Abstract
Pelvic organ prolapse (POP) is a condition wherein one or more of the organs in the pelvis slip down from their original position and protrude into the vagina. Pelvic organ prolapse surgery has increased in the urogynecological field due to higher aging society. POP patients often suffer from bowel dysfunction, such as difficulty of bowel movements and the need to strain or push on the vagina to have a bowel movement. Rectocele is often treated with the same method used for POP, but sometimes it is treated transanally. In the transabdominal approach, the vagina is divided from the rectum, and the mesh is fixed between the vagina and rectum.
On the other hand, rectal prolapse is a condition wherein the rectum slips down from its original position and protrudes from the anus. Like POP surgery, rectal prolapse has been treated laparoscopically. Even though the protruding position is different, both are pelvic conditions, and the concept of treatment is similar. Recently, POP and rectal prolapse have been diagnosed at the same time, and sometimes these diseases have been treated together.
In the higher aging society, incidences of POP and rectal prolapse will increase, and both will have greater chance to be treated. Although POP is a urogynecological disease, coloproctologists need to know the bowel dysfunction in order to treat POP.
Keywords: pelvic organ prolapse (POP), rectal prolapse
Introduction
Pelvic organ prolapse (POP) is a condition, in which one or more organs in the pelvis slip down from their original position and protrude into the vagina.
POP is a common problem affecting up to 50% of parous women, and 6%-20% of them may undergo surgical treatment for pelvic organ prolapse by the age of 80[1]. The importance of prolapse surgery has increased in the urogynecological field due to higher aging society. In fact, the number of prolapse surgery cases has risen up to the same level of continence surgery.
Rectal prolapse is a condition in which the rectum (in some cases including other pelvic organs) slips down from its original position and protrudes from the anus. Due to the prevalence of laparoscopic surgeries, POP and rectal prolapse have been treated laparoscopically. Indeed, their techniques are similar. In addition to the management of POP, we also discuss the management of rectal prolapse in this study.
POP
Definition
In 1996, the International Continence Society (ICS) first determined a standardized definition of POP condition[2]. ICS's Pelvic Organ Prolapse Quantification (POPQ) examination classified prolapse by measuring the descent of specific segments of the reproductive tract during Valsalva strain. Determining POP based on self-reported symptoms is difficult due to the lack of specification and sensitivity as most symptoms are related to multiple pelvic organs.
Epidemiology
A woman's lifetime risk of surgery for POP is 12%-19% with over 300,000 prolapse surgeries performed annually in the United States.
Lack of vaginal or uterine support is seen in 30%-76% of women presenting routine gynecologic care, with 3%-6% of them with complicated descent beyond the vaginal opening (level of the hymen). One population-based study found that about 3% of 1961 adult women reported symptomatic vaginal bulging.
Other studies reported that Hispanic and European women appear to be at higher risk for POP than those of African, Asian, and other races[3,4].
A recent US study reported similar surgical rates: 0.07% for women aged 18-39, 0.24% for women aged 40-59, and 0.31% for women aged 60-79[5]. Surgical rates drop significantly after age 80.
Etiology
DeLancey has introduced the concept of dividing the connective tissue support of the pelvis into three levels, namely, levels I, II, and III, representing apical, midvaginal, and distal support, respectively (Figure 1)[6]. The upper portion of the paracolpium (level I) consists of uterosacral ligament and cardinal ligament. These ligaments suspend the cervix by attaching them to both lateral sides of the pelvic wall. It is also important to suspend the apex of the vagina prior to hysterectomy. In the middle third of the vagina, the pubocervical fascia attaches to the bladder anteriorly, and the rectovaginal fascia attaches to the rectum. These fascias then attach to the arcus tendineus and fascia of the levator ani muscles (level II). This attachment stretches the vagina transversely between the bladder and the rectum. The pubocervical fascia that supports the bladder is composed of the anterior vaginal wall and its attachment through the endopelvic fascia to the pelvic wall. Similarly, the rectovaginal fascia supports the posterior vaginal wall, preventing the rectum from protruding forward. The vagina's lower third (level III) fuses with the perineal membrane, levator ani muscles, and perineal body, without any involving paracolpium.
Figure 1.
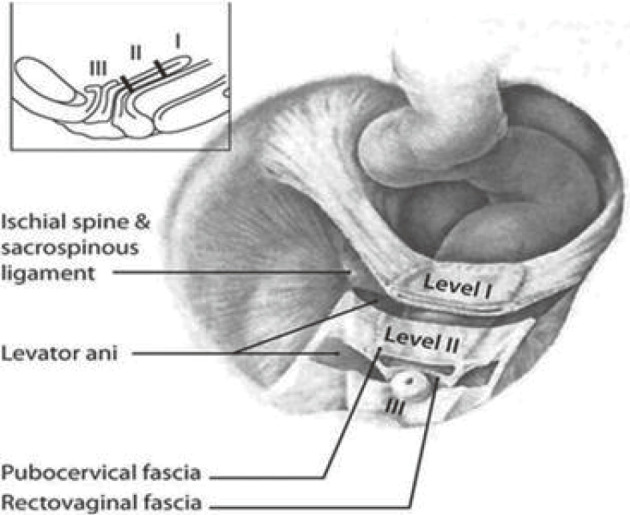
DeLancey’s classification (ref. [6]). Obtained reprint permission.
Causes risk factors
There are several risk factors. The damages of the connective tissues are the highest risk factor. These damages can be triggered by pregnancy and childbirth. Pelvic surgeries also cause connective tissue damages. The connective tissues might become weaker due to aging and menopause. Obesity and heavy lifting give strong pressure to the pelvic organs. Moreover, heavy straining and cough give strong pressure, which might also be risk factors.
Bowel dysfunction is more common in women and includes a wide variety of symptoms, including constipation, rectal emptying difficulties, incomplete defecation, manually assisted defecation, and fecal urgency. However, the prevalence of constipation and its associated symptoms in women with pelvic organ prolapse has been observed in 20%-53% depending on the definition of disorders[7-9]. It is then difficult to say that these symptoms are risk factors or results.
In general, it has widely been accepted that hysterectomy has high risk for POP. Moreover, since there are many types of hysterectomy, there has been no evidence of what has a lower risk of POP.
In a nationwide cohort study, Altman et al. reported that 3.2% of women who had undergone hysterectomy had pelvic organ prolapse surgery. This shows higher morbidity compared with 2.0% in non-hysterectomized controls, corresponding to a risk of 1.7 (95% CI)[10].
According to an Oxford-Family Planning Association study, childbirth was the single strongest risk factor for the development of prolapse in women under 59 years of age, with increased risk for every childbirth. On the other hand, caesarean section decreases the risk for pelvic organ prolapse compared with vaginal birth[2]. Diabetes mellitus, smoking habits, and alcohol consumption are not risk factors; however, these are not good for women's health and increase the risk of postoperative complications.
Symptoms
Although most women with POP often have no symptom, some women experience discomfort, such as unusual pressure or lower abdominal fullness. Bleeding can be seen when the exposed lesion comes into contact with the underwear. In the anterior side, urinary symptoms can be seen, such as urinary incontinence, and frequent urinary tract infections appear. When women feel the difficulty of bowel movements, the need to strain or push on the vagina to have a bowel movement.
These symptoms usually do not appear in the morning but will gradually increase. These symptoms will then be apparent in the evening. Someone may feel like pinching something between the legs.
Examination
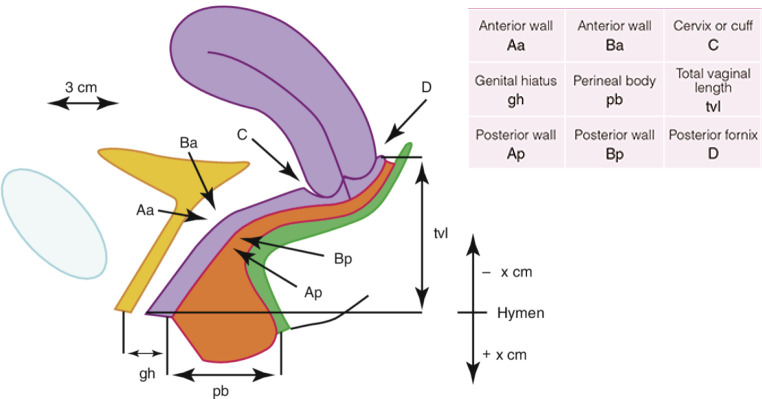
POP is typically diagnosed by a simple pelvic examination during a complete physical examination. Using the Pelvic Organ Prolapse Quantification (POPQ), POP severity is measured (Figure 2)[2], and POP stage is determined (Figure 3)[11]. In addition, other examinations or studies may help assess symptoms associated with prolapse and its conditions.
Figure 2.
Points and landmark for POPQ system examination (ref. [2]). Obtained reprint permission.
Figure 3.
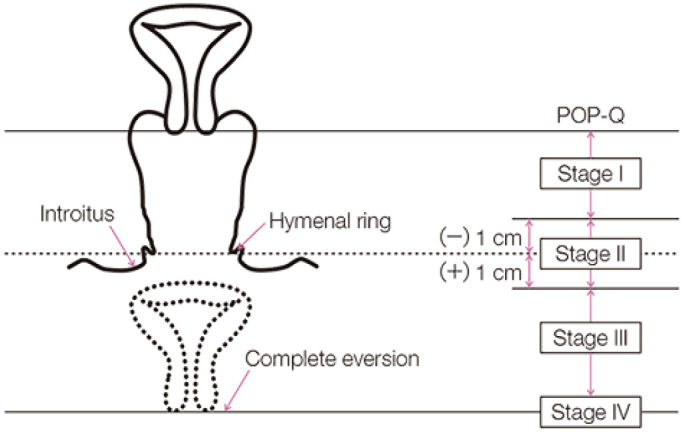
POP-Q staging based on lowest everted lesion (ref. [11]). Obtained reprint permission.
Urodynamics test for bladder function and cystoscopy to look for bladder disease are required for choosing the treatment procedure.
Gynecological palpation and pelvic ultrasound are needed to diagnose gynecological disease and functional disorder.
For coloproctological examination, cine defecography (radiologic study to look at the prolapse associated with bowel function) has been used to identify the degree of prolapsed organs. Motion pelvic floor MRI (radiologic study that assesses the muscles, organs, and support of the pelvic floor) helps to evaluate pelvic floor functions when straining. CT scan of the abdomen and pelvis is useful to rule out malignancy and other diseases. Colonoscopy can be used to check for colorectal malignant diseases.
Treatment
Conservative treatment
Several treatments have been deployed to prevent the prolapse from becoming worse, to decrease the frequency or severity of symptoms caused by the prolapse (pelvic pressure, vaginal bulging, backache, urinary, bowel and sexual dysfunction), and to discard or delay the need for surgery.
Avoiding the heavy pressure to the abdomen (carry the heavy things, constipation, severe cough) and losing body weight may improve prolapse condition. Smoking may have no evidence of an adverse effect for POP, but quitting smoking is very important for the treatment (avoiding cough, improving general condition).
Pelvic floor muscle training (PFMT)
There is no evidence that PFMT is effective in reducing the severity of prolapse based on POP-Q stage. However, regular daily exercising the pelvic muscles can improve and even prevent urinary incontinence. This may be particularly helpful for younger women. Though pelvic floor exercises do not cure the prolapse, they may help control symptoms and limit the worsening of prolapse. Biofeedback therapy is one therapy that uses sensors (anal manometry or surface electromyograph) attached to patients' body to measure key body functions. Usual PFMT cannot confirm the muscle's movement, but biofeedback is intended to help patients learn more about how patients' body works. Biofeedback therapy might be more effective than usual PFMT because they can see how the pelvic floor works correctly.
Pessaries
Prospective case-controlled cohort studies suggest that pessaries are a viable option for women who complain of symptomatic prolapse. Pessaries have many types and sizes, and choosing the correct size and shape is important. However, no significant difference in results was observed between ring pessaries and the Gellhorn pessary.
PFMT with pessary may be concluded to be as effective as PFMT alone in reducing symptoms.
External support
There are some cushioning products to support the perineum externally. It is very easy to fit and wash, but there is no evidence for its effectiveness, and a little bit longer time is needed to take it out.
Surgical treatment
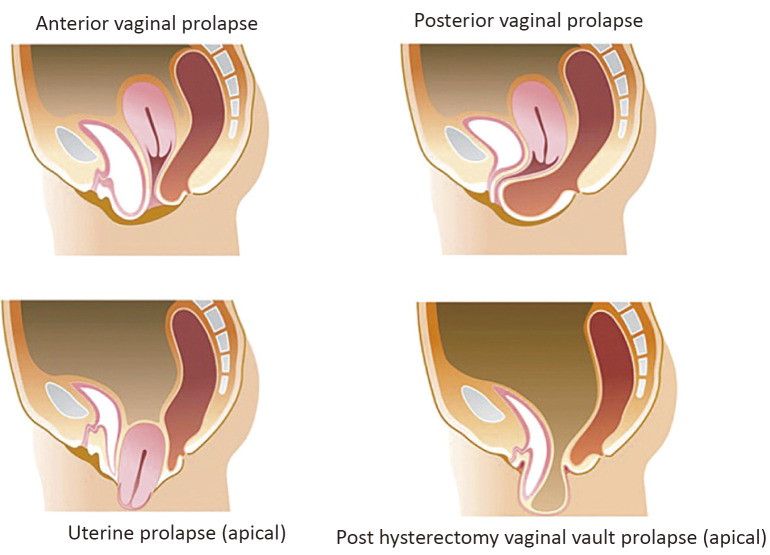
During the examination, anterior compartment prolapse is the most frequently detected, which is twice as many as posterior compartment defects and three times as many as apical prolapse. Following hysterectomy, when vaginal vault prolapse occurs, multi-compartment prolapse is often present. The types of surgeries are divided into two methods: native tissues repair and mesh repair. From the point of surgical approach, they are divided into three types, namely, transvaginal, transanal, and transabdominal. From the point of prolapsed lesion, they are divided into three types, namely, anterior, apical, and posterior lesions (Figure 4).
Figure 4.
Type of pelvic organ prolapse.
Anterior compartment surgery
Native tissue repair
Anterior colporrhaphy is used in cases of bulging anterior vaginal defect.
When the paravaginal defect can be visualized, the paravaginal repair is performed.
Synthetic grafts in anterior compartment surgery
De Tayrac et al. found at 3rd year, in 79 women with grade 3-4 cystocele, the anatomic success rate is 95%, satisfaction rate is 98%, and the mesh exposure rate is 1.3% using lightweight (28 g/m2) polypropylene mesh[12]. However, pain and dyspareunia were the leading causes of adverse events that triggered the 2011 Food and Drug Administration (FDA) warnings on the safety of transvaginal mesh[13].
In the point of anatomical and subjective outcomes, using polypropylene mesh has more improved outcomes compared to anterior colporrhaphy. Moreover, using lightweight polypropylene mesh has very favorable results, as indicated by experienced operators during treatment. However, even if such doctors operate, severe side effects such as greater blood loss and urethral and bladder injury still exist. In the native tissue repair, the procedure is so simple, and the operative field is easy to recognize. Though the success rate could be lower, in total, the native tissue repair has given better results than mesh repair.
Apical compartment surgery
There are numerous options for primary treatment of uterovaginal prolapse. Hysteropexy is reasonable in women undergoing surgery for uterovaginal prolapse. To avoid vaginal prolapse after hysterectomy, apical suspension repair is important. Transvaginal sacrocolpopexy is useful in reducing the risk of recurrent prolapse repeat surgery. McCall culdoplasty, iliococcygeus fixation, and colpocleisis are relatively safe and effective surgical interventions[14].
Tension-free vaginal mesh (TVM) procedure has a concept of total repair and mesh placement without tension; however, fixation in the correct position by fixing the arm to the arcus tendineus fasciae pelvis is difficult. When the arm is fixed to the arcus tendineus fasciae pelvis, we have to find and fix the position blindly. Urethral injury and vascular damage might appear.
In the transabdominal approach, laparoscopic sacrocolpopexy (LSC) (Figure 5)[15] has been able to give lower blood loss, longer operating time, and shorter hospital stay than abdominal sacrocolpopexy. Robotic sacrocolpopexy has longer operating times and greater cost than LSC. Open, laparoscopic, and robotic surgeries have equally given good cure rates.
Figure 5.
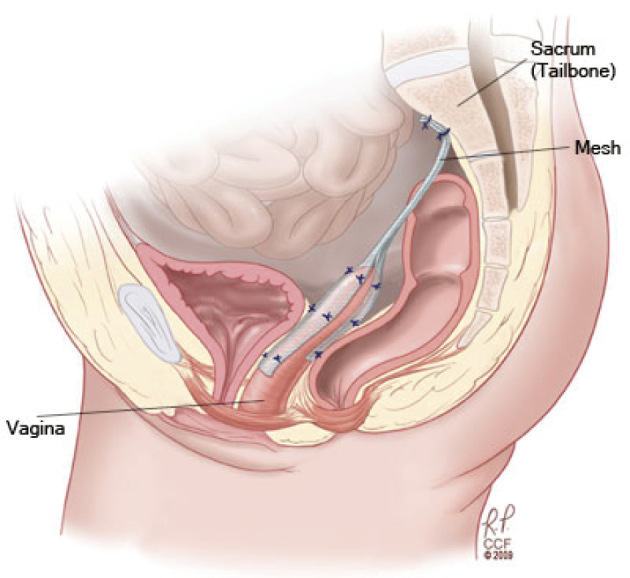
Laparoscopic sacrocolpopexy (ref. [15]). Obtained reprint permission.
Maher et al. reported results from a randomized trial comparing LSC (n = 53) and TVM (Prolift, Ethicon, Sommerville NJ, USA) (n = 55). LSC was associated with longer operating time (mean difference: +52 min), decreased hospital stay (mean difference: −0.5 days), and quicker return to normal activities (mean difference: 5.3 days). Two years after surgery, objective success (overall POP-Q stage 0 or 1) was seen in 77% of the LSC group compared with only 43% of the TVM group. Moreover, reoperations were significantly higher in the TVM group (22%) than in the LSC group (5%, p = 0.006)[16].
Posterior prolapse surgery
Transvaginal repair of posterior vaginal wall defects includes midline fascial plication with or without levatorplasty or site-specific repair.
Transvaginal approach is superior to the transanal approach for repair of posterior wall prolapse. To date, no study has shown any benefit to graft or mesh overlay or augmentation of a suture repair for posterior vaginal wall prolapse[17]. While modified abdominal sacrocolpopexy results have been reported, data on how these results would compare to traditional transvaginal repair of posterior vaginal wall prolapse is lacking. Data are conflicting regarding the efficacy of posterior vaginal repair on improving defecatory symptoms, and the association is understood incompletely[18,19].
Severe complication of pelvic organ prolapse reconstructive surgery is rare; however, once it happens, the recovery from the complication is very difficult, and the severe side effect is a patient's burden, with or without the use of mesh.
Preoperatively, patients should be informed that a mesh is considered a permanent implant, and removal of mesh or correction of mesh-related complications may involve subsequent surgeries. Furthermore, complete removal of mesh may not be possible, and additional surgeries may not fully correct some complications. Patients must also be informed of conservative and alternative surgical techniques. Patients should be informed that transvaginal meshes have a higher reoperation rate than native tissue vaginal repairs. If a synthetic mesh is placed by the vaginal route, it is recommended that a macroporous polypropylene monofilament mesh should be used. Laparoscopic approach is recommended for sacrocolpopexy. The use of polyester (without silicone coating) or polypropylene meshes without coating silicone is recommended.
Our treatment strategy for POP
In our hospital, highly specialized coloproctologists for transvaginal approach treat the POP transvaginally. On the other hand, laparoscopic approach specialists treat the POP laparoscopically. When diagnosed as POP, patients' general conditions are examined. As most patients belong to the elder generation, we examine the existence of the abdominal and pelvic neoplasm. Transvaginal or laparoscopic procedures have been selected for surgical treatments of anterior or apical component surgery. When TVM procedure with transvaginal approach had performed, we preferred to use relatively larger size mesh as the outcome has been favorable. However, due to the FDA's alert, the size of the mesh has gradually become smaller, and the number of native tissue repair cases has gradually increased. At the same time, patients have been better informed concerning conservative and alternative surgical techniques as well as the risk of mesh treatment. On the other hand, LSC cases have increased due to its benefits of safer and easier learning procedure. Urogynecologists and colorectal surgeons work together in cases of LSC. When we operate on the posterior component, conservative treatments are firstly selected in the case of rectocele with outlet obstruction. When conservative treatments had not been completed, the transanal approach has been selected for better operative outcome. In the outlet obstruction cases with rectocele, not only rectocele but also many other causes for the outlet obstruction are related. Therefore, it is very important to figure out the patient's condition correctly and inform the patient of the risk of the surgical treatment. Transvaginal or laparoscopic approach has been applied in the case of stage IV rectocele.
Rectal Prolapse
Like the vagina and uterus, ligaments and muscles securely usually attach the rectum to the pelvis. Rectal prolapse is a condition in which the supporting structures relax or detach from the rectal wall, and the rectum falls out through the anus. Patients may notice a soft, red tissue protruding from the anus after a bowel movement. It can be confused with a large hemorrhoid. Other symptoms may include pain during bowel movements, mucus or blood discharge from the protruding tissue, and loss of control of bowel movements. Unlike the POP, other organ prolapse cannot be seen in the posterior side of the rectum. On the other hand, the sigmoid colon, small intestine, and, very rarely, uterus are protruding in the anterior side of the rectum.
Definition
In general, rectal prolapse is defined as the protrusion of the rectum from the anus. Indeed, there are three types of rectal prolapse, namely, full-thickness rectal prolapse, mucosal prolapse, and internal intussusception.
Full-thickness rectal prolapse is the entire rectal prolapse protruding through the anus. Mucosal prolapse is rectal mucosal prolapse. Internal intussusception is the rectal wall invaginating into the lumen of the rectum, while rectal intussusception does not extrude from the anus.
In terms of rectal prolapse, there seems to be two types, namely, extruding from the anus with full thickness of the rectum and extruding from the anus with only mucosal layer.
Epidemiology
More than 70% of patients with rectal prolapse are women, and the incidence peaks in women older than 70 years old. POP and rectal prolapse may occur concurrently. In recent study, 30~48% of patients treated for rectal prolapse developed genital prolapse at the same time.
Etiology
The etiology of rectal prolapse is still unknown, but there are two major theories. One is Moschcowitz's theory that rectal prolapse occurs as sliding hernia through pelvic fascial defect. Another is Broden and Snellman's theory. They confirmed that rectal intussusception occurred with circumferential rectal intussusception under the defecography observation.
However, neither of them has not been generally accepted.
Risk factors
These are several risk factors, including the long sigmoid colon and rectum, deep Douglas pouch, pelvic floor dysfunction, and increased intra-abdominal pressure due to constipation and straining. In addition, rectal cancer surgery, hysterectomy, pelvic organ prolapse, and mental illness are also considered to be risk factors.
Symptoms
In terms of symptoms, pain during bowel movements and mucous or blood discharge from the protruding intestine have been most commonly observed. Fecal incontinence and no urge for defecation are also commonly observed. Patients experience something protruding upon wiping and also complain constipation, severe anal pain, tenesmus, and obstructed defecation.
Examinations
The following examinations are needed to diagnose rectal prolapse and determine the therapy. The first one is visual inspection to confirm the existence of rectal prolapse. If it cannot be visually confirmed at examination room, straining by sitting position on the Japanese style toilet or taking photo at home is recommended.
The second one is anal function test. To examine the anal sphincters' function, we measure maximum resting pressure and maximum squeeze pressure. The third one is radial-type endosonography to examine the sphincter injury by visualizing rectal mucosa, internal anal sphincter, conjoined longitudinal muscle, and external anal sphincter.
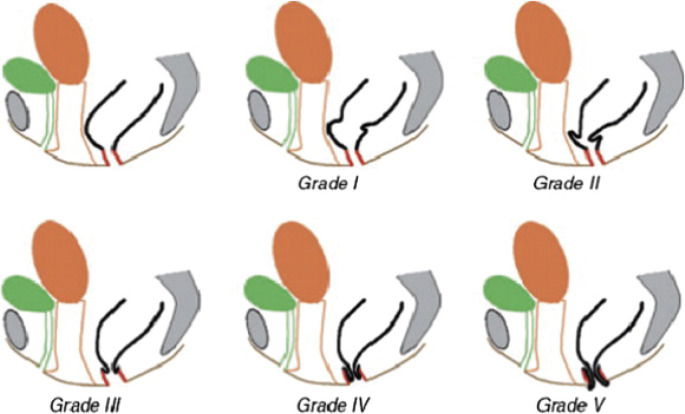
The fourth one is defecation test. Defecography uses 200 ml of soft stool-like barium paste to be injected into the anus. According to the results of defecography, we determine the grade of prolapse based on the Oxford prolapse grade (Figure 6)[20]. To measure transit time of stool in the large intestine, we use SITXMARKSⓇ. MRI is feasible for observing bowel movement and detecting the abnormality of other adjacent organs.
Figure 6.
The Oxford Prolapse Grade for proctographic grading of internal and external rectal prolapse (ref. [20]). Obtained reprint permission.
In addition, a series of tests must be performed to identify complicating illness such as colorectal cancer and other pelvic organ prolapse. For this purpose, CT scan, colonoscopy, and esophagogastroduodenoscopy are deployed.
Treatments
Data comparing Delorme's procedure and laparoscopic ventral rectopexy (LVR)(Figure 7) for external rectal prolapse are conflicting with a single RCT demonstrating no statistical difference, while level 3 data is supportive of LVR performed laparoscopically or robotically, with low rates of recurrent rectal prolapse and improved rates of fecal incontinence and constipation. Ventral mesh repair appears superior to other abdominal rectopexies (posterior mesh rectopexy, Ripstein, Orr-Loygue) with different rectal mobilizations to treat rectal prolapse in terms of functional outcome. Patients with combined rectal and vaginal prolapse benefit from colorectal surgeons and urogynecologist collaborating closely.
Figure 7.
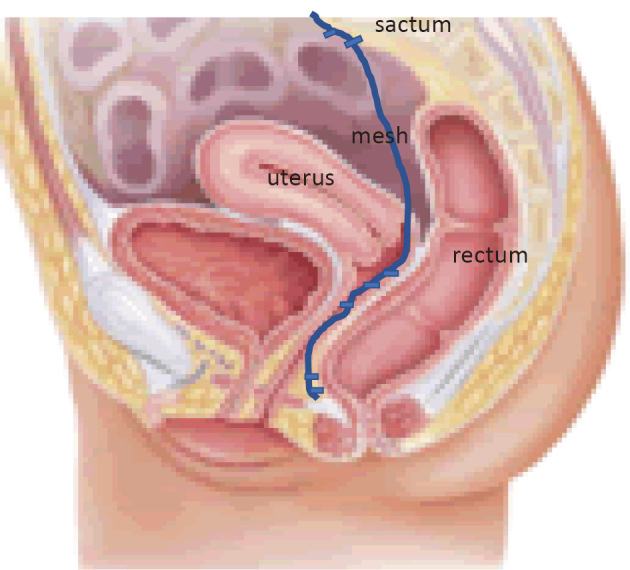
Laparoscopic anterior rectopexy.
Our strategies
This is how we determine operative procedures for the treatments of rectal prolapse. Since the etiology is unknown, we determine the procedure depending on the length of the prolapsed rectum. As the length of prolapsed rectum in many cases was about 3 cm long, we have mostly performed Delorme's procedures. In cases of longer rectal prolapse with 3 cm or longer, we apply laparoscopic rectopexy. If the patient's general condition is poor, a combination therapy of sclerosing therapy and Thiersch's operation is applied (Figure 8).
Figure 8.
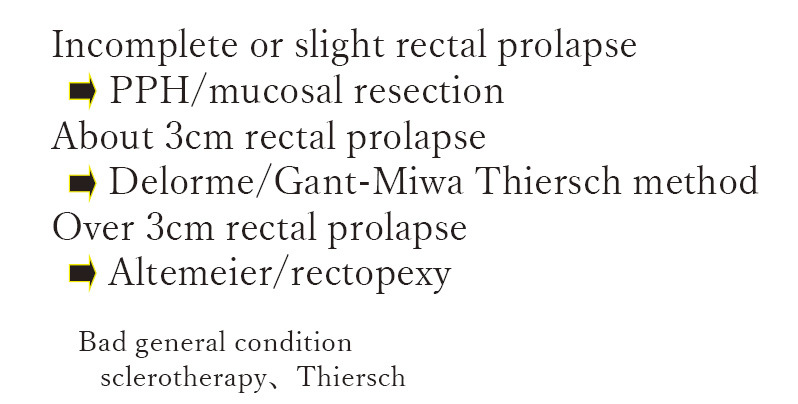
Indications of rectal prolapse.
For younger patients, under 20s, Delorme's procedure has mainly applied to control defecation.
In cases of laparoscopic surgeries, for patients under 60s, laparoscopic sutured rectopexy has been mostly applied, because these patients have higher risk of developing rectal cancer, prostate cancer, and uterine cancer.
We have selected laparoscopic ventral rectopexy in elderly patients and patients with mental illness (Figure 9).
Figure 9.

Indications of rectal prolapse by ages.
In pelvic organ prolapse complicated cases, laparoscopic anterior rectopexy and sacrocolpopexy have been applied simultaneously. In the perineal approach, TVM and Delorme procedure have been selected, with favorable results.
We have rarely chosen laparoscopic posterior rectopexy. But in cases of high risk of prostate cancer, laparoscopic posterior rectopexy has been reluctantly applied.
Conclusion
POP and rectal prolapse may occur in all generation, whereas most patients are in elder age especially over 70s. The reason is considered that the number of healthy elderly has increased. POP and rectal prolapse may deteriorate the patients' QOL. Unfortunately, these diseases may be more common in the near future. Therefore, we should develop more effective treatments by gathering out experience and data.
Conflicts of Interest
There are no conflicts of interest.
Disclaimer
Junichi Koike is one of the Associate Editors of Journal of the Anus, Rectum and Colon and on the journal's Editorial Board. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997 Apr; 89(4): 501-6. [DOI] [PubMed] [Google Scholar]
- 2.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996 Jul; 175(1): 10-7. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HP. Do Asian women have less pelvic organ mobility than Caucasians? Int Urogynecol J Pelvic Floor Dysfunct. 2003 Oct; 14(4): 250-3. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Harvey MA, Johnston S. A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynaecol Can. 2005 Mar; 27(3): 251-9. [DOI] [PubMed] [Google Scholar]
- 5.Bradley CS, Zimmerman MB, Qi Y, et al. Natural history of pelvic organ prolapse in postmenopausal women. Obstet Gynecol. 2007 Apr; 109(4): 848-54. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992 Jun; 166(6 Pt 1): 1717-24. [DOI] [PubMed] [Google Scholar]
- 7.Jelovsek JE, Barber MD, Paraiso MF, et al. Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. Am J Obstet Gynecol. 2005 Dec; 193(6): 2105-11. [DOI] [PubMed] [Google Scholar]
- 8.Kahn MA, Breitkopf CR, Valley MT, et al. Pelvic organ support study (POSST) and bowel symptoms: straining at stool is associated with perineal and anterior vaginal descent in a general gynecologic population. Am J Obstet Gynecol. 2005 May; 192(5): 151622. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DM, DeLancey JO, Guire KE, et al. Symptoms of anal incontinence and difficult defecation among women with prolapse and a matched control cohort. Am J Obstet Gynecol. 2007 Nov; 197(5): 509 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman D, Falconer C, Cnattingius S, et al. Pelvic organ prolapse surgery following hysterectomy on benign indications. Am J Obstet Gynecol. 2008 May; 198(5): 572.e1-6. [DOI] [PubMed] [Google Scholar]
- 11.Theophrastus JP, Swift SE. The clinical evaluation of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998 Dec; 25(4): 783-804. [DOI] [PubMed] [Google Scholar]
- 12.Mant J, Painter, R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford family planning association study. Br J Obstet Gynaecol. 1997 May; 104(5): 579-85. [DOI] [PubMed] [Google Scholar]
- 13.de Tayrac R, Brouziyne M, Priou G, et al. Transvaginal repair of stage III-IV cystocele using a lightweight mesh: safety and 36-month outcome. Int Urogynecol J. 2015 Mar; 26(8): 1147-54. [DOI] [PubMed] [Google Scholar]
- 14.FDA. Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse 2011 15/10/2013; (July 13, 2011):[1-15 pp.]. Available from: http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf.
- 15.Clifton MM, Pizarro-Berdichevsky J, Goldman HB. Robotic female pelvic floor reconstruction: a review. Urology. 2016 May; 91: 33-40, 2016. Elsevier [DOI] [PubMed] [Google Scholar]
- 16.Colombo M, Milani R. Sacrospinous ligament fixation and modified McCall culdoplasty during vaginal hysterectomy for advanced uterovaginal prolapse. Am J Obstet Gynecol. 1998 Jul; 179(1): 13-20. [DOI] [PubMed] [Google Scholar]
- 17.Maher C, Feiner BM, DeCuyper E, Nichlos C, et al. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011 Apr; 204(4): 360e1-7. [DOI] [PubMed] [Google Scholar]
- 18.Grimes CL, Tan-Kim J, Nager CW, et al. Outcome measures to assess anatomy and function of the posterior vaginal compartment. Int Urogynecol J. 2014 Jan; 25(7): 8939. [DOI] [PubMed] [Google Scholar]
- 19.Grimes CL, Lukacz ES. Posterior vaginal compartment prolapse and defecatory dysfunction: are they related? Int Urogynecol J. 2012 Jan; 23(5): 537-51. [DOI] [PubMed] [Google Scholar]
- 20.Christopher H, Oliver J. The evolution of laparoscopic surgery for rectal prolapse. Int. J. Surg. 2011 Dec; 9(5): 370-3. [DOI] [PubMed] [Google Scholar]