Abstract
Short term rapid eye movement (REM) sleep deprivation produced a decrease in waking discharge rates of presumably noradrenergic pontine 'REM sleep-off' cells and an increase in waking discharge rates of pontine 'REM sleep-on' cells. These changes can be viewed as a correlate of increased REM sleep pressure. Slowing of REM sleep-off cells in waking is hypothesized to counteract the functional effects of REM sleep loss on noradrenergic receptor sensitivity This slowing and the resulting reduction in norepinephrine release may contribute to the loss of vigilance seen with sleep deprivation.
Keywords: Rapid eye movement sleep, Pons, Norepinephrine, Locus ceruleus, Reticular
INTRODUCTION
Rapid eye movement (REM) sleep deprivation produces a number of behavioral changes including hyperphagia, hypersexuality9,10,36, increased aggressiveness 15, increased activity levels34, reduction of seizure threshold8, and reversal of endogenous depression37. These changes are parallelled by alterations in evoked responses 18,27,35. REM sleep deprivation may also produce shifts in receptor sensitivity1,22,23,32. However, most of these changes take as long as 2 weeks to develop, indicating that the brain is able to compensate for the effects of short-term REM sleep deprivation. Total REM sleep deprivation in rats is lethal after 16–54 days19.
Despite the large number of studies on the behavioral effects of REM sleep deprivation, there have not, to our knowledge, been any studies of neuronal activity during sleep deprivation. The chronic microwire technique14,31 is capable of maintaining stable single-unit recordings for periods of several days. We have taken advantage of this extraordinary stability to study neuronal activity during REM sleep deprivation using the platform deprivation technique36. Our target for these initial studies was the dorsolateral pons, an area known to be critically involved in generating REM sleep, and to contain neurons that selectively increase or decrease their activity during this state29
MATERIALS AND METHODS
Three mongrel cats served as subjects Surgical and recording procedures were as described previously31.
Before surgery the cats were habituated to the experimental chamber and slept on a 'large' (30 × 35 cm) platform mounted 27 cm above the surface of a 5 cm deep pool of water. They were also habituated to a 'small' (12 × 12 cm) platform for 6 h a day for at least two sessions spaced at 1–2 day intervals When a unit with signal/noise ratio > 3/1 was discriminated, it was recorded for at least 1 h to confirm the stability of the signal The cat was then transferred to the large control platform. The platforms were in a well ventilated 55 × 58 × 85 cm chamber Baseline recordings with the cat on the large platform were continued until at least 3 REM sleep episodes were obtained
In order to classify cells into types on the basis of their sleep discharge pattern during baseline recordings, the discharge rate of neurons for 2 min continuously during REM sleep and equal periods during the preceding non-REM sleep and quiet waking33 periods was determined Unit discharge during 3–8 episodes of REM and non-REM sleep for each neuron were counted using a MINC 1123 computer and compared by t-test A mean of 13 6 h of baseline data was collected for the determination of baseline sleep state distribution.
After the control recording was completed, the large platform was replaced, in the same chamber, by the small platform, where the cat was able to stand, turn and sit Though the cat could go into non-REM sleep on the small platform, it was unable to assume the side-down posture required for REM sleep During the deprivation procedure the cat was fed once a day during a 30 min period between 12.00 and 13 00 h For feeding, the cat was removed from the platform and given one can (160 g) of moist Kal Kan cat food and water ad lib The cat was under constant observation during the feeding period to be sure that no sleep occurred In two cases, polygraph recording was carried out during the feeding period to confirm the absence of sleep. The deprivation period lasted a mean of 28.8 h. At the end of deprivation, the smaller platform was replaced by the larger one used during the baseline recording, for the sleep recovery period, which lasted a mean of 32.4 h.
Stability of unit waveforms was assessed by oscilloscopic monitoring of the waveform at least once every 15 min, throughout the studies. The unit waveforms were periodically photographed for comparison with the initially observed waveform. Unit potentials from cells used in the study either showed no change or gradual change in signal-to-noise ratio throughout the experiment. Cells with abrupt changes in signal to noise ratio or waveshape were dropped from the study. The pattern of change in discharge rate with sleep state, the waveshape, and the behavioral correlates of discharge31, were in every case the same after recovery from deprivation as they were during the baseline The duration of the recovery observation period was equal to the duration of the deprivation period, except for those cases in which the unit recording was lost. Once a unit was studied in the deprivation paradigm, a period of at least 7 days was allowed for recovery before any new unit was studied in the same cat.
Sleep states were scored, using the Ursin-Sterman critena33. Scoring epochs were 60 s long. Four 1 h periods were independently scored by two investigators and interscorer reliability was 91% (97% for REM sleep, 97% for waking, 82% for slow wave sleep 1 (SWS1) and 74% for SWS2). To determine the effect of the REM sleep deprivation procedure on waking firing rate, unit firing rates from the last h of the predeprivation (baseline) condition, last h of deprivation and last h of the recovery period were counted. In each 1 h period, the first 5 episodes of quiet waking33 were scored for unit discharge rate (average total duration of quiet waking samples in each 1 h period = 17.5 min) The significance of differences was determined with the BMDP statistical package. A y = log(x + 0.5) transformation was made to reduce skewness and increase variance homogeneity between cells and cell types A repeated measure, two-way analysis of variance (module 2V) was then used to determine the significance of observed changes.
Recording sites were labelled with iron deposition by passing a 15 μA current for 5 s at the bottom of each microwire track. Cats were sacrificed with an overdose of sodium pentobarbital, perfused with saline and 10% formalin and the brains were histologically analyzed with standard techniques.
RESULTS
Effects of platform on sleep
Confinement to the small platform completely eliminated REM sleep (Table I). The small platform restriction also reduced the total time in non-REM sleep, with the most marked effects on SWS2. While REM sleep is always accompanied by a complete loss of muscle tone, the nuchal muscles are often atonic in SWS229,31,33. Thus this atonia, which would be impossible to maintain on the small platform, could account for the reduction in SWS2 along with REM sleep. Previous studies of the platform deprivation technique in cats have not separately scored for SWS1 and SWS227,36. The hand awakening technique for human REM sleep deprivation, is known to reduce non-REM sleep time38. Thus while REM sleep deprivation techniques produce a disproportionate reduction in REM sleep, non-REM sleep stages are also altered. Since REM sleep-off cells show greatly reduced activity levels during REM sleep and the immediately preceding SWS2 state5,17, our deprivation conditions greatly reduced both of the states during which locus coeruleus cells are inactive. REM sleep-on cells start discharging in SWS2 prior to REM sleep onset28, and therefore this period of acceleration was also eliminated by the deprivation. Total time in REM sleep and SWS2, and proportion of sleep devoted to REM sleep and SWS2 increased to above baseline levels during the recovery period (Table I).
TABLE I.
Sleep state changes during deprivation and recovery in REM sleep-off cells in % total time
| %Awake | %Sleep | %SWS1 | %SWS2 | %REM | Hours | |
|---|---|---|---|---|---|---|
| Baseline | 41.3 | 58.7 | 27.2 | 17.0 | 14 6 | 6.8 |
| Deprivation | 78 7 | 21.3 | 17.8 | 3.5 | 0 0 | 20.4 |
| Recovery | 47.2 | 52.8 | 16 4 | 18.6 | 17.8 | 14.0 |
Effects of REM sleep deprivation on discharge rate
Sixteen cells were each held for an average of 74.8 h of continuous recording during which time REM sleep was deprived for an average of 28.8 h (Table II). During this period an experimenter was continuously present to monitor unit waveshape. Cells which could not be held for at least 48 h continuously, or which showed changes in unit waveform as described above, were dropped from the study. For purposes of data analysis, cells were divided into 3 groups on the basis of their baseline discharge patterns, 'REM sleep-off' (n = 5), 'REM sleep-on' (n = 3), and 'other' (n = 8) cell types. REM sleep-off cells were defined as those with significantly (p < 0.05, t-test) lower rates in REM sleep than in both non-REM sleep and active waking33. Two of the 3 tested REM sleep-off cells stopped firing during the scruff immobility reflex, as described by Reiner26. REM sleep-on cells were defined as those with significantly higher discharge rates in REM sleep than in both non-REM sleep and active waking. All cells not meeting the criteria for REM sleep-on or REM sleep-off cells were classified as 'other'. The most common pattern seen in the 'other' cells was elevated activity during both waking and REM sleep when compared to non-REM sleep. Recorded cells were distributed throughout the dorsolateral pons between P1 and P5, L1.5 and L3.0, H0 and H-5. REM sleep-on and -off cells were both located in the locus coeruleus and in the subcoeruleus region, as previously described2,5,16,17,28.
TABLE II.
Duration of studies in hours
| n | Baseline | Deprivation | Recovery | |
|---|---|---|---|---|
| REM sleep-off cells | 5 | 6.8 | 20.4 | 14.0 |
| REM sleep-on cells | 3 | 15.7 | 41 7 | 39.0 |
| Other cells | 8 | 17.0 | 26.4 | 41.4 |
| Overall | 16 | 13.6 | 28.8 | 32.4 |
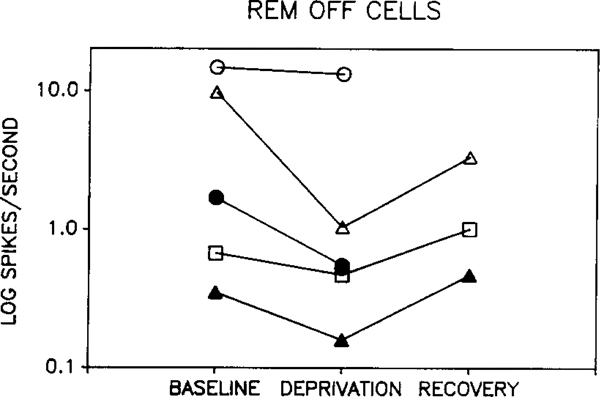
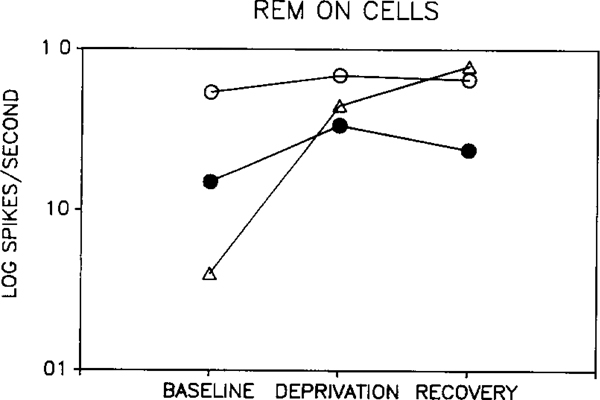
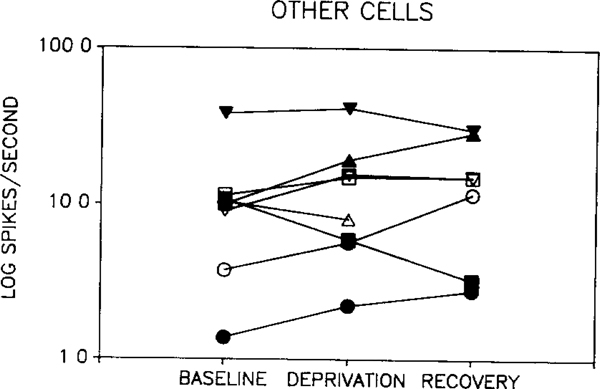
Two-way ANOVA revealed no significant difference between waking discharge rate in baseline, deprivation and recovery periods when considering all cell types. There was also no significant difference between baseline and recovery periods for any single cell type. However, there was a significant interaction between cell type and the change induced by deprivation (p < 0.002, two-way repeated measure ANOVA, deprivation vs baseline + recovery/2 × cell type). This effect was due to opposite directions of change in REM sleep-off and REM sleep-on cells. Fig. 1 shows the response of REM sleep-off cells to REM sleep deprivation. Waking discharge rate was reduced during deprivation in all 5 cells. Rates returned towards baseline levels in all 3 cells that could be recorded after recovery sleep. The opposite pattern was seen in two of the 3 REM sleep-on cells (Fig. 2). The third REM sleep-on cell did not show a return to baseline levels by the end of the recovery period. We were unable to find any variable that distinguished this cell from the cells whose waking discharge rates returned towards baseline levels after recovery sleep. As a group, the 'other' cell types showed no consistent change in discharge rate with deprivation (Fig. 3).
Fig. 1.
Effect of REM sleep deprivation on waking discharge rates of 'REM sleep-off' cells. Each tracing shows the rate changes seen in a single unit during baseline, REM sleep deprivation and recovery. Deprivation reduced discharge rate in all recorded cells, with return towards baseline levels with recovery sleep.
Fig. 2.
Effect of REM sleep deprivation on waking discharge rates of 'REM sleep-on' cells Deprivation increased discharge rate in all recorded cells. After recovery sleep, waking discharge levels decreased in two cells, but continued to increase in third
Fig. 3.
Effect of REM sleep deprivation on waking discharge rates in 'other' cell types. No consistent pattern was seen in this group.
DISCUSSION
We have found that REM sleep deprivation alters waking firing rates in pontine REM sleep-on and REM sleep-off cells, while having no consistent effect on other cell types. In any sleep deprivation experiment, the issue of stress as a cause of the observed effects arises. In the present study, we have taken the approach of using a short period of REM sleep deprivation to minimize stress. We have also used a large platform control that produced minimal REM sleep deprivation, while providing a comparable behavioral environment. Recovery and baseline REM sleep occurred on the large platform, showing that the changes observed during deprivation were not secondary to platform restriction. Nevertheless, one could still argue that the small platform was more stressful than the large platform and that this accounts for the altered unit activity rates. However, the rate changes we observed in REM sleep-off units were not consistent with such an explanation. A number of studies have shown that stress tends to increase firing in REM sleep-off neurons, and to also increase norepinephrine (NE) release2,3,4,5,11,12,17. REM sleep-off cells in the dorsolateral pons are thought to be predominantly noradrenergic cells of the locus coeruleus complex5, 16,17,25 In the present study we find that REM sleep deprivation produced a decrease in unit firing, the opposite of the change predicted by a stress explanation.
The discharge rates during baseline and deprivation conditions were taken during desynchronized EEG conditions, utilizing the usual scoring criteria33. Thus the altered discharge rate could not result from increased sampling at times when the EEG was synchronized in the REM sleep deprivation condition. Nevertheless, one could view the REM sleep-off unit activity change as a result of a more subtle 'state' change during deprivation. That is, sleepiness could be hypothesized to produce a slowing of discharge in REM sleep-off cells without observable change in the EEG, in addition to the previously reported intrusion of 'microsleep' episodes accompanied by EEG changes13. Subtle changes in the EEG might be observable using power spectral analysis or other techniques. Conversely, one could view the altered activity in REM sleep-off cells as a cause of a subtle state change. There is ample evidence that NE release by the locus coeruleus is critically involved in the regulation of states of alertness3,5,17. The present data do not allow us to choose between these two alternatives. Indeed, since the locus coeruleus is in active interaction with adjacent cells of the cholinergic pontine nuclei in the regulation of level of arousal4,6,7,20,21, it is not likely that the cause/effect distinction is useful in this case. In any event, the present results demonstrate that diminished activity in REM sleep-off cells and increased activity in REM sleep-on cells is a correlate of REM sleep deprivation.
One of the key early observations after the discovery of REM sleep was that REM sleep deprivation produced increased REM sleep 'pressure' that was manifest in more frequent attempts to enter REM sleep and a large REM sleep rebound when the deprived subject was left undisturbed9,24,36. The changes in unit activity that we report here can best be seen as a correlate of this increased REM sleep pressure. Thus, REM sleep-off cells are by definition those cells which decrease rate, and REM sleep-on cells those which increase rate, in REM sleep. We have found that during REM sleep deprivation, both cell types show rate changes in the direction of their normal rate changes during REM sleep. These changes can be seen as either a cause or as a result of the greater REM sleep pressure that develops during deprivation, as well as being a correlate of changes in arousal brought about by sleep deprivation.
Although we are seeing a shift in activity towards the REM sleep pattern in the REM sleep-on and REM sleep-off cell groups, we must emphasize that these changes are much smaller than those normally occurring in REM sleep. However, it is possible that longer-term REM sleep deprivation would produce even more marked changes in unit activity in these cells. We have recently hypothesized30 that a key function of REM sleep is to reduce activity in noradrenergic REM sleep-off cells, and thereby upregulate or prevent downregulation of NE receptors.
Two strategies by which evolution would have compensated for the loss of the hypothesized REM sleep receptor regulation effect during sleep disruption can be envisioned. In the first strategy, a reduction in the waking discharge rate of noradrenergic cells during sleep deprivation, would tend to produce changes in receptors in the same direction as occur in REM sleep, albeit to a lesser extent. Such rate changes during deprivation would be the expression of an adaptive mechanism tending to attenuate the neural effects of REM sleep loss, by producing in waking some of the same reduction in NE release normally seen in REM sleep. This would slow the downregulation of NE receptors hypothesized to occur during waking. In emergency situations, an increased NE release would act on receptors whose sensitivity has been only slightly reduced. The disadvantage of this strategy would be a reduced level of tonic vigilance.
A second physiological strategy by which the organism might cope with the effects of REM sleep deprivation and the hypothesized NE receptor downregulation that would result, would be by increased tonic NE release. This would be mediated by increased discharge rates in noradrenergic cells and would tend to compensate for the reduction in NE receptor sensitivity. However, this strategy, by increasing NE availability would tend to accelerate receptor downregulation. Therefore, while this would initially be adaptive, by maintaining normal levels of vigilance, over a long period it would progressively reduce the responsiveness of the NE system to phasic release. Our data indicate that it is the first strategy which has evolved.
The present results demonstrate the feasibility of combining unit recording with sleep deprivation. Further studies using these techniques should help to shed light on the changes in neuronal activity and responsiveness resulting from sleep loss.
Acknowledgements
Supported by the Medical Research Service of the Veterans Administration, USPHS Grants MH43811, NS14610 and the American Narcolepsy Association.
REFERENCES
- 1.Abel MS, Villegas F, Abreu J, Gimino E, Steiner S, Beer B and Meyerson LR, The effect of rapid eye movement sleep deprivation on cortical beta-adrenergic receptors, Brain Res Bull, 11 (1983) 729–734. [DOI] [PubMed] [Google Scholar]
- 2.Abercrombie ED and Jacobs BL, Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli, J. Neurosci, 7 (1987) 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abercrombie ED and Jacobs BL, Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli, J Neurosci, 7 (1987) 2844–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral DG and Sinnamon HM, The locus coeruleus: neurobiology of a central noradrenergic nucleus, Prog. Neurobiol, 9 (1977) 147–196. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G and Bloom FE, Activity of norepinephrine-contaimng locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle, J Neurosci, 8 (1981) 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aston-Jones G, Foote SL and Bloom FE, Anatomy and physiology of locus coeruleus neurons: functional implications In Ziegler M and Lake R (Eds.), Norepinephrine, Williams and Wilkins, Baltimore, 1984, pp 92–116. [Google Scholar]
- 7.Clark TK, The locus coeruleus in behavior regulation: evidence for behavior-specific versus general involvement, Behav. Neural Biol, 25 (1979) 271–300. [DOI] [PubMed] [Google Scholar]
- 8.Cohen HB and Dement WC, Prolonged tonic convulsions in REM deprived mice, Brain Research, 22 (1970) 421–422 [DOI] [PubMed] [Google Scholar]
- 9.Dement WC., The effect of dream deprivation, Science, 131 (1960) 1705–1707. [DOI] [PubMed] [Google Scholar]
- 10.Dement WC, Recent studies on the biological role of rapid eye movement sleep, Am. J. Psychiatry, 122 (1965) 404–408. [DOI] [PubMed] [Google Scholar]
- 11.Glavin GB, Stress and brain noradrenaline: a review, Neurosci. Biobehav, 9 (1985) 233–243 [DOI] [PubMed] [Google Scholar]
- 12.Grant S and Redmond D, Neuronal activity of the locus ceruleus in awake Macaca arctiodes, Exp Neurobiol, 84 (1984) 701–708. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Billard M, Montplaisir J and Dement WC, Altered states of consciousness in disorders of daytime sleepiness, J. Neurol. Sci, 26 (1975) 377–393. [DOI] [PubMed] [Google Scholar]
- 14.Harper RM and McGinty DJ, A technique for recording single neurons from unrestrained animals In Phillips MI (Ed.), Brain Unit Activity during Behavior, Thomas, Springfield, IL, 1973, pp. 80–104. [Google Scholar]
- 15.Hicks RA, Moore JD, Hayes C, Phillips N and Hawkins J, REM sleep deprivation increases agressiveness in male rats, Physiol. Behav, 22 (1979) 1097–1100 [DOI] [PubMed] [Google Scholar]
- 16.Hobson JA, McCarley RW and Nelson JP, Location and spike-train characteristics of cells in anterodorsal pons having selective decreases in firing rate during desynchronized sleep, J. Neurophystol., 50 (1983) 770–783. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs BL, Single unit activity of locus coeruleus neurons in behaving animals, Prog Neurobiol, 27 (1986) 183–194. [DOI] [PubMed] [Google Scholar]
- 18.Kopell BS, Zarcone V, De La Pena A and Dement WC, Changes in selective attention as measured by the visual averaged evoked potential following REM deprivation in man, Electroenceph. Clin. Neurophysiol, 32 (1972) 322–325 [DOI] [PubMed] [Google Scholar]
- 19.Kushida CA, Bergmann BM and Rechtschaffen A, Sleep deprivation in the rat. IV Paradoxical sleep deprivation, Sleep, 12 (1989) 22–30. [DOI] [PubMed] [Google Scholar]
- 20.Mason S, Noradrenaline in the brain: progess in theories of behavioural function, Prog. Neurobiol, 16 (1981) 263–303 [DOI] [PubMed] [Google Scholar]
- 21.MeNaughton N and Mason S, The neuropsychology and neuropharmacology of the dorsal ascending noradrenergic bundle -- a review, Prog. Neurobiol, 14 (1980) 157–219 [DOI] [PubMed] [Google Scholar]
- 22.Mogilnicka E, Arbilla S, Depoortere H and Langer SZ, Rapid-eye-movement sleep deprivation decreases the density of 3H-dihydroalprenolol and 3H-imipramine binding sites in the rat cerebral cortex, Eur. J. Pharmacol, 65 (1980) 289–292. [DOI] [PubMed] [Google Scholar]
- 23.Mogilnicka E, Przewlocka B, Van Luijtelaar ELJM, Klimek V and Coenen AML, Effects of REM sleep deprivation on central alpha 1- and beta-adrenoceptors in rat brain, Pharmacol Biochem. Behav, 25 (1986) 329–332 [DOI] [PubMed] [Google Scholar]
- 24.Pivik RT, Blysma FW and Cooper P, Effects of paradoxical sleep deprivation in the rabhit, Physiol Behav, 36 (1986) 671–676. [DOI] [PubMed] [Google Scholar]
- 25.Pompeiano O and Hoshino K, Tonic inhibition of dorsal pontine neurons during the postural atonia produced by an anticholinesterase tn the decerebrate cat, Arch Ital. Biol, 114 (1976) 310–340. [PubMed] [Google Scholar]
- 26.Reiner PB, Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats, Brain Research, 378 (1986) 86–96. [DOI] [PubMed] [Google Scholar]
- 27.Satinoff E, Drucker-Colin RR and Hernandez-Peon R, Paleocortical excitability and sensory filtering during REM sleep deprivation, Physiol. Behav, 7 (1971) 103–106. [DOI] [PubMed] [Google Scholar]
- 28.Shiromani PJ, Armstrong DM, Bruce G, Hersh LB, Groves PJ and Gillin C, Relation of pontine choline acetyltranferase immunoreactive neurons with cells which increase discharge during REM sleep, Brain Res. Bull, 18 (1987) 447–455. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JM, Brainstem mechanisms generating REM sleep In Kryger MK, Roth T and Dement WC (Eds.), Principals and Practice of Sleep Medicine, Saunders, Philadelphia, 1989, pp. 104–120. [Google Scholar]
- 30.Siegel JM and Rogawski MA, A function for REM sleep: regulation of noradrenergic receptor sensitivity, Brain Res. Rev, 13 (1988) 213–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel JM and Tomaszewski KS, Behavioral organization of reticular formation, studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements, J Neurophysiol., 50 (1983) 696–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troncone LRP, Braz S, Benedito MAC and Tufik S, REM sleep deprivation induces a decrease in norepinephrine-stimulated 3H-cyclic AMP accumulation in slices from rat brain, Pharmacol. Biochem. Behav, 25 (1986) 223–225. [DOI] [PubMed] [Google Scholar]
- 33.Ursin R and Sterman MB, A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat, Brain Information Service/Brain Research Institute, University of California, Los Angeles, 1981 [Google Scholar]
- 34.Van Hulzen ZJM and Coenen AML, Paradoxical sleep deprivation and locomotor activity in rats, Physiol. Behav, 27 (1981) 741–744. [DOI] [PubMed] [Google Scholar]
- 35.Van Hulzen ZJM and Coenen AML, Photically evoked potentials in the visual cortex following paradoxical sleep deprivation in rats, Physiol Behav, 32 (1984) 557–563 [DOI] [PubMed] [Google Scholar]
- 36.Vimont-Vicary P, Jouvet-Mounier D and Delorme F, Effets et comportementaux des privations de sommeil paradoxal chez le chat, Electroenceph. Clin. Neurophysiol, 20 (1966) 439–449. [DOI] [PubMed] [Google Scholar]
- 37.Vogel GW, Thurmond A, Gibbons P, Sloan K, Boyd M and Walker M, REM sleep reduction effects on depression syndromes, Arch Gen Psychiatry, 32 (1975) 765–777. [DOI] [PubMed] [Google Scholar]
- 38.Zarcone V, De La Pena A and Dement WC, Heightened sexual interest and sleep disturbance, Percept. Mot. Skills, 39 (1974) 1135–1141 [Google Scholar]