Abstract
Pseudomonas mendocina is a Gram-negative bacillus from the family Pseudomonadaceae. The first P. mendocina-related infection was reported in 1992. Although a rare cause of infections, P. mendocina has been known to cause severe infections that require intensive treatment. We present the first documented case of urinary tract infection caused by P. mendocina.
An 83-year-old male with a past medical history of diabetes, hypertension, coronary artery disease, and prostate cancer with bone metastases, currently being treated with abiraterone and prednisone, presented with subjective fever, fatigue, altered mental status, dysuria, and hematuria of one-week duration. He was found to have a complicated urinary tract infection with an incidental asymptomatic COVID-19 infection on admission. The patient was empirically treated with ceftriaxone and switched to cefepime for broader coverage on day two of hospitalization. Urine culture reported the presence of P. mendocina with resistance only to fluoroquinolones. Ceftriaxone was reinstated. The patient was successfully treated with a seven-day course of ceftriaxone (days 1-3, days 6-7) and cefepime (days 4-5) but continued to remain inpatient for a later symptomatic COVID-19 pneumonia with discharge on day 15.
The majority of P. mendocina infections present as skin and soft tissue infections, infective endocarditis, meningitis, and bacteremia. Ours is the first documented case of urinary tract infection caused by P. mendocina, particularly in an immunocompromised COVID-19 patient, and the second to report P. mendocina with resistance to fluoroquinolones. This report contributes to the growing literature regarding P. mendocina-related infections.
Keywords: urinary tract infection, pseudomonas infections, pseudomonas mendocina, mendocina, pseudomonas
Introduction
Pseudomonas mendocina is a motile, Gram-negative, aerobic bacillus, belonging to the family Pseudomonadaceae [1]. P. mendocina is found ubiquitously in soil and water and can grow in various temperatures ranging from 25 ºC to 42 ºC [1,2]. P. mendocina is a rare cause of human infections. The first case of P. mendocina-related infection was reported in Mendoza, Argentina in 1992 [2]. Since then, P. mendocina-related infections have been seldomly documented in Asia [3-7], Europe [8-10], Middle East [11,12] North America [13-16], and South America [17]. Despite its low incidence of pathogenicity, P. mendocina has been known to cause severe infections, requiring hospitalization and intensive treatment.
A systematic review of current literature reveals endocarditis, meningitis, and bacteremia to be the most common presentations of P. mendocina infections [18]. In the United States, there have been four documented cases of P. mendocina-related infections, which include three reports of bacteremia and one report of infective endocarditis [13-16]. In this case report, we present the first documented case of urinary tract infection caused by P. mendocina.
Case presentation
An 83-year-old male presented to the emergency department with subjective fever, fatigue, altered mental status, dysuria, and hematuria of one-week duration. His past medical history included diabetes, hypertension, coronary artery disease, and prostate cancer with bone metastases. He had been receiving prostate cancer treatment for at least 10 years, consisting of prednisone 5 mg daily and abiraterone 1000 mg daily with an injection every three months.
On arrival, he had a temperature of 38.7 °C (101.7 °F), a heart rate of 79/minute, a blood pressure of 119/72 mmHg, and a respiratory rate of 18/minute with an oxygen saturation of 99% on room air. On the physical exam, he appeared cachectic with bilateral pale conjunctivae. Oropharyngeal mucous membranes were dry. Suprapubic or costovertebral tenderness was not elicited on the abdominal exam. A genitourinary exam revealed no masses, blood, or discharge at the penile meatus. There was no edema, erythema, lesions, or warmth noted in the penis or scrotum.
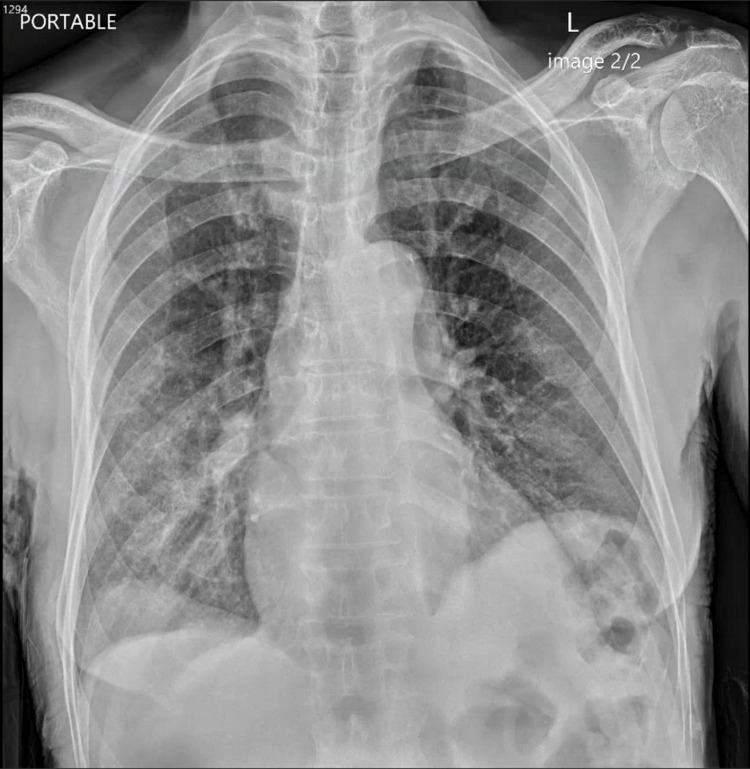
Laboratory evaluation revealed pancytopenia with a WBC count of 2530/μL, absolute neutrophil count of 1200/μL, hemoglobin of 11.1 g/dL, and a platelet count of 106/μL (Table 1). A urinalysis demonstrated RBC 16/hpf, WBC 13/hpf, protein 100 mg/dL, urobilinogen 4 mg/dL, with small amounts of blood, negative nitrite, trace leukocyte esterase, and few bacteria (Table 2). A standard pre-admission COVID-19 screening test was positive. Chest X-rays showed mild right basilar hazy reticulation and minimal left basilar opacification (Figure 1). The patient was admitted with a complicated urinary tract infection and a concurrent asymptomatic incidental COVID-19 infection. Therapy was initiated with ceftriaxone.
Table 1. Laboratory findings of our patient.
WBC: white blood cell, RBC: red blood cell, MCV: mean corpuscular volume, MCH: mean cell hemoglobin, MCHC: mean cell hemoglobin concentration, RDW: red cell distribution width
| Laboratory test | On admission | Day 2 of admission | Day 7 of admission | Reference ranges |
| WBC (×103 μL) | 2.53 | 5.06 | 4.68 | 3.50–10.80 |
| RBC (×106 μL) | 3.59 | 3.54 | 3.43 | 4.70–6.10 |
| Hemoglobin (g/dL) | 11.1 | 10.7 | 10.8 | 14.0–18.0 |
| Hematocrit (%) | 32.2 | 31.7 | 30.8 | 42.0–52.0 |
| MCV (fL) | 89.9 | 89.5 | 89.7 | 80.0–95.0 |
| MCH (pg) | 31.0 | 30.3 | 31.6 | 27.0–31.0 |
| MCHC (%) | 34.5 | 33.9 | 35.2 | 33.0–37.0 |
| RDW | 14.8 | 14.8 | 14.6 | 11.5–14.5 |
| Platelets (×103 μL) | 106 | 126 | 198 | 130–400 |
| Neutrophil (%) | 47.3 | 84.9 | 80.5 | 40.0–74.0 |
| Lymphocyte (%) | 42.2 | 9.9 | 14.6 | 19.0–48.0 |
| Monocyte (%) | 6.4 | 4.6 | 3.3 | 0.0–9.0 |
| Eosinophil (%) | 1.3 | 0.0 | 0.4 | 0.0–7.0 |
| Basophil (%) | 0.6 | 0.1 | 0.1 | 0.0–1.5 |
| Neutrophil (×103 μL) | 1.2 | 4.3 | 3.8 | 1.7–7.0 |
| Lymphocyte (×103 μL) | 1.1 | 0.5 | 0.7 | 0.9–2.9 |
| Monocyte (×103 μL) | 0.2 | 0.2 | 0.2 | 0.0–1.0 |
| Eosinophil (×103 μL) | 0.03 | 0.0 | 0.0 | 0.0–0.80 |
| Basophil (×103 μL) | 0.0 | 0.0 | 0.0 | 0.0–0.2 |
Table 2. Urinalysis of our patient.
| Component | On admission | Day 7 of admission | Reference ranges |
| Dipstick analysis | |||
| Appearance | Cloudy | Cloudy | Clear |
| Color | Amber | Yellow | Yellow |
| Glucose level | Negative | Negative | Negative |
| Bilirubin (mg/dL) | Negative | Negative | Negative |
| Ketones | 5 | 5 | Negative |
| Specific gravity | 1.017 | 1.017 | 1.005–1.030 |
| Blood | Small | Moderate | Negative |
| pH | 6.0 | 5.0 | 5.0–7.0 |
| Protein UA (mg/dL) | 100 | 100 | Negative |
| Urobilinogen (mg/dL) | 4.0 | <2.0 | <2.0 |
| Nitrite | Negative | Negative | Negative |
| Leukocyte esterase | Trace | Negative | Negative |
| Urine microscopy | |||
| RBC (per high-power field) | 16 | 2 | 0–4 |
| WBC (per high-power field) | 13 | 4 | 0–5 |
| Bacteria (per high-power field) | Few | None | None |
| Squamous epithelial cells (per high-power field) | Rare | Rare | None |
Figure 1. Chest X-ray of our patient on admission.
The patient remained febrile during the first three days of admission with a maximum temperature of 38.5 °C (101.4 °F). Absolute neutrophil count and WBC increased to 4300/μL and 5060/μL, respectively, on day 2 of admission (Table 1). Preliminary urine culture reported >100,000 CFU/mL of Gram-negative rods. Multiple blood cultures were negative for the presence of bacteria. Due to the persistence of fever, the antibiotic coverage was broadened to cefepime while awaiting final speciation.
A urine culture report from our hospital microbiology department demonstrated the presence of P. mendocina with resistance to ciprofloxacin and levofloxacin. The provided antibiotic susceptibilities of the P. mendocina isolate from our patient are summarized in Table 3. MICs were not available to us retrospectively. Following the report, cefepime was discontinued and ceftriaxone was reinstated. By day 7 of admission, the patient no longer endorsed hematuria or dysuria. Absolute neutrophil count and WBC were 3800/μL and 4680/μL on day seven of admission (Table 1). The patient received a total of seven days of ceftriaxone (days 1-3, days 6-7) and cefepime (days 4-5). The patient was intermittently febrile, likely due to underlying malignancy and COVID-19 infection. A repeat urinalysis obtained due to the persistence of fevers showed no bacteria, with negative nitrite, negative leukocyte esterase, and moderate blood (Table 2). Complete resolution of urinary symptoms post-antibiotic therapy suggested that these symptoms were due to his urinary tract infection and less likely his prostate cancer. The hospital course was complicated by hypoxic respiratory failure due to COVID-19 infection, requiring prolonged hospitalization. The patient was successfully discharged after a 15-day hospital stay.
Table 3. Antibiotics susceptibility profile of isolated Pseudomonas mendocina.
| Antibiotics | Susceptibility |
| Amikacin | Susceptible |
| Aztreonam | Susceptible |
| Cefepime | Susceptible |
| Ceftriaxone | Susceptible |
| Ciprofloxacin | Not susceptible |
| Gentamicin | Susceptible |
| Levofloxacin | Not susceptible |
| Meropenem | Susceptible |
| Piperacillin/tazobactam | Susceptible |
| Tetracycline | Susceptible |
| Tobramycin | Susceptible |
| Trimethoprim/sulfamethoxazole | Susceptible |
Discussion
Pseudomonas aeruginosa has been known to cause severe nosocomial and opportunistic infections in immunocompetent and immunocompromised adults [19]. P. mendocina, however, is a rare cause of human infections and is less frequently reported in the literature.
A literature search was performed on PubMed using the terms "Pseudomonas mendocina" and "Pseudomonas mendocina infection." The query returned 14 case reports of P. mendocina-related infections in humans. Additional case reports were identified by cross-referencing previously discovered case reports. A total of 20 cases of P. mendocina-related infections were documented. Seven were from Asia (Taiwan [3,4], Singapore [5,6], and India [7]), three were from Europe (Denmark [8], France [9], and Portugal [10]), two were from the Middle East (Israel [11] and Turkey [12]), four were from North America (USA [13-16]), and two were from South America (Argentina [2,17]. P. mendocina can cause various infections, including infective endocarditis, meningitis, skin and soft tissue infections (burn wound infections, leg wound infections, and spondylodiscitis), peritonitis, septic arthritis, osteomyelitis, and bacteremia. Ours is the fifth case report of P. mendocina infection in the United States and the first documented case of P. mendocina urinary tract infection. A systematic literature review by Ioannou and Vougiouklakis in 2020 demonstrated that previous cases of P. mendocina had low mortality [18]. No deaths directly attributed to P. mendocina were reported.
Previous cases of P. mendocina infections reported successful treatments with various antibiotics, including penicillins, aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, and trimethoprim-sulfamethoxazole [1-17]. Across all cases, third- or fourth-generation cephalosporins and fluoroquinolones were commonly used agents for the treatment of P. mendocina. Documented P. mendocina isolates have shown susceptibility to non-traditional antipseudomonal antibiotics, such as ampicillin, cefazolin, and trimethoprim-sulfamethoxazole, allowing for a broader range of antibiotic selection compared to that of P. aeruginosa. Some cases reported susceptibility to all antibiotics tested, including aminoglycosides, ampicillin, carbapenems, later-generation cephalosporins, fluoroquinolones, and piperacillin/tazobactam [3,9,12-14,17]. However, some cases are also reported to have resistance to a variety of antibiotics, including ampicillin, amikacin, aztreonam, cephalothin, cefazolin, ceftazidime, aztreonam, ciprofloxacin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole [2,4-6,7,11,15]. Gupta et al. reported an isolate that had resistance to multiple antibiotics, including ciprofloxacin, ceftazidime, amikacin, piperacillin-tazobactam, and aztreonam [7]. Our isolate was resistant to ciprofloxacin and levofloxacin, making this the second documented isolate of P. mendocina that showed resistance to fluoroquinolones. Our patient was successfully treated with a seven-day course of ceftriaxone and cefepime.
P. mendocina can infect both immunocompromised and immunocompetent hosts. Most of the reported P. mendocina-related infections occurred in immunocompetent adults with several comorbidities, as shown in Table 4. A few cases were reported in immunocompromised adults. Gani et al. reported bacteremia in a patient with resistant HIV/AIDS [16]. Huang et al. reported meningitis in a patient with a history of diabetes mellitus type 2 and buccal cancer [3]. Gupta et al. previously reported a wound infection in a patient with diabetes mellitus type 2 and a prolonged history of asthma and intermittent corticosteroid use [7]. Our patient had a 10-year history of prostate cancer with bone metastases and was receiving treatment with abiraterone 1000 mg daily with an injection every three months and prednisone 5 mg daily. His immune system might be compromised due to his prostate cancer and treatment. It can be argued that P. mendocina caused an opportunistic urinary tract infection in our immunocompromised patient.
Table 4. Current literature reports on P. mendocina.
| Publication year | Author | Location | Age | Sex | Comorbidities | Infection type | Antibiotic resistance |
| 1992 | Aragone et al.[2] | Argentina | 63 | Male | Diabetes mellitus type 2, aortic valve replacement, poliomyelitis | Infective endocarditis | Ampicillin, cephalothin |
| 2001 | Johansen et al. [8] | Denmark | 28 | Female | Situs inversus, double-outlet right ventricle, ventricular septal defect (VSD), pulmonary stenosis, multiple cardiovascular surgeries | Infective endocarditis | No available data, culture unable to be obtained from abscess |
| 2005 | Chi et al. [4] | Taiwan | 65 | Male | Alcoholic hepatitis, chronic renal disease | Spondylodiscitis | Trimethoprim/sulfamethoxazole |
| 2007 | Mert et al. [12] | Turkey | 36 | Male | Mental retardation | Infective endocarditis | No known resistance |
| 2011 | Suel et al. [9] | France | 79 | Female | Atrial fibrillation, transient ischemic attack, hypertension | Infective endocarditis | No known resistance |
| 2011 | Nseir et al. [11] | Israel | 31 | Male | Healthy | Bacteremia | Ceftriaxone and aztreonam |
| 2013 | Howe et al. [6] | Singapore | 86 | Female | Vertebral compression fractures, tibial plateau stress fracture | Osteomyelitis | No available data, polymicrobial infection |
| 2013 | Chiu and Wang [5] | Singapore | 34 | Male | Healthy | Septic arthritis | Ampicillin ampicillin/sulbactam |
| 2016 | Rapsinski et al. [15] | United States | 57 | Male | Gout, chronic alcohol use | Infective endocarditis | Ampicillin/sulbactam, cefazolin |
| 2017 | Jerónimo et al. [10] | Portugal | 22 | Male | Chronic kidney disease, peritoneal dialysis | Peritonitis | No available data |
| 2018 | Almuzara et al. [17] | Argentina | 56 | Male | Alcohol use disorder, vascular insufficiency | Burn wound infection | No known resistance |
| 2018 | Almuzara et al. [17] | Argentina | 36 | Male | Alcohol use disorder | Burn wound infection | No known resistance |
| 2018 | Huang et al. [3] | Taiwan | 55 | Male | Diabetes mellitus type 2, buccal cancer, community-acquired infection | Meningitis | No known resistance |
| 2018 | Huang et al. [3] | Taiwan | 66 | Female | Spontaneous intracerebral hemorrhage, external ventricular drainage | Meningitis | No known resistance |
| 2018 | Huang et al. [3] | Taiwan | 79 | Male | Chronic obstructive pulmonary disease, respiratory failure, nosocomial infection | Meningitis | No known resistance |
| 2018 | Huang et al. [3] | Taiwan | 78 | Female | Healthy | Meningitis | No known resistance |
| 2019 | Gani et al. [16] | United States | 63 | Male | Resistant HIV/AIDS | Bacteremia | No resistance against cefepime, ceftazidime, levofloxacin, meropenem; resistance against piperacillin/tazobactam unable to be determined |
| 2020 | Goldberg et al. [14] | United States | 72 | Male | End-stage renal disease, immunoglobulin A (IgA) nephropathy, atrial fibrillation, heart failure with reduced ejection fraction, obesity, chronic venous stasis | Bacteremia | No known resistance |
| 2021 | Ezeokoli et al. [13] | United States | 81 | Male | Coronary artery disease, atrial fibrillation, heart failure, chronic kidney disease, diabetes mellitus type 2, CVA | Bacteremia | No known resistance |
| 2021 | Gupta et al. [7] | India | 53 | Male | Diabetes mellitus type 2, asthma | Leg wound infection | Ciprofloxacin, ceftazidime, amikacin, piperacillin-tazobactam, aztreonam |
| 2022 | This case report | United States | 83 | Male | Diabetes mellitus type 2, hypertension, coronary artery disease, prostate cancer, COVID-19 pneumonia | Urinary tract infection | Ciprofloxacin, levofloxacin |
Recent literature has documented the increase in opportunistic infections in patients with concomitant COVID-19 infections [20]. In particular, fungal infections remain the most common opportunistic infections amongst immunocompromised adults with COVID-19 [21,22]. Studies have also reported the association of COVID-19 with bacterial infections [23]. These infections are classified as nosocomial infections and are associated with increased morbidity and mortality among COVID-19 patients. In these patients, Staphylococcus aureus and Haemophilus influenzae are the most common bacterial infections [24]. Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Legionella pneumophila are other important bacterial pathogens that were detected among COVID-19 patients [22]. However, to date, no report has shown infection of P. mendocina in those with COVID-19 infections. We suspect that the combination of active COVID-19 infection and our patient's cancer status and treatment increases the risk for P. mendocina infection.
Multiple sources of P. mendocina were proposed but never confirmed in previous case reports. In the first-ever report of P. mendocina, Aragone et al. proposed that P. mendocina caused infective endocarditis by entering through thorn pricks and handling of damp earth as the patient was a florist with previous aortic valve placement and a permanent pacemaker [2]. Johansen et al. suspected that bacteria was introduced during one of the three cardiac operations, resulting in infective endocarditis [8]. Gupta et al. proposed that P. mendocina was present in soil and water, which gained entry into a leg wound when the patient fell while working on his farm [7]. In the case reported by Nseir et al., since the patient owned "a new pet cockatiel that he fed and watered directly from his mouth," the shared drinking water might be the source of P. mendocina [11]. In our patient, the source of P. mendocina was not identified.
Our case report adds to the current literature regarding P. mendocina infections. Although further research is required to identify the underlying pathogenicity and mechanism of P. mendocina infections, our report contributes to the growing body of publications that can help guide clinical management and treatment of P. mendocina infections in the future.
Conclusions
P. mendocina is a gram-negative bacillus that rarely causes infections in humans. When it does, P. mendocina has been known to cause skin and soft tissue infections, infective endocarditis, meningitis, and bacteremia. Our case is the first to report a urinary tract infection caused by P. mendocina and the second with fluoroquinolone resistance. In particular, this is also the first report on P. mendocina infection in an immunocompromised patient with COVID-19. Our report is the fifth documented case of P. mendocina-related infections in the United States, contributing to the growing literature regarding P. mendocina-related infections.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. Palleroni NJ, Doudoroff M, Stanier RY, Solánes RE, Mandel M. J Gen Microbiol. 1970;60:215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- 2.Pseudomonas mendocina, an environmental bacterium isolated from a patient with human infective endocarditis. Aragone MR, Maurizi DM, Clara LO, Navarro Estrada JL, Ascione A. J Clin Microbiol. 1992;30:1583–1584. doi: 10.1128/jcm.30.6.1583-1584.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: A clinical comparison with Ps. aeruginosa meningitis. Huang CR, Lien CY, Tsai WC, et al. Kaohsiung J Med Sci. 2018;34:49–55. doi: 10.1016/j.kjms.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Pseudomonas mendocina spondylodiscitis: a case report and literature review. Chi CY, Lai CH, Fung CP, Wang JH. Scand J Infect Dis. 2005;37:950–953. doi: 10.1080/00365540500263177. [DOI] [PubMed] [Google Scholar]
- 5.A case of unusual Gram-negative bacilli septic arthritis in an immunocompetent patient. Chiu LQ, Wang W. Singapore Med J. 2013;54:0–8. doi: 10.11622/smedj.2013162. [DOI] [PubMed] [Google Scholar]
- 6.A case of an atypical femoral fracture associated with bacterial biofilm--pathogen or bystander? Howe TS, Ehrlich GD, Koh JS, Ng AC, Costerton W. Osteoporos Int. 2013;24:1765–1766. doi: 10.1007/s00198-012-2222-4. [DOI] [PubMed] [Google Scholar]
- 7.Pseudomonas mendocina: wound infection in a farmer: a rare case. Gupta V, Singhal L, Pal K, Attri AK, Chander J. J Clin Diagn Res. 2021;15:1–3. [Google Scholar]
- 8.Pseudomonas mendocina as a cause of chronic infective endocarditis in a patient with situs inversus. Johansen HK, Kjeldsen K, Høiby N. Clin Microbiol Infect. 2001;7:650–652. doi: 10.1046/j.1198-743x.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.[A case of Pseudomonas mendocina endocarditis] Suel P, Martin P, Berthelot G, Robaday S, Etienne M, Chibani A. Med Mal Infect. 2011;41:109–110. doi: 10.1016/j.medmal.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Pseudomonas mendocina: the first case of peritonitis on peritoneal dialysis. Jerónimo TM, Guedes AM, Stieglmair S, Guerreiro R, Laranjo C, Bernardo I, Neves PL. Nefrología (English Edition) 2017;37:647–649. doi: 10.1016/j.nefro.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Pseudomonas mendocina sepsis in a healthy man. Nseir W, Taha H, Abid A, Khateeb J. https://pubmed.ncbi.nlm.nih.gov/21809738/ Isr Med Assoc J. 2011;13:375–376. [PubMed] [Google Scholar]
- 12.Native valve endocarditis due to Pseudomonas mendocina in a patient with mental retardation and a review of literature. Mert A, Yilmaz M, Ozaras R, Kocak F, Dagsali S. Scand J Infect Dis. 2007;39:615–616. doi: 10.1080/00365540601071883. [DOI] [PubMed] [Google Scholar]
- 13.A case of Pseudomonas mendocina bacteremia in an elderly man with bilateral leg lesions. Ezeokoli EU, Polat MU, Ogundipe O, Szela J. Cureus. 2021;13:0. doi: 10.7759/cureus.17777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pseudomonas mendocina bacteremia in a hemodialysis patient with a central venous catheter. Goldberg ME, Blyth M, Swiatlo E. Cureus. 2020;12:0. doi: 10.7759/cureus.10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pseudomonas mendocina native valve infective endocarditis: a case report. Rapsinski GJ, Makadia J, Bhanot N, Min Z. J Med Case Rep. 2016;10:275. doi: 10.1186/s13256-016-1057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pseudomonas mendocina bacteremia: a case study and review of literature. Gani M, Rao S, Miller M, Scoular S. Am J Case Rep. 2019;20:453–458. doi: 10.12659/AJCR.914360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical cases of VIM-producing Pseudomonas mendocina from two burned patients. Almuzara M, Montaña S, Carulla M, et al. J Glob Antimicrob Resist. 2018;14:273–274. doi: 10.1016/j.jgar.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 18.A systematic review of human infections by Pseudomonas mendocina. Ioannou P, Vougiouklakis G. Trop Med Infect Dis. 2020;5 doi: 10.3390/tropicalmed5020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Sadikot RT, Blackwell TS, Christman JW, Prince AS. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVID-19-associated opportunistic infections: a snapshot on the current reports. Abdoli A, Falahi S, Kenarkoohi A. Clin Exp Med. 2021 doi: 10.1007/s10238-021-00751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hidden killers: human fungal infections. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Sci Transl Med. 2012;4:165. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 22.Bacterial co-infections with SARS-CoV-2. Mirzaei R, Goodarzi P, Asadi M, et al. IUBMB Life. 2020;72:2097–2111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? Lai CC, Wang CY, Hsueh PR. J Microbiol Immunol Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Co-infection with respiratory pathogens among COVID-2019 cases. Zhu X, Ge Y, Wu T, et al. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]