 ms. During non-REM sleep, the amplitude of the early excitation decreased, with only the suppression or suppression followed by the late excitation remaining. As non-REM sleep progressed, the late excitatory response also decreased while the duration of the phasic EMG suppression increased. As demonstrated in Fig. 8, the duration of the silent period evoked by a 105 dB click increased from 23 ms, during QW to 34 ms during the transition from non-REM to REM. The duration of stimulus-evoked muscle atonia during QW, non-REM and the transition period was systematically studied in 2 cats (Table I). In both cats, the duration of the silent period significantly increased from QW to non-REM sleep, to the transition from non-REM to REM. On many occasions during the transition period, the duration of the evoked EMG suppression lasted over 100 ms.
Fig. 8.
Oscilloscope traces of EMG and LGN spike response to an 105 dB click during periods of quiet waking (QW) and the transition from non-REM to REM (transition). The arrow indicates stimulus onset.
TABLE I.
Average duration for stimulus-evoked muscle atonia during quiet waking, non-REM sleep and the transition from non-REM to REM
| QW | non-REM | TRANSITION | |
|---|---|---|---|
| Cat W1 | 20.44 ± 1.17 | 32.95 ± 1.26** | 53.00 ±8.64***,+ |
| Cat T2 | 13.08 ±2.10 | 25.45 ± 1.62** | 34.48 ±2.97**,+ |
Note: Values are means ±S.E.M. Statistics are t-test.
P < 0.01, compared to QW
P < 0.001, compared to QW
P < 0.05, compared to non-REM.
Other factors which affected the startle EMG morphology included the posture of the animal relative to the muscle group from which EMG was taken, and the extent of habituation. The primary response was more easily elicited from active muscles than from relaxed muscles. Repeated presentation of the same eliciting stimulus also decreased the probability of eliciting the primary response, and increased the incidence and duration of the silent period in EMG.
Startle amplitude was larger during quiet waking than during active waking or non-REM sleep (Fig. 9). The amplitude also decreased from quiet waking to non-REM sleep. In contrast to the evoked LGN response, the startle response was greatly depressed and overt response was rarely elicited during REM, presumably due to the motoneuron inhibition of this state 15. During transition, auditory stimuli evoked muscle suppression rather than excitation. On a few rare occasions in which startle responses did occur during the transition and REM, the animal responded with a vigorous body jerk which generally interrupted the transition to REM or awakened the animal from REM. The repeated measures ANOVA of startle amplitude on 2 cats showed significant quadratic trend of state function, F1,1 = 20.21, P < 0.05.
Fig. 9.
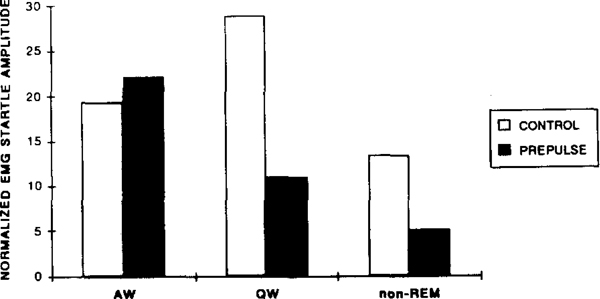
Startle amplitude to a 115 dB click with (prepulse) or without (control) a preceding 70 dB click stimulus during active waking (AW), quiet waking (QW) and non-REM sleep. Data are the average of 2 cats and normalized as the percentage of total response level across states.
The effect of auditory prestimuli on startle amplitude was also a function of the animal’s state (Fig. 9). Prestimuli were less effective in reducing the startle response when the animal was in active waking, and were most effective during QW and non-REM sleep. (As discussed above, startle was greatly suppressed in transition and REM.) The repeated measures ANOVA on 2 cats showed a significant main effect of prestimulus, F1,1 = 279.6, P < 0.04, and a significant interaction of prestimulus and state function, F2,2 = 27.7, P < 0.04.
Startle and LGN response habituation in QW and non-REM
The startle and the LGN response had very different habituation rates. At interstimulus interval of 50 s, startle amplitude habituated to an average of 50% within 40 trials and to 24% within 60 trials compared to the amplitude of the initial 10 trials during QW. On the other hand, LGN response to the same stimulus presented at the same interval showed comparably little habituation even after several hundred stimulus repetitions. The amplitude of LGN response during the last 10 trials in a 300-trial series was on the average 86% (range 65–101%) of that of the initial 10 trials of the same state. However, there was a correlation between startle and LGN spike amplitudes for the initial 50 trials when startle was not totally habituated during quiet waking and non-REM sleep. A large startle response during these states almost always guaranteed a large concurrent LGN response. The correlation coefficients between startle amplitude and the amplitude of the evoked LGN spike during waking and non-REM sleep for 3 cats examined was 0.28 (n = 50, P < 0.05), 0.56 (n = 22, P < 0.01) and 0.65 (n = 50, P < 0.001) respectively.
DISCUSSION
The present study demonstrated that PGO-like waves, which had similar wave forms to the spontaneous ones, can be evoked in the cat LGN in all sleep–waking states by auditory stimuli, consistent with previous studies7,32, and that the amplitude varied depending on the state of the animal. The amplitude of the evoked LGN spike corresponded to that of the spontaneous waves during that state, and was maximal in transition and REM and minimal in waking and non-REM. This is in contrast to that reported previously in the rat25,26 in which the number and amplitude of the elicited PGO waves recorded from locus coeruleus (LC) were lowest during REM and highest immediately after REM. In the rat LC, the amplitude of the PGO wave, when elicited, was maximal during waking. In the cat LGN, however, the evoked PGO amplitude during waking was less than half of the size of the spontaneous waves in transition and REM, even with a 115 dB stimulus. The LC has been shown to inhibit PGO wave generation13. It is likely then the difference between our LGN recording and previous LC studies represents differential excitation of these two structures in different states.
Auditory stimuli may, elicit LGN spikes in waking and non-REM sleep through the same underlying mechanism which generates spontaneous PGO waves during transition and REM. This is suggested by the fact that both the evoked and spontaneous LGN spikes were recorded from the same electrodes, had similar wave forms, showed similar state dependency of amplitude change, and were closely associated with eye movements. The tonic inhibitory control of PGO generating mechanisms during waking and non-REM sleep from other brainstem sources, e.g., dorsal raphe and locus coeruleus13,40, may contribute to the reduced LGN spike amplitude during waking and non-REM sleep. During transition and REM, the PGO generators are free from inhibitory influences from the dorsal raphe neurons and locus coeruleus, and thus larger LGN spikes are evoked.
Within the range of intensities of somatic stimuli used in the present study, LGN responses could only be elicited during the transition and REM but not during waking and non-REM sleep. A similar effect was seen in the elicitation of LGN responses by electrical stimulation of the reticular formation4,10,17,29. This suggests that whether or not the PGO wave is an ‘all-or-none’ phenomenon or a ‘graded’ process may depend upon how PGO waves are evoked, and that the all-or-none gating phenomenon occurs prior to the point at which auditory and somatic sensory signal converge on the PGO generator.
When startle and LGN spikes were concurrently elicited during waking and non-REM sleep, their amplitudes were correlated. Both startle and LGN spike responses could be reduced by a preliminary stimulus. These results indicate that there is a common prestimulus modulation mechanism for these two processes. The modulation may occur early at the sensory pathway as has been suggested for startle43,45. Auditory prestimuli were more effective in suppressing the subsequently evoked LGN response than somatic prestimuli, which suggests that the modulation by prestimuli may be modality specific. The fact that the evoked LGN spike and startle have very different threshold and habituation rates, as also indicated in other studies25,26, however, suggests that these two phenomena, although sharing common sensory inputs, have independent mechanisms triggering them and modulating their amplitude. Anatomical and physiological evidence have suggested that the startle triggering mechanism is located at the medial pontomedullary reticular formation18,45, while the PGO generator is located at the dorsolateral pontine tegmentum38. The startle and the LGN response became totally dissociated as the animal passed from non-REM to REM, with evoked LGN response amplitude increasing while startle response amplitude greatly decreased.
The loss of both somatic and auditory prepulse inhibition of PGO waves during transition and REM is a striking phenomenon. One may hypothesize that this results from a central reorganization of inhibitory processes during this state. Chase and McGinty14 have shown that orbital cortex stimulation effective in suppressing the masseteric reflex in waking is ineffective in REM sleep. A similar process may be operative in the loss of prepulse suppression of the PGO spike. One may also hypothesize that the attenuation of sensory input in REM sleep3,12,23 renders the prepulse stimulus, which is of relatively low intensity, ineffective but is not sufficient to block the response elicited by more intense startle eliciting stimulus. Further work using electrical prepulse stimulation of brainstem regions would be necessary to test these two hypotheses, which are not mutually exclusive.
During waking and non-REM sleep the amplitude of the evoked LGN spike was a function of the intensity of the stimulus. However, the same stimulus which evoked the PGO response in both states would elicit either muscle suppression or excitation, depending upon the state of the animal. The startle response could be elicited during waking and non-REM sleep but rarely during transition and REM periods. The size of the excitatory EMG response was maximal in quiet waking while EMG suppression was maximal during transition. Our findings are consistent with reports of motor inhibition produced by auditory stimulation during transition20, and also with a recent study of diaphragmatic EMG response to auditory stimuli27. The present study also demonstrated that the inhibitory process has a lower threshold than the excitatory process. Furthermore, the latency for the silent period in EMG remained relatively constant, regardless of whether or not an excitatory response preceded it. These results suggest that the excitatory and inhibitory process are activated independently, and that the suppression is not a consequence of prior excitation. The mechanism for the transient, stimulus-evoked, muscle atonia is not known at present. Indirect evidence27 suggests that it may be mediated in part by the same mechanism which is responsible for muscle atonia during REM, since lesioning of the dorsolateral pons, which induces REM-without-atonia, also eliminates the stimulus-evoked suppression of diaphragm muscles.
In summary, we have identified 3 phasic responses to auditory stimulation: muscle suppression, muscle excitation, and PGO response. The present evidence suggests that these 3 phasic events are mediated by 3 independent generator mechanisms, and are modulated independently by state, stimulus intensity, and habituation processes. The final outcome of muscle response to the stimulus is a result of summation of the excitatory and the inhibitory process, and depending on the intensity of the stimulus and the state of the organism, the response may be pure inhibition or excitation superimposed on the inhibition. PGO-like waves can be evoked by somatic and auditory stimuli over a wide range of intensities, and the amplitude of these waves is also a function of stimulus intensity and the state of the animal. The initial muscle atonia in response to weak stimuli may represent a primary component of the ‘orienting reflex’, which functions to alert the animal by interrupting the ongoing behavior and allowing it to attend to the stimulus. The startle reflex to sudden, intense stimuli may be the extreme of the response continuum which primes the organism for subsequent movement2. PGO waves may represent an internal alerting response32 which depending upon the strength of the evoking stimulus (external or internal) and arousal state of the organism, can be associated with either activation or suppression of muscle activity. Thus these 3 parallel systems may serve to coordinate sensorimotor responses appropriate to sudden onset stimuli.
ACKNOWLEDGEMENTS
This research was supported by the Medical Research Service of the Veterans Administration and PHS Grants MH43811 and NS14610. The authors would like to thank E. Kleiger for his assistance in scoring some of the data, and Dr. D.J. McGinty, Dr. N. Shouse and G. Arankowsky-Sandoval for allowing us to use some of their cats.
REFERENCES
- 1.Barnes CD and Thomas JS, Effects of acoustic stimulation on the spinal cord, Brain Research, 7 (1968) 303–305. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MVL, Escapism: some startling revelations. In Eaton RC (Ed.), Neural Mechanisms of Startle Behavior, Plenum, New York, 1984, pp. 353–363. [Google Scholar]
- 3.Berlucchi G, Munson JB and Rizzolatti G, Changes in click-evoked responses in the auditory system and the cerebellum of free-moving cats during sleep and waking, Arch. Ital. Biol, 105 (1967) 118–135. [PubMed] [Google Scholar]
- 4.Bizzi E and Brooks DC, Functional connections between pontine reticular formation and lateral geniculate nucleus during deep sleep, Arch. Ital. Biol, 101 (1963) 666–680. [PubMed] [Google Scholar]
- 5.Bowker RM, The awakening of the sleeping ponto-geniculo-occipital wave. In Koell WP (Ed.), Sleep, S. Karger, Basel, 1980, pp. 304–306. [Google Scholar]
- 6.Bowker RM, Variability in the characteristics of ponto-geniculo-ocipital spikes during paradoxical sleep, Exp. Neurol, 87 (1985) 212–224. [DOI] [PubMed] [Google Scholar]
- 7.Bowker RM and Morrison AR, The startle reflex and PGO spikes, Brain Research, 102 (1976) 185–190. [DOI] [PubMed] [Google Scholar]
- 8.Bowker RM and Morrison AR, The PGO spike: an indicator of hyperalertness. In Koella WP and Levin P (Eds.), Sleep, Karger, Basle, 1976, pp. 23–27. [Google Scholar]
- 9.Brooks DC, Waves associated with eye movement in the awake and sleeping cat, Electroencephalogr. Clin. Neurophysiol, 24 (1968) 532–541. [DOI] [PubMed] [Google Scholar]
- 10.Brooks DC and Bizzi E, Brainstem electrical activity during deep sleep, Arch. Ital. Biol, 101 (1963) 648–665. [PubMed] [Google Scholar]
- 11.Brooks DC and Gershon MD, Eye movement potentials in the oculomotor and visual systems of the cat: a comparison of reserpine induced waves with those present during wakefulness and rapid eye movement sleep, Brain Research, 27 (1971) 223–239. [DOI] [PubMed] [Google Scholar]
- 12.Carli G, Diete-Spiff K and Pompeiano O, Transmission of sensory information through the lemniscal pathway during sleep, Arch. Ital. Biol, 105 (1967) 31–51. [PubMed] [Google Scholar]
- 13.Cespuglio R, Gomez ME, Faradji H and Jouvet M, Alternations in the sleep—waking cycle induced by cooling of the locus coeruleus area, Electroencephalogr. Clin. Neurophysiol, 54 (1982) 570–578. [DOI] [PubMed] [Google Scholar]
- 14.Chase MH and McGinty DJ, Modulation of spontaneous and reflex activity of the jaw musculature by orbital cortical stimulation in the freely-moving cat, Brain Research, 19 (1970) 117–126. [DOI] [PubMed] [Google Scholar]
- 15.Chase MH and Morales ER., Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep, Science, 221 (1983) 1195–1198. [DOI] [PubMed] [Google Scholar]
- 16.Cirignotta E, Montagna P, Mondini S and Lugaresi E, Inhibitory phenomena during NREM sleep related to auditory stimuli and epileptic spikes, Electroencephalogr. Clin. Neurophysiol, 55 (1983) 165–167. [DOI] [PubMed] [Google Scholar]
- 17.Cole AM, Griffin AE and Burke W, All-or-nothing and graded slow waves in the lateral geniculate nucleus to stimulation of the midbrain reticular formation, Exp. Neurol, 69 (1980) 528–542. [DOI] [PubMed] [Google Scholar]
- 18.Davis M, A primary acoustic startle circuit: lesion and stimulation studies, J. Neurosci, 2 (1982) 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drucker-Colin R, Bernal-Pedraza J, Fernandez-Cancino F and Morrison AR, Increasing PGO spike density by auditory stimulation increases the duration and decreases the latency of rapid eye movement (REM) sleep, Brain Research, 278 (1983) 308–312. [DOI] [PubMed] [Google Scholar]
- 20.Glenn LL, Brainstem and spinal control of lower limb motoneurons with special reference to phasic events and startle reflexes. In McGinty DJ, Drucker-Colin R, Morrison A and Parmeggiani PL (Eds.), Brain Mechanisms of Sleep, Raven Press, New York, 1985, pp. 81–95. [Google Scholar]
- 21.Glenn LL and Dement WC, Motoneuron properties during electromyogram pauses in sleep, Brain Research, 243 (1982) 11–23. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HS and Ison JR, Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input, Psychol. Rev, 87 (1980) 175–189. [PubMed] [Google Scholar]
- 23.Huttenlocher PR, Effects of state of arousal on click responses in the mesencephalic reticular formation, Electroencephalogr. Clin. Neurophysiol, 12 (1960) 819–827. [DOI] [PubMed] [Google Scholar]
- 24.Jeannerod M and Sakai K, Occipital and geniculate potentials related to eye movements: saccade-locked changes in dark and in light, Brain Research, 19 (1970) 361–377. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman LS, PGO waves in rats in the non-paradoxical sleep states, Brain Research, 276 (1983) 73–80. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman LS and Morrison AR, Ponto-geniculo-occipital waves in rats reflect the state-dependent modulation of sensory input to the locus coeruleus. In Spiegelstein MY and Levy A (Eds.), Behavioral Models and the Analysis of Drug Action, Elsevier, Amsterdam, 1982, pp. 459–465. [Google Scholar]
- 27.Kline LR, Hendricks JC, Silage DA, Morrison AR and Pack AI, State-dependent changes in diaphragmatic activity produced by startling stimuli, Sleep Res, 17 (1988) 8. [Google Scholar]
- 28.Landis C and Hunt WA, The Startle Pattern, Farrar and Tinehart, New York, 1939. [Google Scholar]
- 29.Malcolm LJ, Watson JA and Burke W, PGO waves as unitary events, Brain Research, 24 (1970) 130–133. [DOI] [PubMed] [Google Scholar]
- 30.Meier-Ewert K, Gleitsman K and Reiter F, Acoustic jaw reflex in man: its relationship to other brain-stem and microreflexes, Electroencephalogr. Clin. Neurophysiol, 36 (1974) 629–637. [DOI] [PubMed] [Google Scholar]
- 31.Morrison AR, Relationships between phenomena of paradoxical sleep and their counterparts in wakefulness, Acta Neurobiol. Exp, 39 (1979) 567–583. [PubMed] [Google Scholar]
- 32.Morrison AR, Brainstem regulation of behavior during sleep and wakefulness. In Sprague JM and Epstein AN (Eds.), Progress in Psychobiology and Physiological Psychology, Vol. 8 Academic Press, New York, 1979, pp. 91–131. [Google Scholar]
- 33.Morrison AR and Bowker RM, Cerebellar and spinal contributions to the regulation of muscle tone and movement during sleep. In Jovanovic UJ (Ed.), The Nature of Sleep, Gustav Fischer, Stuttgart, 1973, pp. 270–277. [Google Scholar]
- 34.Morrison AR and Bowker RM, The biological significance of PGO spikes in the sleeping cat, Acta Neurobiol. Exp, 35 (1975) 821–840. [PubMed] [Google Scholar]
- 35.Pivik T and Dement WC, Phasic changes in muscular and reflex activity during non-REM sleep, Exp. Neurol, 27 (1970) 115–124. [DOI] [PubMed] [Google Scholar]
- 36.Pivik RT and Metz J, Phasic EMG inhibition and spinal reflex modulation during synchronized sleep in the cat, Exp. Neurol, 48 (1975) 493–501. [DOI] [PubMed] [Google Scholar]
- 37.Rossignol S, Startle responses recorded in the leg of man, Electroencephalogr. Clin. Neurophysiol, 39 (1975) 389–397. [DOI] [PubMed] [Google Scholar]
- 38.Sakai K, Some anatomical and physiological properties of pontomesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In Hobson JA and Brazier MAB (Eds.), The Reticular Formation Revisited, Raven, New York, 1980, pp. 429–447. [Google Scholar]
- 39.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of pontine gigantocellular field neurons, Exp. Neurol, 56 (1977) 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon R, Gershon MD and Brooks DC, The role of the raphe nuclei in the regulation of PGO wave activity, Brain Research, 58 (1973) 313–330. [DOI] [PubMed] [Google Scholar]
- 41.Ursin R and Sterman MB, A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat, Brain Information Service/Brain Research Institute, University of California, Los Angeles, 1981. [Google Scholar]
- 42.Wright CG and Barnes CD, Audio-spinal reflex responses in decerebrate and chloralose anesthetized cats, Brain Research, 36 (1972) 307–331. [DOI] [PubMed] [Google Scholar]
- 43.Wu M-F, Modulation by prestimulus and background noise of multiple-unit activities in the inferior colliculus of the rat: relationship with the acoustic pinna reflex, Soc. Neurosci. Abstr, 13 (1987) 61. [Google Scholar]
- 44.Wu M-F, Mallick BN and Siegel JM, Lateral geniculate spikes, muscle atonia and startle response elicited by auditory stimuli are a function of stimulus parameters and sleep-waking state, Sleep Res, 17 (1988) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M-F, Suzuki SS and Siegel JM, Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle, Brain Research, 457 (1988) 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]