ABSTRACT
Interferon gamma (IFNG/IFNγ)-induced adaptive immune resistance remains a challenge for tumor therapy. We observed that the chaperone heat shock protein 90 (HSP90) stabilizes the transcription factor signal transducer and activator of transcription 1 (STAT1), resulting in IFNγ-induced expression of immunosuppressive indoleamine 2,3-dioxygenase 1 (IDO1) and programmed death-ligand 1 (PD-L1/CD274). Pharmacological inhibition of HSP90 enhances the efficacy of programmed cell death 1 (PDCD1/PD-1) targeting immunotherapy in suitable mouse models without any toxicity.
KEYWORDS: Adaptive immune resistance, immune checkpoint, molecular chaperone, pancreatic cancer, protein degradation
Interferon gamma (IFNG, best known as IFNγ) is a pleiotropic cytokine that mediates antiviral effects and acts as a central coordinator of antitumor immune responses.1 In addition to activating the cytotoxic function of CD8+ T cells, IFNγ is a strong inducer of the expression of multiple immune checkpoint molecules, presumably by activating the signal transducer and activator of transcription 1 (STAT1) pathway, leading to adaptive immune resistance.2 Our recent study identifies the therapeutic vulnerability of pancreatic ductal adenocarcinoma (PDAC) by showing that heat shock protein 90 (HSP90) plays a new role in mediating IFNγ-induced adaptive resistance to immunotherapies targeting programmed cell death 1 (PDCD1, best known as PD-1) (Figure 1).3
Figure 1.
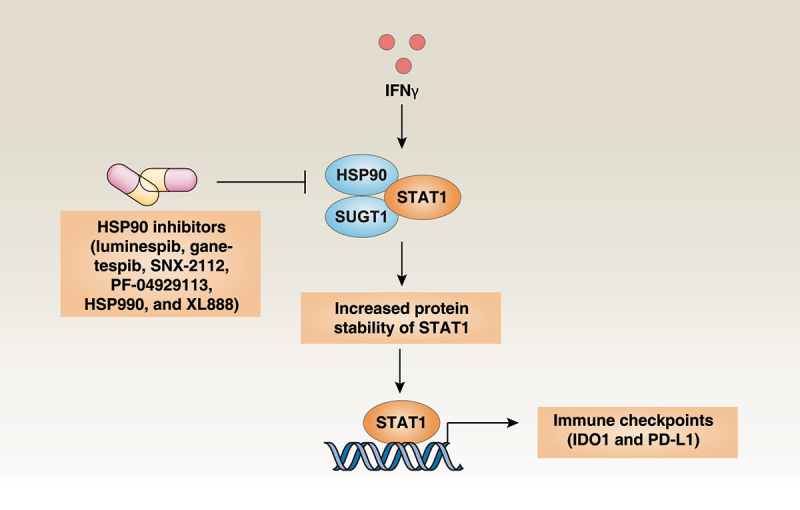
The HSP90 chaperone machinery modulates IDO1 and PD-L1 expression. IFNγ-induced the expression of immune checkpoint molecules (IDO1 and PD-L1) requires increased protein stability of the transcription factor STAT1 mediated by the HSP90-SUGT1 chaperone complex. Abbreviations: HSP90, heat shock protein 90; IDO1, indoleamine 2,3-dioxygenase 1; IFNγ, interferon gamma; PD-L1, programmed death-ligand 1; STAT1, signal transducer and activator of transcription 1; SUGT1, SGT1 homolog, MIS12 kinetochore complex assembly cochaperone.
PDAC is one of the deadliest gastrointestinal cancers driven by KRAS mutations, with a 5-year overall survival rate of 5% to 10% that has not been ameliorated over the past 30 years. PDAC is resistant to therapy with immune checkpoint inhibitors (ICI), but the mechanisms underlying this resistance are largely unknown.4 We implanted KPC cells (which are PDAC cells derived from Pdx1-Cre;KrasG12D/+;Tp53R172H/+ mice) into C57BL/6 J mice and examined the expression of 16 common immune checkpoint molecules in isolated tumor after treatment with anti-PD-1 antibody (αPD-1). Compared with the minority of responder mice (~25%), animals that did not respond to PD-1 blockade (~75%) selectively upregulated the expression of indoleamine 2,3-dioxygenase 1 (IDO1), rather than other immune checkpoint molecules, in cancer cells in an IFNγ-dependent manner.3 Given that the functions of different immune checkpoint molecules are complementary, dynamic monitoring of their expression in the tumor microenvironment may be important for identifying suitable therapeutic targets.5
Next, we used the KRAS and tumor protein p53 (TP53, best known as p53)-mutated human PDAC cell line CFPAC1 to screen for compounds that inhibit IFNγ-induced IDO1 as well as expression of CD274 molecule (best known as programmed death-ligand 1 [PD-L1]). Of note, we found that 24 compounds (used at 10 µM) that blocked IFNγ-induced IDO1 and PD-L1 expression in CFPAC1 cells. The vast majority (71%) of these agents were HSP90 inhibitors.3 In addition, nanomolar amounts of six HSP90 inhibitors (luminespib, ganetespib, SNX-2112, PF-04929113, HSP990 and XL888) suppressed IFNγ-induced IDO1 and PD-L1 expression in 16 human tumor cell lines (corresponding to 11 different types of cancer) and primary PDAC cells from patients and KPC mice.3 Mechanistically, we demonstrated that the binding of HSP90 to its partner SGT1 homolog, MIS12 kinetochore complex assembly cochaperone (SUGT1) resulted in increased protein stability of STAT1, a key transcription factor for the expression of immune checkpoint molecules. Expression of dominant-negative HSP90 (D88N) led to inhibition of STAT1-mediated IFNγ signaling, suggesting that the aforementioned HSP90 inhibitors act on target.3 Altogether, these results point to a broad role for HSP90 in mediating the expression of inducible immune checkpoint molecules.
IDO1 is a rate-limiting metabolic enzyme that converts the essential amino acid tryptophan to a downstream immunosuppressor, kynurenine, thereby inhibiting the proliferation and function of cytotoxic CD8+ T cells. However, the mechanism of action of IDO1-mediated kynurenine production and secretion remains poorly understood. We found that tetraspanin 5 (TSPAN5), a transmembrane protein of the tetraspanin family, plays a critical role in mediating IFNγ-induced kynurenine secretion, but not IFNγ-induced IDO1 expression.3 We also verified that the enzymatic activity of IDO1 requires iron (but not other metal ions) to trigger kynurenine production. Consequently, iron-enhanced kynurenine release (but not kynurenine synthesis) was inhibited in TSPAN5-deficient CFPAC1 cells.3 These findings reveal a role for iron in promoting IDO1-dependent kynurenine production and subsequent TSPAN5-mediated kynurenine release.
Finally, we evaluated the efficacy and safety of combinations of αPD-1 with the HSP90 inhibitor ganetespib, the IDO1 inhibitor BMS-986205, or the iron chelator desferoxamine in the treatment of transplanted or a transgene-induced PDAC. Compared with the αPD-1 alone group, the combination of αPD-1 with ganetespib, BMS-986205, or desferoxamine was more efficient in reducing tumor growth and enhancing the infiltration of PDAC by CD8+ T cells and dendritic cells (but not CD4+ T cells, macrophages, and natural killer cells). Depletion of CD8+ T cells abolished deferoxamine and αPD-1-mediated tumor suppression, demonstrating that this combination therapy relies on the contribution of tumor-specific cytotoxic T lymphocytes. This new combination therapy had an acceptable safety profile and did not affect liver and kidney function in mice.
In summary, our study uncovers a new strategy through which PDAC cells hide from the immune system. Moreover, IDO1 emerges as a potential biomarker for predicting treatment responses to anti-PD-1. We provide proof-of-concept for future clinical applications of HSP90 inhibitors, IDO1 inhibitors, or iron chelators to enhance the anticancer activity of PD-1 blockade. In addition to STAT1, the expression of immune checkpoint molecules is controlled by other transcriptional factors, such as TP53,6 hypoxia inducible factor 1 subunit alpha,7 and MYC.8 It is important to further define the crosstalk of gene transcription and protein degradation pathways in coordinating the expression of immune checkpoint molecules by PDAC cells.9 Potentially, distinguishing different clients of the HSP90 chaperone machinery in tumor immunity remains a challenge. Regardless, the hypothesis that inducers of immunogenic stress and death,10 including HDP90 inhibitors, may sensitize PDAC cells to immunotherapy should be explored in future clinical assays.
Funding Statement
The authors reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Gocher AM, Workman CJ, Vignali DAA.. Interferon-gamma: teammate or opponent in the tumour microenvironment? Nat Rev Immunol. 2022.22(3):158–3. doi: 10.1038/s41577-021-00566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas AP. Adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017.168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K, Huang J, Liu J, Li C, Kroemer G, Tang D, Kang R. HSP90 mediates IFNgamma-induced adaptive resistance to anti-PD-1 immunotherapy. Cancer Res. 2022. doi: 10.1158/0008-5472.CAN-21-3917. [DOI] [PubMed] [Google Scholar]
- 4.Bear AS, Vonderheide RH, O’Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020.38(6):788–802. doi: 10.1016/j.ccell.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehnert JM, Monjazeb AM, Beerthuijzen JMT, Collyar D, Rubinstein L, Harris LN. The challenge for development of valuable immuno-oncology biomarkers. Clin Cancer Res. 2017.23(17):4970–4979. doi: 10.1158/1078-0432.CCR-16-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiem A, Hesbacher S, Kneitz H, Di Primio T, Heppt MV, Hermanns HM, Goebeler M, Meierjohann S, Houben R, Schrama D, et al. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J Exp Clin Cancer Res. 2019;38(1):397. doi: 10.1186/s13046-019-1403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, Peng Q, Liu Z-X, Li C, Kroemer G, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70(5):890–899. doi: 10.1136/gutjnl-2019-320441. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022.23(4):487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


