Abstract
Background
There are few evidence-based interventions for long COVID; however, holistic approaches supporting recovery are advocated. We assessed whether an online breathing and wellbeing programme improves health related quality-of-life (HRQoL) in people with persisting breathlessness following COVID-19.
Methods
We conducted a parallel-group, single-blind, randomised controlled trial in patients who had been referred from one of 51 UK-based collaborating long COVID clinics. Eligible participants were aged 18 years or older; were recovering from COVID-19 with ongoing breathlessness, with or without anxiety, at least 4 weeks after symptom onset; had internet access with an appropriate device; and were deemed clinically suitable for participation by one of the collaborating COVID-19 clinics. Following clinical assessment, potential participants were given a unique online portal code. Participants were randomly assigned (1:1) to either immediate participation in the English National Opera (ENO) Breathe programme or to usual care. Randomisation was done by the research team using computer-generated block randomisation lists, with block size 10. The researcher responsible for randomisation was masked to responses. Participants in the ENO Breathe group participated in a 6-week online breathing and wellbeing programme, developed for people with long COVID experiencing breathlessness, focusing on breathing retraining using singing techniques. Those in the deferred group received usual care until they exited the trial. The primary outcome, assessed in the intention-to-treat population, was change in HRQoL, assessed using the RAND 36-item short form survey instrument mental health composite (MHC) and physical health composite (PHC) scores. Secondary outcome measures were the chronic obstructive pulmonary disease assessment test score, visual analogue scales (VAS) for breathlessness, and scores on the dyspnoea-12, the generalised anxiety disorder 7-item scale, and the short form-6D. A thematic analysis exploring participant experience was also conducted using qualitative data from focus groups, survey responses, and email correspondence. This trial is registered with ClinicalTrials.gov, NCT04830033.
Findings
Between April 22 and May 25, 2021, 158 participants were recruited and randomly assigned. Of these, eight (5%) individuals were excluded and 150 participants were allocated to a treatment group (74 in the ENO Breathe group and 76 in the usual care group). Compared with usual care, ENO Breathe was associated with an improvement in MHC score (regression coefficient 2·42 [95% CI 0·03 to 4·80]; p=0·047), but not PHC score (0·60 [–1·33 to 2·52]; p=0·54). VAS for breathlessness (running) favoured ENO Breathe participation (−10·48 [–17·23 to –3·73]; p=0·0026). No other statistically significant between-group differences in secondary outcomes were observed. One minor self-limiting adverse event was reported by a participant in the ENO Breathe group who felt dizzy using a computer for extended periods. Thematic analysis of ENO Breathe participant experience identified three key themes: (1) improvements in symptoms; (2) feeling that the programme was complementary to standard care; and (3) the particular suitability of singing and music to address their needs.
Interpretation
Our findings suggest that an online breathing and wellbeing programme can improve the mental component of HRQoL and elements of breathlessness in people with persisting symptoms after COVID-19. Mind–body and music-based approaches, including practical, enjoyable, symptom-management techniques might have a role supporting recovery.
Funding
Imperial College London.
Introduction
COVID-19 can cause long-term illness and disability,1, 2, 3 which is increasingly appreciated as a major global challenge.4, 5 Various case definitions are currently in use—long COVID refers to symptoms that continue or develop beyond 4 weeks after the start of acute COVID-19,6 whereas post-COVID condition refers to symptoms 3 months from initial infection and lasting at least 2 months.7 Long COVID is a heterogeneous condition that can involve multiple organs, resulting in numerous, often debilitating symptoms.8, 9 People with long COVID commonly have breathlessness, anxiety, and reduced quality of life.3, 10 At least 10% of people who develop COVID-19 have one or more symptoms for 12 weeks or longer,11 with some estimates considerably higher.9 Currently, approximately 1·3 million people in the UK (2% of the population), are estimated to have long COVID.12
Research in context.
Evidence before this study
We searched PubMed using the search terms “post-COVID-19 syndrome”, “long COVID” and “trial” or “treatment”, from inception to Feb 20, 2022, with no limits on language, and found no randomised clinical trial data addressing interventions or treatments in people with ongoing symptoms following COVID-19. Previous research has demonstrated large numbers of people experience long-term illness and disability due to COVID-19, with breathlessness being a common symptom. Individualised holistic approaches to rehabilitation have been widely advocated given potentially relevant research in other conditions.
Added value of this study
To our knowledge, this is the first randomised controlled trial to evaluate an intervention for people with long COVID. We found that participation in an online breathing and wellbeing programme resulted in improvements in the mental component of health-related quality of life, and elements of breathlessness, in people with persisting breathlessness after COVID-19.
Implications of all the available evidence
Our findings suggest that mind–body and music-based approaches, including practical, enjoyable symptom-management techniques, might have a role supporting recovery for people with persisting breathlessness following COVID-19. Research into other related approaches would be valuable, as would better characterisation of long COVID subgroups to identify those most likely to benefit. Further randomised controlled trials of interventions targeting both symptoms and underlying pathology are required to create a portfolio of evidence-based management options adaptable to the specific needs of individual patients.
A key research priority is addressing the absence of evidence-based interventions.3, 13, 14, 15 Long COVID management guidelines have so far largely been based on expert opinion, and advocate personalised, holistic approaches to support recovery.6, 16 Arts-in-health interventions can promote health and wellbeing for people with long-term health conditions.17 Music and singing based activities have been shown to improve health related quality of life (HRQoL)18 and are popular for people with long-term respiratory conditions and breathlessness.19, 20, 21, 22, 23, 24 Additionally, the pandemic has seen successful online adaptation and delivery of many activities, including dance and Singing for Lung Health programmes,25, 26 because singing is an activity associated with increased aerosol generation.27
The English National Opera (ENO) created ENO Breathe in collaboration with health-care professionals, as part of its long-standing commitment to social prescribing. ENO Breathe is an online breathing retraining and wellbeing programme, which aims to support people recovering from COVID-19 with persistent breathlessness, with or without anxiety. Since the programme started on Oct 1, 2020, more than 500 people have taken part in ENO Breathe, and many other organisations and individuals globally have begun developing related programmes. The primary aim of this study was to test the hypothesis that ENO Breathe would improve mental and physical HRQoL, and breathlessness, in people with long COVID.
Methods
Study design and participants
This parallel-group, single-blind, randomised controlled trial was conducted in patients who had been referred from one of the 51 UK-based collaborating long COVID clinics in which multidisciplinary team assessment and management took place. This included investigations such as lung function testing, echocardiography, and further imaging as appropriate for the individual. As such, treatable pathology was identified and managed to the best extent possible given current knowledge. Individuals with ongoing breathlessness (ie, who had chronic breathlessness syndrome28) despite the investigation and management steps taken, could be considered for referral to ENO Breathe. Patients were given a unique login code by the clinic team. The exact proportion of codes that were used from those given out was not monitored during the trial period itself. However, during the subsequent 3 months as the clinical programme has continued, the rate has been stable at 43–44%.
Eligible participants were aged 18 years or older; were recovering from COVID-19 with ongoing breathlessness, with or without anxiety; had internet access with an appropriate device (eg, computer or tablet); and were deemed clinically suitable for participation by a specialist collaborating COVID-19 clinic after appropriate clinical evaluation and investigation. Participants were excluded if they were (in their or the clinical team's judgment) too unwell, had excessive fatigue, had concerning upper airways symptoms, or were unable to participate due to comorbidities. The long COVID diagnosis required patients to have ongoing symptoms at least 4 weeks following the onset of COVID-19, as per current guidelines,6 and was made by the collaborating specialist clinic using a combination of laboratory investigations and comprehensive clinical assessment. Recruitment continued until the target sample size was met. All participants provided written informed consent.
This study is reported following the CONSORT 2010 guidelines.29 Ethics approval was granted by the National Health Service Health Research Authority, Stanmore Research Ethics Committee (19/LO/0418). The study was conducted in accordance with the 1975 Declaration of Helsinki.
Randomisation and masking
Participants were randomly assigned (1:1) to immediate participation in ENO Breathe or to usual care (in effect a 6-week delay to participation in ENO Breathe until after they had exited the trial) using computer-generated block randomisation lists, with block size 10 (using SealedEnvelope). Randomisation by the research team took place when the potential participant used their individual code to log in to the ENO online system, which in practice resulted in five random assignments of individuals who then did not consent to participate in the research study, although two went on to participate in the programme after the research was completed. These individuals were not allocated to a study group or told which group they would have been allocated to.
Masking of participants was not possible given the nature of the intervention. However, outcome measures were collected using a self-completion online form, with the researcher responsible for randomisation masked to responses.
Procedures
The intervention group participated in ENO Breathe, a breathing and wellbeing programme developed by the ENO's learning and participation team, with clinical input and ongoing support from members of the Imperial College Healthcare Trust (ICHT) Respiratory Medicine Team. The programme is intended to support people recovering from COVID-19 with breathlessness, with or without anxiety, and focuses on breathing retraining through singing techniques and utilising lullabies, delivered online via a video conferencing application. Lullabies were intentionally selected given their accessibility, appropriateness for non-specialist singers, and inherent suitability given their core purpose to calm and soothe. This choice of repertory was identified as central to the programme's success in evaluation of earlier groups before this study (unpublished). The programme consists of an initial online 20-min one-to-one discussion with the ENO team to establish rapport, assess symptoms and expectations, confirm current suitability, and answer questions. This is followed by six, once weekly, 1 h online group workshop sessions led by an ENO vocal specialist. Participants also receive a welcome pack, containing a welcome note from the ENO Breathe team and items including an ENO mug, tea, biscuits, and a reusable straw (the latter provided for the straw phonation exercises on the programme). The pack fulfils a dual intention of providing resources required for participation in sessions, alongside fostering a sense of intimacy and connection that would be experienced in an in-person session environment (eg, tea and biscuits in break times). Participants have access to specially developed online audio-visual resources to support learning between sessions as well as regular emails with new music and other supportive content to encourage them to practise the techniques (table 1 ). Intervention components are tailored to the individual participant's capabilities and needs through continuous feedback, including the exercises and tools selected, and the intensity, rests, and suggested homework. As such, the programme content is personalised, whereas the core components, duration, and methods of delivery are kept constant.
Table 1.
Overview of the components of the ENO Breathe programme
| People present | Description | Content | |
|---|---|---|---|
| One-to-one | Two ENO Breathe team members (ENO vocal specialist and ENO group coordinator) and participant | Overview and review current individual participation suitability (20 min) | Introduce course content in more depth; discuss suitability for the programme at current time; answer questions; address technical difficulties for those with limited online video conferencing software experience to ensure full participation regardless of initial technological proficiency |
| Welcome pack | .. | Welcome pack delivered by mail at beginning of programme | Contains a welcome card, ENO tote bag, ENO mug, tea, biscuits, and a reusable straw (for straw phonation exercises) |
| Weekly online group sessions | Led by an ENO vocal specialist with up to 20 participants | Encourage participation in exercises and activities to support breathing control, providing tools for self-management of breath and anxiety (six 1 h sessions) | Warm-up exercises to prepare the body and mind; practical tools to support improvement in posture and breath control, encouraging self-management of anxiety and breathlessness; guided and supported singing of culturally diverse lullabies selected for their power to calm and soothe (memorable and accessible to all); a moment for participants to connect with each other in a safe and supportive environment; homework (session leaders give participants homework each week, encouraging participants to focus on particular exercises, and to engage with the material in a specific way, learning the exercise and integrating it into their daily routine); related online supporting resources (below) are provided |
| Midpoint focus group | Participants who attended the third group session | Semi-structured participant discussion about how they are finding the programme | Provides an opportunity to feedback in more depth to session leaders; sharing of experiences between participants; identify any issues arising |
| Online resources | Participant self-directed | Bespoke online digital resources to support participants between sessions (as per participant preference) | Lullabies to sing along with or listen to from a specially recorded playlist of lullabies used in the ENO Breathe programme; lullabies from operas performed by ENO singers and players, selected and recorded especially for ENO Breathe participants to watch and listen to; downloadable playlists for bedtime or moments of anxiety or panic; filmed exercise videos explaining and guiding viewers through breathing, vocal, and warm-up techniques; further exercises and tools for daily practice, or as frequently as the participant feels is appropriate for them; in addition, towards the end of a session, the session leader shows the relevant week's online page by sharing their screen and guiding participants to the exercises and lullabies for that week |
| Regular emails and point of contact provided | All participants during the 6-week course | Emails (minimum of three per week) from group leaders to participants | Encouraging engagement with online resources; weekly release of a specially filmed opera lullaby performed by the ENO, emailed directly to participants; pre-session email reminding to attend upcoming session, sheet of music and lyrics of any lullabies to be covered, and the link to the session; group text reminder is sent to all participants within a group before a session starts to remind them to join the online link for the session; email following session recapitulates session content and reminds them of key exercise homework with links to online resources; other emails, throughout the week, depending on the needs of each participant; all emails received from participants are answered by their group coordinator; group coordinators are provided with a mobile telephone by ENO; participants have the phone number of their group coordinator and can call and text |
| Twilight sessions | Open to all previous ENO Breathe participants | Fortnightly evening sessions (these were not included as a component of the research study intervention, which was limited to the core 6-week programme) | Open to anyone who has previously completed the core ENO Breathe programme, to provide ongoing sessions for individuals wanting to continue |
ENO=English National Opera.
At the time the study commenced, 192 people with long COVID had taken part in the ENO Breathe programme, including continuous evaluation by an external independent evaluator (THH), which suggested the intervention was acceptable and safe. Adherence to, and potential adaptations from, the standard format are monitored and discussed in reflective programme provider discussions facilitated by a health psychologist (AMA) focused on clinical issues and potential emotional and relational pitfalls emerging from the group's dynamics. Fortnightly meetings involving ENO Breathe session leaders and clinicians at ICHT (respiratory physiotherapists and speech and language therapists) were scheduled to continue discussion of exercises delivered during sessions. Every 2 months, ENO Breathe Steering Group meetings also took place and involved a range of relevant stakeholders, including participant representatives. No substantial changes to the intervention took place during the study period, or since, at the time of writing.
Adherence to the programme is monitored with a register, with reasons for non-attendance recorded when possible. Participants are informed that full attendance at sessions is strongly recommended to get the full benefits of the programme, with additional adherence support including emails and phone calls to individuals who missed sessions, with support from either the ENO or clinical teams as appropriate.
People in the usual care group continued their clinical management as directed by their COVID-19 clinic and any other clinical services, going on to take part in the ENO Breathe programme once they had exited the trial. Given the current absence of randomised controlled trials in people with long COVID, usual care is not standardised; rather, current guidelines suggest holistic, individualised, multidisciplinary approaches, responsive to varied symptoms experienced by this group. For example, participants reported varied components of physiotherapy, including breathing exercises, physical rehabilitative exercises, balance training, and fatigue management.
All data collection was via an online form, which required a response to each question to progress through the form, resulting in no data missing for people who completed data collection. Participants started ENO Breathe or usual care within 1 week of baseline data collection and completed the follow-up data collection within 1 week of completion of the intervention period.
Qualitative data consisted of transcriptions of three focus groups conducted after the third group session in the ENO Breathe group, and data from email correspondence and free-text responses from the end of programme evaluation form for both the ENO Breathe group and the usual care group. KEJP and HO did a thematic analysis based on the approach described by Braun and Clarke.30 Qualitative results and interpretation were reviewed by all co-authors and patient experts. Full qualitative methods are shown in the appendix (pp 5–11).
Outcomes
The primary outcome measure was change in HRQoL from baseline to the end of the 6-week course, assessed using the RAND 36-item short form survey instrument (SF-36) mental health composite (MHC) and physical health composite (PHC) scores,31, 32 comparing the ENO Breathe and usual care groups. Secondary outcome measures were the chronic obstructive pulmonary disease (COPD) assessment test score (CAT), visual analogue scales (VAS) for breathlessness (scored 0–100; participants asked to “rate the following levels of your breathlessness over the past 2 weeks: [1] at rest, [2] walking around the house, [3] climbing stairs, and [4] running”), and scores on the dyspnoea-12 (its two subscales), the generalised anxiety disorder 7-item (GAD-7) scale, the eight RAND SF-36 subscales to support interpretation of the MHC and PHC, and the short form-6D (SF-6D), which can be useful for economic evaluations. The qualitative component was prespecified, aiming to explore participant experience in the programme.
Participants were actively asked about any adverse events, experiences, and reasons for non-attendance or engagement.
Statistical analysis
Our initial recruitment target of 120 participants was based on sample size calculations using pilot data from a singing-based intervention for COPD.33 Subsequently, evaluation data from previous ENO Breathe participants became available, which was used to revise the recruitment target before any research participant follow-up data were collected. The revised sample calculation indicated that 108 participants were required to have a 90% chance of detecting as significant, at the 5% level, a difference of 5 points in the SF-36 MHC or PHC, between the control group and the experimental group. Allowing for 30% dropout, the revised recruitment target was 158 participants. This change was prospectively documented on the ClinicalTrials.gov record.
Outcomes were compared between study groups using linear regression, including the baseline level of the variable of interest as a covariate. Regression coefficients correspond to the effect size related to intervention participation. Primary prespecified analyses were in the intention-to-treat population and included a responder analysis defined as having a 10% increase in MHC or PHC score from baseline. Suggested MHC and PHC minimal clinically important difference (MCID) is 3 to 5 points.34 Baseline RAND MHC and PHC scores for ENO Breathe participants before the trial started were in the 30–50 range, corresponding with the 10% improvement responder threshold used. An additional, post-hoc responder analysis using a 5-point threshold34 was also conducted. Given the differential withdrawal rate between study groups, an additional post-hoc sensitivity analysis was conducted imputing missing data using the baseline observation carried forward method.
The control group participants went on to take part in open-label ENO Breathe immediately after the conclusion of the study. This allowed us to do a post-hoc modified per-protocol analysis. We compared only those participants in ENO Breathe who participated in all intervention sessions, with only those in the control group who had gone on to participate in all the intervention sessions when offered the programme subsequently. This allowed us to assess effectiveness of the intervention in groups likely to be better matched in terms of programme suitability, health status, and engagement.
An α of 0·05 was taken to indicate statistical significance. Statistical analysis was completed using Stata (version 14). This trial is registered with ClinicalTrials.gov, NCT04830033.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
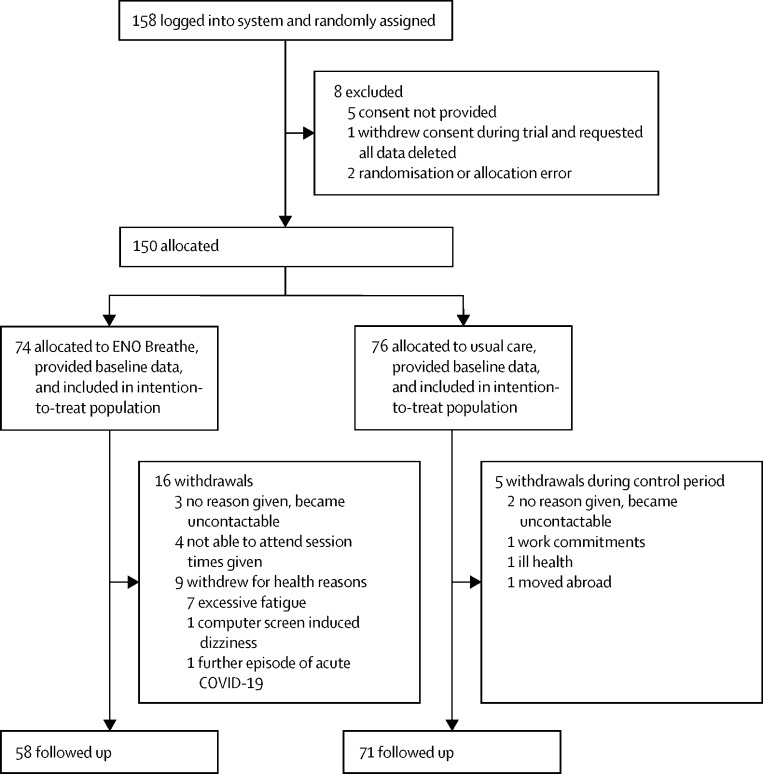
Between April 22 and May 25, 2021, 158 participants were recruited and randomly assigned (figure 1 ). Of these, eight (5%) individuals were excluded: five (3%) did not complete study participation consent; two (1%) were entered into the incorrect study group (allocation error); and one (1%) completed the baseline data collection then withdrew, requesting all data be deleted and not analysed. Therefore, 150 participants were allocated to a treatment group (74 in the ENO Breathe group and 76 in the usual care group). At baseline, mean age was 49 years (SD 12), 121 (81%) participants were female, 26 (17%) were male, and three (2%) selected other gender or preferred not to say (table 2 ). Across the two groups there was a mean of 320 days (SD 127) since the onset of first COVID-19 symptoms to randomisation. The study groups were well matched at baseline on demographic, clinical, and baseline outcome measures (table 2).
Figure 1.
Trial profile
Table 2.
Baseline characteristics
| ENO Breathe (n=74) | Usual care (n=76) | |||
|---|---|---|---|---|
| Age, years | 49 (12) | 50 (12) | ||
| Gender | ||||
| Female | 58 (78%) | 63 (83%) | ||
| Male | 14 (19%) | 12 (16%) | ||
| Other gender or prefer not to say | 2 (3%) | 1 (1%) | ||
| Ethnicity | ||||
| White | 60 (82%) | 62 (82%) | ||
| Black or Black British | 1 (1%) | 7 (9%) | ||
| Asian or British Asian | 4 (5%) | 3 (4%) | ||
| Mixed or multiple ethnic backgrounds | 3 (4%) | 2 (3%) | ||
| Other | 4 (5%) | 1 (1%) | ||
| Prefer not to say | 2 (3%) | 1 (1%) | ||
| English spoken as other language | 6 (10%) | 5 (8%) | ||
| Body-mass index,* kg/m2 | 26·85 (5·95) | 28·11 (8·10) | ||
| Number of comorbidities | 1 (0–1) | 1 (0–1) | ||
| Number of days since COVID-19 symptoms started | 330 (124) | 311 (130) | ||
| Admitted to hospital | 11 (15%) | 15 (20%) | ||
| Not admitted to hospital | 63 (85%) | 61 (80%) | ||
| Current treatments for long COVID, components of usual care | ||||
| Physiotherapy | 28 (38%) | 29 (38%) | ||
| Speech and language therapy | 1 (1%) | 1 (1%) | ||
| Occupational therapy | 1 (1%) | 2 (3%) | ||
| Clinical psychology | 2 (3%) | 2 (3%) | ||
| Respiratory nurse | 0 | 1 (1%) | ||
| Dietician | 0 | 1 (1%) | ||
| Complementary therapies† | 5 (7%) | 4 (5%) | ||
| Medications | ||||
| Oral medications‡ | 6 (8%) | 3 (4%) | ||
| Inhalers | 3 (4%) | 7 (9%) | ||
| Waiting for physiotherapy or speech and language therapy | 6 (8%) | 8 (11%) | ||
| No current active treatments directed at long COVID symptoms | 29 (39%) | 33 (43%) | ||
| Self-reported perceived barriers to participation at baseline | ||||
| None | 70 (95%) | 71 (93%) | ||
| Perceived barrier§ | 4 (5%) | 5 (7%) | ||
| Outcome measures | ||||
| RAND SF-36 MHC score | 30·89 (10·20) | 33·21 (9·83) | ||
| RAND SF-36 PHC score | 31·77 (7·44) | 32·18 (7·41) | ||
| CAT score | 21·30 (7·18) | 19·34 (6·60) | ||
| Anxiety (GAD-7 score) | 8·57 (5·54) | 7·38 (5·54) | ||
| Dyspnoea-12 score | 16·08 (7·25) | 15·83 (7·86) | ||
| VAS breathlessness rest | 23·32 (20·73) | 26·67 (22·41) | ||
| VAS breathlessness walking | 38·51 (23·88) | 41·07 (26·78) | ||
| VAS breathlessness stairs | 60·18 (24·12) | 59·09 (26·96) | ||
| VAS breathlessness running | 83·53 (23·19) | 81·71 (24·62) | ||
Data are mean (SD), n (%), or median (IQR). CAT=chronic obstructive pulmonary disease assessment test. ENO=English National Opera. GAD-7=generalised anxiety disorder-7 questionnaire. MHC=mental health composite. PHC=physical health composite. RAND SF-36=RAND 36-item short form survey instrument. VAS=visual analogue scale.
Body-mass index data were missing for one participant in each group.
Complementary therapies in the ENO Breathe group: yoga (n=1), osteopathy (n=2), acupuncture (n=1), vitamin supplements (n=1); in the usual care group: yoga (n=1), acupuncture (n=1), vitamin supplements (n=1), meditation (n=1).
Oral medications included analgesics, β-blockers, and proton-pump inhibitors.
Perceived barriers in the ENO Breathe group: has smartphone but no computer (n=2), autism (n=1), barrier not specified (n=1); in usual care group: mobility limitation (n=1), limited technical access (n=1), eye strain on extended screen use (n=1), severe dyslexia (n=1), not previously used video conferencing application (n=1).
All ENO Breathe participants who consented to study participation attended their initial one-to-one session. Mean attendance at group sessions was 4·5 (SD 2·3) of 6 sessions. 16 (22%) participants in the ENO Breathe group withdrew due to being unable to attend the session times offered (n=4); levels of fatigue being deemed excessive during one-to-one session (n=7; after discussion with the clinical team it was felt that participation risked exacerbating symptoms); new acute COVID-19 (n=1); and becoming uncontactable without providing a reason (n=3). In the ENO Breathe group, one self-limiting minor adverse event attributable to the intervention resulting in study withdrawal was reported, which was dizziness due to looking at the computer screen. There were five (7%) withdrawals from the usual care group due to work commitments (n=1); moving abroad (n=1); feeling too symptomatic to participate (n=1); and becoming uncontactable without providing a reason (n=2).
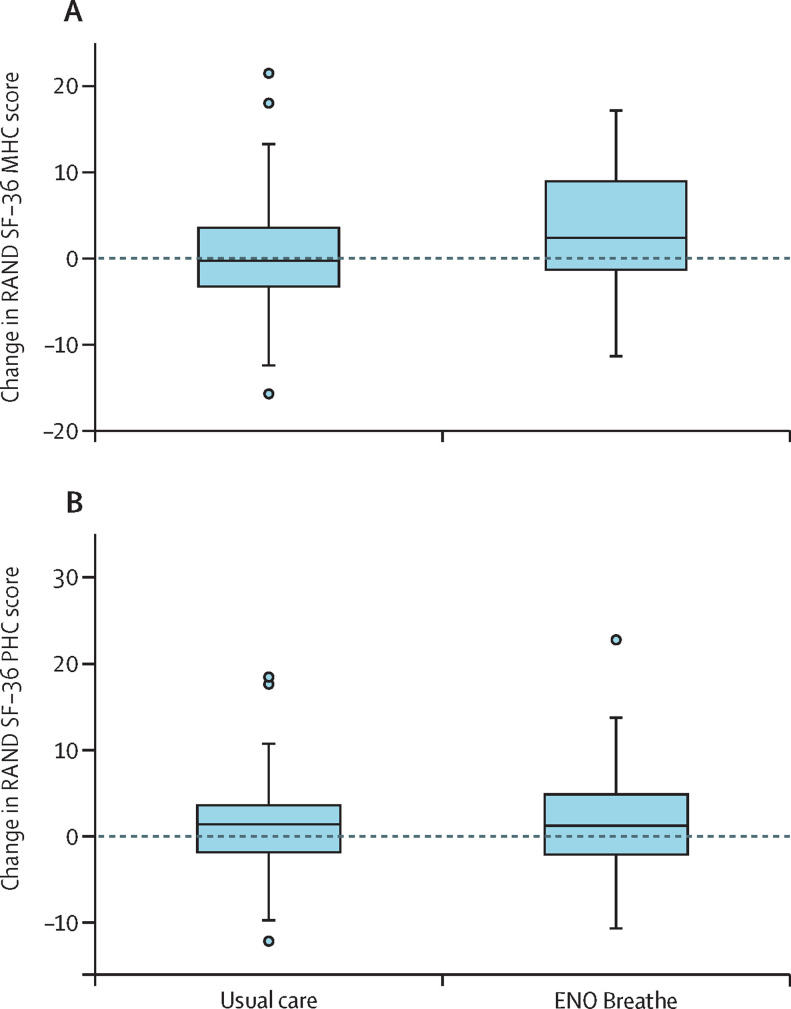
Participants in the ENO Breathe group had an improvement in the SF-36 MHC compared with the usual care group (regression coefficient 2·42 [95% CI 0·03 to 4·80]; p=0·047), but the SF-36 PHC did not differ significantly between the groups (0·60 [–1·33 to 2·52]; p=0·54; figure 2 and table 3 ). None of the individual SF-36 subscales showed statistically significant differences, although those used to calculate the MHC were all numerically more improved in the ENO Breathe group (table 3).
Figure 2.
Change in RAND SF-36 scores from baseline to week 6 follow-up
(A) Change in RAND SF-36 MHC score. (B) Change in RAND SF-36 PHC score. Boxes indicate 25th to 75th percentile, central line is the median, whiskers are upper and lower adjacent values, outliers are values beyond the upper and lower adjacent values. ENO=English National Opera. MHC=mental health composite. PHC=physical health composite. SF-36=36-item short form survey instrument.
Table 3.
Comparison of outcomes between study groups in the intention-to-treat population
|
ENO Breathe (n=58) |
Usual care (n=71) |
Regression coefficient (95% CI) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=74) | Follow-up (n=58) | Change (n=58) | Baseline (n= 76) | Follow-up (n=71) | Change (n=71) | ||||
| RAND SF-36 scores | |||||||||
| RAND SF-36 MHC score | 30·89 (10·20) | 34·40 (11·97) | 3·56 (7·22) | 33·21 (9·83) | 34·17 (10·37) | 0·78 (6·63) | 2·42 (0·03 to 4·80) | 0·047 | |
| RAND SF-36 PHC score | 31·77 (7·44) | 34·02 (9·39) | 1·73 (5·79) | 32·18 (7·41) | 33·30 (8·51) | 1·14 (5·27) | 0·60 (−1·33 to 2·52) | 0·54 | |
| SF-36 physical function | 41·55 (22·71) | 48·10 (26·17) | 4·05 (13·72) | 42·76 (23·42) | 46·41 (23·61) | 4·37 (14·56) | −0·08 (−4·99 to 4·82) | 0·97 | |
| SF-36 role limitation, physical | 8·78 (21·67) | 14·22 (29·66) | 5·60 (26·92) | 8·22 (18·88) | 14·79 (27·57) | 6·34 (26·63) | −0·67 (−9·70 to 8·36) | 0·88 | |
| SF-36 pain | 47·82 (25·12) | 55·95 (24·48) | 6·41 (21·95) | 50·58 (25·51) | 50·58 (23·51) | −0·18 (20·01) | 6·11 (−0·34 to 12·57) | 0·063 | |
| SF-36 general health | 40·57 (19·49) | 40·97 (20·59) | 0·03 (12·17) | 40·89 (17·45) | 41·01 (20·71) | 0·30 (13·95) | −0·23 (−4·79 to 4·32) | 0·92 | |
| SF-36 energy | 22·03 (17·69) | 27·59 (21·40) | 5·26 (16·26) | 20·26 (16·12) | 22·93 (16·90) | 2·08 (12·23) | 3·50 (−1·32 to 8·32) | 0·15 | |
| SF-36 role limitation, emotional | 43·24 (42·28) | 45·40 (44·02) | 1·15 (38·98) | 51·76 (45·67) | 47·89 (43·18) | −3·29 (45·83) | 1·08 (−12·03 to 14·19) | 0·87 | |
| SF-36 emotional wellbeing | 54·65 (19·92) | 58·76 (23·41) | 5·03 (14·21) | 59·05 (19·23) | 60·28 (19·52) | 0·39 (12·38) | 3·65 (−0·91 to 8·22) | 0·12 | |
| SF-36 social functioning | 33·28 (24·59) | 44·40 (26·82) | 10·56 (22·56) | 41·15 (26·41) | 44·37 (26·29) | 3·84 (19·30) | 4·75 (−2·13 to 11·63) | 0·17 | |
| SF-6D | 0·56 (0·09) | 0·59 (0·09) | 0·02 (0·06) | 0·58 (0·10) | 0·59 (0·09) | 0·01 (0·06) | 0·01 (−0·01 to 0·03) | 0·16 | |
| CAT score | 21·30 (7·18) | 17·43 (7·95) | −2·88 (5·84) | 19·34 (6·60) | 17·76 (7·15) | −1·41 (4·61) | −1·25 (−3·02 to 0·52) | 0·17 | |
| Anxiety (GAD-7 score) | 8·57 (5·54) | 7·29 (5·81) | −1·14 (2·99) | 7·38 (5·54) | 7·34 (5·37) | −0·03 (4·02) | −1·03 (−2·21 to 0·14) | 0·085 | |
| Dyspnoea-12 total score | 16·08 (7·25) | 12·05 (7·88) | −3·10 (5·29) | 15·83 (7·86) | 13·41 (7·43) | −2·46 (5·67) | −0·82 (−2·64 to 1·00) | 0·38 | |
| Dyspnoea-12 affective | 5·88 (3·78) | 3·98 (3·56) | −1·55 (3·21) | 5·42 (3·90) | 4·70 (3·72) | −0·73 (3·14) | −0·78 (−1·76 to 0·20) | 0·12 | |
| Dyspnoea-12 physical | 10·20 (4·14) | 8·07 (4·79) | −1·55 (3·20) | 10·41 (4·65) | 8·70 (4·23) | −1·73 (3·29) | −0·02 (−1·10 to 1·06) | 0·97 | |
| VAS breathlessness rest | 23·32 (20·73) | 26·55 (24·71) | 3·12 (20·24) | 26·67 (22·41) | 23·41 (23·33) | −3·00 (19·08) | 5·23 (−1·29 to 11·75) | 0·12 | |
| VAS breathlessness walking | 38·51 (23·88) | 30·21 (24·66) | −5·91 (21·06) | 41·07 (26·78) | 35·49 (25·26) | −4·08 (18·02) | −2·86 (−9·20 to 3·49) | 0·38 | |
| VAS breathlessness stairs | 60·18 (24·12) | 47·57 (28·21) | −11·07 (23·20) | 59·09 (26·96) | 52·66 (26·50) | −5·92 (21·06) | −5·13 (−12·33 to 2·06) | 0·16 | |
| VAS breathlessness running | 83·53 (23·19) | 71·47 (28·12) | −10·03 (22·59) | 81·71 (24·62) | 81·48 (22·22) | 0·70 (19·92) | −10·48 (−17·23 to −3·73) | 0·0026 | |
Data are mean (SD). CAT=chronic obstructive pulmonary disease assessment test. ENO=English National Opera. GAD-7=generalised anxiety disorder-7 questionnaire. MHC=mental health composite. PHC=physical health composite. RAND SF-36=RAND 36-item short form survey instrument. SF-6D=short form-6D. VAS=visual analogue scale.
There was a significant difference in the improvement in VAS breathlessness (running) score between the groups, favouring the ENO Breathe group (table 3). Although most other secondary outcome measures were numerically better in the ENO Breathe group, differences were not statistically significant (table 3).
The responder analysis based on a 10% change showed no significant difference between the ENO Breathe group and the usual care group for SF-36 MHC or PHC score (table 4 ). A post-hoc responder analysis of those with a 5-point improvement in SF-36 MHC or PHC score showed statistically significant benefits for SF-36 MHC score in the ENO Breathe group compared with the usual care group, but not for SF-36 PHC score (table 4).
Table 4.
Numbers of participants with an improvement in the RAND SF-36 MHC score
| ENO Breathe (n=58) | Usual care (n=71) | Total (n=129) | p value | |
|---|---|---|---|---|
| Improvement of 10% or more from baseline | ||||
| RAND SF-36 MHC score | 26 (45%) | 21 (30%) | 47 (36%) | 0·073 |
| RAND SF-36 PHC score | 20 (35%) | 19 (27%) | 39 (30%) | 0·34 |
| 5-point improvement from baseline | ||||
| RAND SF-36 MHC score | 23 (40%) | 12 (17%) | 35 (27%) | 0·0038 |
| RAND SF-36 PHC score | 13 (22%) | 12 (17%) | 25 (19%) | 0·43 |
ENO=English National Opera. MHC=mental health composite. PHC=physical health composite. RAND SF-36=RAND 36-item short form survey instrument.
Baseline characteristics of those included in the post-hoc, modified per-protocol analysis, which was limited to individuals in both groups who showed compliance with the ENO Breathe intervention, either during the trial itself or after trial completion in the case of those in the usual care group, were well matched at baseline (appendix pp 2–3). Statistically significant improvements favouring ENO Breathe were observed for the SF-36 MHC score, CAT score, GAD-7, dyspnoea-12 (affective component), and VAS breathlessness (running; table 5 ); and for the 10% responder analysis for the SF-36 MHC score (20 [50%] of 40 participants in the ENO Breathe group vs ten [24%] of 42 in the usual care group; p=0·014), but not for the SF-36 PHC score (14 [35%] in the ENO Breathe group vs 12 [29%] in the usual care group; p=0·53)
Table 5.
Comparison of outcomes between study groups in the modified per-protocol population
|
ENO Breathe (n=40) |
Usual care (n=42) |
Regression coefficient (95% CI) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | ||||
| RAND SF-36 scores | |||||||||
| RAND SF-36 MHC score | 30·88 (10·41) | 35·11 (12·00) | 4·22 (6·83) | 36·23 (7·78) | 36·63 (8·82) | 0·40 (5·17) | 3·50 (0·72 to 6·27) | 0·014 | |
| RAND SF-36 PHC score | 32·97 (7·41) | 35·02 (9·30) | 2·05 (6·23) | 33·83 (6·74) | 35·40 (8·23) | 1·56 (5·06) | 0·45 (−2·06 to 2·95) | 0·72 | |
| SF-36 physical function | 46·25 (22·32) | 51·25 (25·13) | 5·00 (12·96) | 45·24 (20·78) | 50·24 (22·44) | 5·00 (12·00) | 0·06 (−5·44 to 5·55) | 0·98 | |
| SF-36 role limitation, physical | 8·75 (19·24) | 15·63 (30·32) | 6·88 (29·41) | 9·52 (16·52) | 17·26 (28·42) | 7·74 (27·32) | −1·18 (−13·30 to 10·95) | 0·85 | |
| SF-36 pain | 53·10 (24·52) | 58·70 (24·26) | 5·60 (24·93) | 56·45 (24·70) | 56·64 (23·56) | 0·19 (21·24) | 3·84 (−5·04 to 12·72) | 0·39 | |
| SF36 general health | 40·88 (18·92) | 42·45 (20·20) | 1·57 (12·19) | 44·62 (16·88) | 45·98 (19·35) | 1·36 (13·06) | −0·30 (−5·82 to 5·21) | 0·91 | |
| SF-36 energy | 22·38 (16·76) | 29·38 (21·10) | 7·00 (17·35) | 22·38 (14·99) | 24·00 (16·47) | 1·62 (11·28) | 5·38 (−0·88 to 11·64) | 0·091 | |
| SF-36 role limitation, emotional | 41·67 (40·47) | 50·00 (44·66) | 8·33 (38·30) | 61·11 (44·73) | 58·73 (40·86) | −2·38 (46·21) | 1·09 (−15·67 to 17·85) | 0·90 | |
| SF-36 emotional wellbeing | 54·50 (20·91) | 59·70 (23·14) | 5·20 (13·01) | 65·52 (14·99) | 65·71 (16·72) | 0·19 (10·22) | 4·00 (−1·35 to 9·35) | 0·14 | |
| SF-36 social functioning | 33·75 (25·03) | 44·06 (28·59) | 10·31 (21·54) | 45·83 (26·29) | 47·32 (26·41) | 1·49 (19·17) | 5·91 (−2·91 to 14·73) | 0·19 | |
| SF-6D | 0·57 (0·09) | 0·60 (0·08) | 0·03 (0·05) | 0·57 (0·09) | 0·60 (0·08) | 0·01 (0·06) | 0·01 (−0·01 to 0·03) | 0·33 | |
| CAT score | 20·13 (7·02) | 15·85 (6·85) | −4·28 (5·33) | 17·52 (5·69) | 16·60 (5·96) | −0·93 (4·28) | −2·61 (−4·64 to −0·59) | 0·012 | |
| Anxiety (GAD-7 score) | 8·43 (5·40) | 6·95 (5·60) | −1·48 (2·62) | 5·62 (4·17) | 6·24 (4·64) | 0·62 (3·63) | −1·68 (−3·10 to −0·25) | 0·022 | |
| Dyspnoea-12 total score | 14·40 to (6·90) | 10·40 (7·19) | −4·00 (4·51) | 14·48 (7·26) | 12·43 (6·72) | −2·05 (5·53) | −1·97 (−4·04 to 0·09) | 0·061 | |
| Dyspnoea-12 affective | 5·33 (3·68) | 3·45 (3·34) | −1·88 (2·87) | 4·57 (3·23) | 4·10 (3·24) | −0·48 (2·76) | −1·11 (−2·22 to −0·01) | 0·049 | |
| Dyspnoea-12 physical | 9·07 (3·78) | 6·95 (4·28) | −2·13 (2·52) | 9·90 (4·47) | 8·33 (3·97) | −1·57 (3·39) | −0·78 (−2·01 to 0·46) | 0·22 | |
| VAS breathlessness rest | 22·63 (20·78) | 20·10 (20·83) | −2·52 (16·75) | 23·00 (20·77) | 19·10 (18·57) | −3·90 (12·95) | 1·26 (−4·70 to 7·23) | 0·67 | |
| VAS breathlessness walking | 33·08 (22·25) | 25·77 (22·04) | −7·30 (19·64) | 37·79 (23·24) | 31·60 (21·05) | −6·19 (14·71) | −2·72 (−9·59 to 4·16) | 0·43 | |
| VAS breathlessness stairs | 52·67 (24·59) | 40·50 (24·42) | −12·18 (22·98) | 56·02 (25·42) | 51·02 (24·94) | −5·00 (19·77) | −8·44 (−16·95 to 0·07) | 0·052 | |
| VAS breathlessness running | 76·60 (27·79) | 65·92 (29·80) | −10·68 (24·64) | 82·48 (18·16) | 80·62 (20·73) | −1·86 (13·95) | −10·37 (−18·81 to −1·93) | 0·017 | |
Data are mean (SD). CAT=chronic obstructive pulmonary disease assessment test. ENO=English National Opera. GAD-7=generalised anxiety disorder-7 questionnaire. MHC=mental health composite. PHC=physical health composite. RAND SF-36=RAND 36-item short form survey instrument. SF-6D=short form-6D. VAS=visual analogue scale.
At baseline, participants included in the modified per-protocol analysis, compared with those excluded from the modified per-protocol analysis, had scores indicating better health or less severe symptoms on most measures, including SF-36 MHC and PHC scores, CAT score, GAD-7, dyspnoea-12, and VAS breathlessness walking and stairs, as well as lower body-mass indices (appendix pp 3–4).
Given the differential withdrawal rate between study groups, we conducted additional post-hoc sensitivity analyses imputing missing data using the baseline observation carried forward method. The results indicate slight attenuation of the positive outcome measures, with the p value for the SF-36 MHC score going from 0·047 in the main analysis to 0·095 in the post-hoc analysis, and the VAS breathlessness (running) p value going from 0·0026 in the main analysis to 0·0089 in the post-hoc analysis (appendix pp 4–5).
Patient experience was assessed in 52 participants in the ENO Breathe group who attended the focus groups, and 129 participants across both groups using data collected from email correspondence and the end of programme evaluation form (appendix pp 5–11). Overall, participants reported highly positive experiences. Three key themes were identified through qualitative analysis regarding participant experience of ENO Breathe: improvement in symptoms; feeling that the programme was complementary to standard care; and the particular suitability of singing and music to address their needs. A 44-year-old woman from the ENO Breathe group said “ENO Breathe is … so powerful because it responds to our illness humanely, openly, and richly, through emotions, embodiment, culture, art, ideas ... whereas medical spaces (if we even manage to access them, which is hard enough) can be so alienating and emotionally and spiritually empty—so averse to treating the whole experience, the whole person. I felt that ENO Breathe has been healing for the trauma I have experienced and continue to experience: of having an unknown illness, not knowing if I will ever [be] getting better, and of receiving barely any medical care, for over a year.”
Categorised under the theme of improvements in symptoms, the improvements experienced were most commonly related to breathlessness on exertion and anxiety, although sleep, concentration, and voice abnormalities were noted to improve by some. Improvements were attributed to (1) learning practical and effective techniques for acute symptom management that could be applied in daily life (subtheme 1a); (2) providing calming and enjoyable experiences during the sessions; and (3) changing the way participants experienced their condition. A 32-year-old woman from the ENO Breathe group stated “It has given me the confidence outside of these sessions to remember that I can breathe and rely on the techniques that we're taught.”
The second theme, that the programme was considered complementary to standard health care, related primarily to addressing gaps in the type of care, or the way it is delivered. These strengths were perceived as resulting from the programme being specifically designed for people with long COVID, providing continuity over time, and facilitating interpersonal connections. A 44-year-old woman from the ENO Breathe group stated “there has been so little treatment for so many of us, and I really like that it's a programme designed for us … I'm so glad that someone cares that I have long COVID.”
Under the third theme, participants considered the use of music and song to be a particularly suitable approach to support recovery, even for those who were hesitant or dubious about singing or music-based approaches initially. Underpinning this were interrelated experiences of enjoyment, emotional engagement, and the ability to engage with the breath without consciously focusing on breathlessness. A 60-year-old man from the ENO Breathe group stated “the singing helps—it's like you're breathing without thinking.'
Discussion
We assessed the effect of ENO Breathe on HRQoL for people with long COVID who had breathlessness, with or without anxiety. The intervention appears safe and was effective at improving the mental component of HRQoL and elements of breathlessness. Participants reported symptomatic improvements, felt the programme was complementary to usual care, and that the use of singing and music was particularly suitable for their needs even if initially unsure about this type of activity.
There is a broad consensus that rehabilitative approaches are needed to address the varied effects of long COVID11 and this study is one of the first randomised controlled trials to evaluate such an intervention, addressing an important evidence gap for this patient group.3 There is considerable interest in developing singing based approaches to address long COVID—68 attendees from 12 countries (including Latin America, Canada, the USA, and Europe) attended an international webinar in June, 2021, hosted by the ENO Breathe team to share information about the programme. All attendees expressed the intention of setting up a similar service in their country in the near future or were already in the active process of doing so.
The ENO Breathe programme was accessed via review and suitability assessment by an NHS long COVID clinic, demonstrating a potential point of integration for these types of approaches into clinical services, and our qualitative findings suggest participants felt the integration in this format was appropriate. It is important to ensure that patients, clinicians, and those delivering this type of intervention can be confident that cardiorespiratory or other problems that require specific therapy have been identified.
Although multiple participants who were initially unsure about this type of approach felt they benefited, singing based approaches might not be suitable for or preferred by all, so alternative types of wellbeing programmes should be developed and evaluated. This could build on existing approaches, based on tai chi35 and yoga,36 that have been developed for other conditions. Individualised participant selection would be required, particularly given the potential for other approaches to be physiologically demanding,37 which might lead to worsening of symptoms in some individuals. Comparisons of approaches would also help identify which individual and combinations of intervention components are most beneficial. Future research should consider that, although progress is slow, for many people with long COVID, there is a general trajectory towards improvement.38 This trajectory might influence the way in which intervention impacts are interpreted, and emphasises the importance of having comparator groups whenever possible. Interestingly, the CAT score, GAD-7, dyspnoea-12, and VAS breathlessness scores for walking, stairs, and running all improved more in the ENO Breathe group than in the usual care group, with many close to the predefined statistical significance threshold of p<0·05. This consistent direction of effect complements the main outcome. The study might have been underpowered to detect differences in these secondary outcomes, or it might be that an increased amount of time spent participating in the intervention is required. Of note, in the present study, the mean time from the onset of symptoms to randomisation was 320 days, and earlier intervention might support earlier recovery.
The post-hoc modified per-protocol analysis extends our findings. It showed a greater effect on a range of outcomes compared with the main analysis, comparing individuals in each treatment group who actually completed, or went on to complete, the ENO Breathe programme. This analysis provides useful insights to guide future research identifying specific responder phenotypes, as has been done in rehabilitation research more broadly. Interestingly, the affective, but not the physical component, of the dyspnoea-12 improved, suggesting moderation of how breathlessness was experienced and the emotional impact it had. This impact was possibly through increased confidence and changes in mood, rather than if breathlessness occurred or not, which aligns with qualitative analysis of participant experience regarding changes to how participants experienced their condition. Future research is required to clarify mechanisms of impact.
No serious adverse events were reported. One participant withdrew from the ENO Breathe group due to dizziness that they attributed to looking at the computer screen for too long during the sessions. The qualitative data identified other participants who had fatigue or mild dizziness related to ENO Breathe participation, but chose to continue, as perceived benefits outweighed small negatives. Post-exertional symptom exacerbation is an important consideration, particularly regarding fatigue.39 Participants with excessive fatigue were withdrawn at the end of their one-to-one session, before the more participatory components of the intervention took place, as such the shared decision to withdraw was proactive to avoid causing potential harm. Reassuringly, the SF-36 energy (vitality) subscale did not show worsening of this symptom in either the intention-to-treat or modified per-protocol analysis, with both numerically favouring the treatment group. The difference in withdrawal rate between groups is notable, although perhaps not unexpected given the absence of an active intervention to withdraw from in the usual care group, who were randomly assigned to delayed treatment. Differential withdrawal rates might have contributed slightly to the findings as suggested by the post-hoc sensitivity analyses using the baseline observation carried forward method described. However, this approach might, in this situation, be a cautious one given the trend towards improvement seen in outcome measures of the control group. Overall, the differential withdrawal rate is unlikely to explain the results when considered as a whole, particularly given the findings of the qualitative component and modified per-protocol analysis, which suggest that between-group differences are related to intervention participation.
Our sample size calculation was based on a 5-point change in SF-36 MHC or PHC scores. However, it is not currently clear what constitutes an MCID in people with long COVID. Ranges between 3 and 5 points are typically used in other medical conditions.34 Of note, a systematic review assessing the responsiveness of the SF-36 in randomised controlled trials involving treatments that are otherwise established to be effective in COPD, found that none of the identified studies achieved PHC or MHC score improvements exceeding that MCID.34 It might be the case that a lower threshold for the MCID in long COVID is appropriate too. The particulars of this and other conditions might not be captured by this more generic health status measure. Absolute MCID thresholds also need to be considered in the context of the type of intervention involved. Qualitative data suggest many participants had improvements that were meaningful to them, and even a small improvement in measured HRQoL might be considered to represent a good value intervention if the health system or participant cost is low enough.31 MCIDs for VAS breathlessness scales are generally in the range of 10 to 20,40 indicating our study identified a between-group difference of important magnitude for the VAS breathlessness (running) scale, but not on other dyspnoea measures. VAS breathlessness measures were based on participants' perception over the preceding 2 weeks. Some participants might have estimated what their experience would have been like rather than drawing on a specific recollection. The VAS results are in keeping with the qualitative findings regarding participant experience, which suggested improvements in breathlessness on exertion, rather than at rest or minimal exertion, and might relate to breathing pattern disorder, which appears prevalent post-COVID-19.41 ENO Breathe includes a focus on breathing retraining, which might have been particularly helpful in individuals with breathing pattern disorder, and future research should assess whether formal assessment of the presence of this disorder should be used to guide referral into this kind of programme. The improvements observed could be influenced by the measures selected, the amount of time spent participating in the intervention, or heterogeneity of the participant group. Regarding the last point, the modified per-protocol analysis suggests that in people who fully participate in ENO Breathe, the effect of the intervention was substantially greater than usual care.
Some considerations and limitations are important to discuss. First, given the nature of the intervention, double blinding was not possible. Second, additional measures to further characterise participants might have been useful, such as objective physical performance measures, actigraphy, breathing pattern assessment, and baseline lung function testing. However, the assessments included were selected with expert patient input to carefully balance the number and type of assessments of most use and importance to participants. Baseline lung function and physical performance testing, as well as excessive questionnaires, would have been likely to inhibit participation due to the potential to induce post-exertional symptom exacerbation, which is common in patients with long COVID. Additionally, it is currently unclear how best to phenotypically characterise people with long COVID, both in terms of response to interventions or more generally. Given the limited scope of published trials in patients with long COVID, we opted for qualitative components instead, which, although infrequently included in randomised controlled trials, have provided valuable insights into potential therapeutic mechanisms that would probably not have been identified using the additional clinical assessments considered. Detailing the components of usual care for these participants also facilitates clinical characterisation of the sample. As such, the research methods used were appropriate given these considerations and fit with our intention to integrate the trial into existing clinical pathways without overburdening participants with assessments. Third, it is not clear how our findings transfer to people with long COVID who have not been seen in specialist clinics and then safely referred. That said, we deliberately targeted people who had been reviewed in specialist clinics and then referred. We see this as the most appropriate point to provide such interventions, at least initially, to ensure they are delivered as a component of, not instead of, multidisciplinary investigation and management. Fourth, the study participants were predominantly female, with a mean age of 49 years, and of White ethnicity. In terms of age and gender, this is broadly representative of the demographic composition of patients seen in the 51 UK-based long COVID clinics collaborating with the programme (service evaluation, unpublished), and in keeping with research regarding long COVID more generally.42 However, a higher proportion of minority ethnic participants might have been expected, which might suggest barriers to participation in research, or the intervention. Fifth, given the nature of the intervention, establishing intervention fidelity in practice could present challenges, as with other established non-pharmacological interventions. Clear guidance, training, and auditing or monitoring are likely to be important. Last, although there was limited discussion of barriers to participation in the patient experience component, barriers might exist that were not identified because individuals experiencing the most substantial barriers would have been less likely to participate in the trial or, if they did, they would have been more likely to withdraw before qualitative data collection.
The ENO Breathe online wellbeing programme appears safe and effective at improving the mental component of HRQoL, and elements of breathlessness, for people with long COVID with breathlessness, with or without anxiety. Participants reported symptomatic improvements, felt the programme was complementary to standard care, and felt that the use of singing and music were particularly suitable for their needs. This study suggests mind–body interventions targeting HRQoL could have a potential role as complementary additional elements of long COVID management, particularly in patients who participate most in the intervention. Research into other related approaches would be valuable, as would better characterisation of long COVID subgroups to identify those most likely to benefit.
Data sharing
Fully anonymised data will be provided upon reasonable request to the corresponding author.
Declaration of interests
SM, TP, KB, HB, JM, SZ, THH, and ALa work for ENO who developed and deliver ENO Breathe; however, the programme is delivered free of charge to participants. All other authors declare no competing interests. No authors have been paid to write this article by a pharmaceutical company or other agency.
Acknowledgments
Acknowledgments
KEJP was supported by the Imperial College Clinician Investigator Scholarship. KEJP would like to acknowledge the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London for their support. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The ENO Breathe programme is generously supported by ENO's patrons and donors. We the authors thank the participants for their time and effort, with particular thanks to Sharon Sullivan, Umut Esmer, and Jen Shepherd for contributing to the steering group meetings and providing participant feedback and perspectives on the qualitative aspects of the research. We also thank Laura Moth, Phillip Crisp, Georgina Russell, Boon Lim, Michelle Maguire, Anna Wallin, Helena Klinge, Abi Simpson, Stuart Murphy, Beth Warnock, Emily Smith, Anya Chomacki, Poppy Harrison, Amy Powell, Lea Cornthwaite, and Edmund Jeffery for their expertise, advice, and support in the creation, development, and delivery of the programme. Thanks also to all original SHIELD Trial team members for contribution to the study design.
Contributors
NSH, KEJP, ALe, HO, and SE designed the study. KEJP and NSH wrote the protocol and obtained ethics approval and authorisation for the study. HO and SE provided clinical input and support for the study. The ENO Breathe programme was devised by the English National Opera in collaboration with Imperial College Healthcare Trust. Scope and content were designed by SZ, JM, TP, SE, HO, VP, and ALo. TP, SM, and KB coordinated delivery of ENO Breathe and data collection. KEJP randomly assigned participants. KEJP analysed the data and wrote the first draft of the manuscript. WB provided statistical advice and support for quantitative components. KEJP, NSH, and WB have directly accessed and verified the underlying data reported in the manuscript. All authors contributed to the study design, study conduct, interpretation, revising the manuscript, and agreeing on the final version. No authors were precluded from accessing data in the study, and all authors accept final responsibility to submit for publication.
Supplementary Material
References
- 1.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: a meta-analysis and systematic review. medRxiv. 2021 doi: 10.1101/2021.11.15.21266377. published online Nov 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after COVID-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20 doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buttery S, Philip KEJ, Williams P, et al. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2021-001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward H, Flower B, Garcia PJ, et al. Global surveillance, research, and collaboration needed to improve understanding and management of long COVID. Lancet. 2021;398:2057–2059. doi: 10.1016/S0140-6736(21)02444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs A, Vassall A. Count the cost of disability caused by COVID-19. Nature. 2021;593:502–505. doi: 10.1038/d41586-021-01392-2. [DOI] [PubMed] [Google Scholar]
- 6.NICE NICE guideline (NG188) COVID-19 rapid guideline: managing the long-term effects of COVID-19. Version 1.7 (updated 23 Nov 2021) https://www.nice.org.uk/guidance/NG188
- 7.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID—mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 9.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health Research Living with Covid19—second review. https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review/
- 12.Ayoubkhani D, King S, Bosworth M, Pawelek P. Office for National Statistics; 2022. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 6 January 2022.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6january2022 [Google Scholar]
- 13.Norton A, Olliaro P, Sigfrid L, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis. 2021;21:601–602. doi: 10.1016/S1473-3099(21)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meagher T. Long COVID—an early perspective. J Insur Med. 2021;49:19–23. doi: 10.17849/insm-49-1-1-5.1. [DOI] [PubMed] [Google Scholar]
- 15.Adeloye D, Elneima O, Daines L, et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467–1478. doi: 10.1016/S2213-2600(21)00286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 17.Fancourt D, Finn S. World Health Organisation; 2019. What is the evidence on the role of the arts in improving health and well-being? A scoping review.https://www.euro.who.int/en/data-and-evidence/evidence-informed-policy-making/publications/2019/what-is-the-evidence-on-the-role-of-the-arts-in-improving-health-and-well-being-a-scoping-review-2019 [PubMed] [Google Scholar]
- 18.McCrary JM, Altenmüller E, Kretschmer C, Scholz DS. The impact of music on health-related quality of life, as quantified by the SF-36: a systematic review and meta-analysis. medrxiv. 2021 doi: 10.1101/2021.11.30.21267066. published online Nov 30. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philip K, Lewis A, Hopkinson NS. Music and dance in chronic lung disease. Breathe (Sheff) 2019;15:116–120. doi: 10.1183/20734735.0007-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis A, Cave P, Stern M, et al. Singing for lung health—a systematic review of the literature and consensus statement. NPJ Prim Care Respir Med. 2016;26 doi: 10.1038/npjpcrm.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis A, Cave P, Hopkinson NS. Singing for lung health: a qualitative assessment of a British Lung Foundation programme for group leaders. BMJ Open Respir Res. 2017;4 doi: 10.1136/bmjresp-2017-000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis A, Cave P, Hopkinson NS. Singing for lung health: service evaluation of the British Lung Foundation programme. Perspect Public Health. 2018;138:215–222. doi: 10.1177/1757913918774079. [DOI] [PubMed] [Google Scholar]
- 23.Cave P, Lewis A, Fancourt D. In: The Routledge companion to interdiciplinary studies in singing, volume III: wellbeing. Heydon R, Fancourt D, Cohen AJ, editors. Routledge; New York, NY: 2020. Singing for lung health; pp. 86–97. [Google Scholar]
- 24.Philip KEJ, Katagira W, Jones R. Dance for respiratory patients in low-resource settings. JAMA. 2020;324:921–922. doi: 10.1001/jama.2020.15426. [DOI] [PubMed] [Google Scholar]
- 25.Philip KE, Lewis A, Jeffery E, et al. Moving singing for lung health online in response to COVID-19: experience from a randomised controlled trial. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philip K, Lewis A, Harrison S. Singing and dance for people with chronic breathlessness during the COVID-19 pandemic. 2020. https://www.thoracic.org/members/assemblies/assemblies/pr/quarterly-bite/singing-and-dance-for-people-with-chronic-breathlessness-during-the-covid-19-pandemic.php
- 27.Philip KEJ, Lewis A, Buttery SC, et al. Aerosol transmission of SARS-CoV-2: inhalation as well as exhalation matters for COVID-19. Am J Respir Crit Care Med. 2021;203:1041–1042. doi: 10.1164/rccm.202012-4445LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J. 2017;49 doi: 10.1183/13993003.02277-2016. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 31.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 32.Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Joint Surg Am. 2015;97:1628–1634. doi: 10.2106/JBJS.O.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord VM, Hume VJ, Kelly JL, et al. Singing classes for chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulm Med. 2012;12:69. doi: 10.1186/1471-2466-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frendl DM, Ware JE., Jr Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. 2014;52:439–445. doi: 10.1097/MLR.000000000000010311. [DOI] [PubMed] [Google Scholar]
- 35.Lewis A, Hopkinson NS. Tai chi movements for wellbeing—evaluation of a British Lung Foundation pilot. Perspect Public Health. 2020;140:172–180. doi: 10.1177/1757913919872515. [DOI] [PubMed] [Google Scholar]
- 36.Nolan CM, Rochester CL. Exercise training modalities for people with chronic obstructive pulmonary disease. COPD. 2019;16:378–389. doi: 10.1080/15412555.2019.1637834. [DOI] [PubMed] [Google Scholar]
- 37.Philip KE, Lewis A, Buttery SC, et al. Physiological demands of singing for lung health compared with treadmill walking. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2021-000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Physiotherapy . World Physiotherapy; London: 2021. World Physiotherapy response to COVID-19 briefing paper 9: safe rehabilitation approaches for people living with long COVID: physical activity and exercise. [Google Scholar]
- 40.Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, borg scale, and visual analog scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 41.Stockley JA, Alhuthail EA, Coney AM, et al. Lung function and breathing patterns in hospitalised COVID-19 survivors: a review of post-COVID-19 clinics. Respir Res. 2021;22:255. doi: 10.1186/s12931-021-01834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fully anonymised data will be provided upon reasonable request to the corresponding author.