Abstract
Introduction
Coronavirus disease of 2019 (COVID-19) has resulted in millions of cases worldwide. As the pandemic has progressed, the understanding of this disease has evolved. Its impact on the health and welfare of the human population is significant; its impact on the delivery of healthcare is also considerable.
Objective
This article is another paper in a series addressing COVID-19-related updates to emergency clinicians on the management of COVID-19 patients with cardiac arrest.
Discussion
COVID-19 has resulted in significant morbidity and mortality worldwide. From a global perspective, as of February 23, 2022, 435 million infections have been noted with 5.9 million deaths (1.4%). Current data suggest an increase in the occurrence of cardiac arrest, both in the outpatient and inpatient settings, with corresponding reductions in most survival metrics. The frequency of out-of-hospital lay provider initial care has decreased while non-shockable initial cardiac arrest rhythms have increased. While many interventions, including chest compressions, are aerosol-generating procedures, the risk of contagion to healthcare personnel is low, assuming appropriate personal protective equipment is used; vaccination with boosting provides further protection against contagion for the healthcare personnel involved in cardiac arrest resuscitation. The burden of the COVID-19 pandemic on the delivery of cardiac arrest care is considerable and, despite multiple efforts, has adversely impacted the chain of survival.
Conclusion
This review provides a focused update of cardiac arrest in the setting of COVID-19 for emergency clinicians.
Keywords: Coronavirus-2019, COVID-19, COVID-19 infection, Cardiac arrest, Resuscitation
1. Introduction
Cardiac arrest is a major public health issue, associated with low rates of survival, poor neurologic outcome among survivors, and high resource utilization in both prehospital and hospital environments [1,2]. Despite extensive research and substantial clinical effort, survival rates for out-of-hospital cardiac arrest (OHCA) range from 3 to 10.4%; in-hospital cardiac arrest (IHCA) survival rates range from 12.4–26.4% [[2], [3], [4], [5], [6]]. In the out-of-hospital environment, cardiac issues are the primary etiology, with the majority of OHCA occurring as a result of acute coronary syndrome and the sequelae of past myocardial infarction, referred to as cardiogenic cardiac arrest [7]. Among persons with IHCA, the range of etiologies is broader, consisting of approximately 60% cardiogenic and 40% non-cardiogenic [8].
The challenges faced and difficulties associated with cardiac arrest care have been further compounded by the COVID-19 pandemic. The first outbreak was reported in late 2019 in Wuhan, China before spreading rapidly around the globe, reaching pandemic status on March 11, 2020 [[9], [10], [11], [12]]. As of February 23, 2022, 429 million cases have been diagnosed worldwide with 5.9 million deaths [13]. In the COVID-19 pandemic era, cardiac arrest is occurring more often and is associated with an extremely high mortality rate (as much as 100% for COVID-19 patients experiencing IHCA) and poor resultant functional status (6.3% with good neurologic outcomes at 30 days; 95% confidence interval [CI] 0.4–9.7%) [[14], [15], [16], [17], [18]].
COVID-19 may result in cardiorespiratory compromise in those with severe disease and those with underlying cardiorespiratory conditions [19]. Fear of contagion and limited resource availability have impacted patient access to medical care [[19], [20], [21]]. Furthermore, the high demand for service has understandably delayed urgent treatment, which may result in cardiac arrest [20]. In fact, many centers have reported a reduction in the presentation of ST segment elevation myocardial infarction (STEMI) and cerebrovascular accident (CVA) during the pandemic, with many of these patients staying home with their illness and potentially risking cardiac arrest [21].
For OHCA, the incidence of OHCA has increased while survival rates have declined commensurately with COVID-19 in several studies, which may be due to several important factors [11,[22], [23], [24], [25], [26]]. Due to concerns of viral spread, bystanders may be less willing to intervene in OHCA [[27], [28], [29], [30]]. Because of social distancing and quarantines, the likelihood of a witnessed arrest occurring in public, with a knowledgeable bystander capable of performing CPR and/or applying an automated external defibrillator (AED) may have decreased [30]. Additionally, according to one meta-analysis, field terminations (odds ratio [OR] = 2.46, 95% CI 1.62–3.74) and asphyxial etiologies (OR = 1.17, 95% CI 1.02–1.33) have increased, while rates of shockable rhythm (OR = 0.73, 95% CI 0.60–0.88), ROSC (OR = 0.65, 95% CI 0.55–0.77), and survival to hospital admission (OR = 0.55, 95% CI 0.48–0.89) have decreased compared to the pre-pandemic period [30].
Similarly, the COVID-19 pandemic has adversely affected IHCA care and outcomes. Prior to the COVID-19 era, significant variation existed among hospitals regarding IHCA care and survival outcomes; the pandemic has only accentuated this variation [31]. IHCA is also more likely to be associated with a non-shockable rhythm (76.5%) and occur in the ICU (73.7%) [32]. Factors such as emphasis on healthcare personnel use of personal protective equipment (PPE) prior to contact, increased patient volume, and strained resources impact response times and subsequent outcomes [17,31].
2. Methods
A literature review of PubMed, Ovid Medline, and Google Scholar databases was performed for articles up to January 31, 2022, using the keywords ‘COVID’ OR ‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘coronavirus’ AND ‘cardiac arrest’ OR ‘heart arrest’ for this narrative review. The authors included retrospective and prospective studies, systematic reviews and meta-analyses, clinical guidelines, and other narrative reviews. Commentaries and letters were also included. The literature search was restricted to studies published in English. Authors reviewed all relevant articles and decided which studies to include for the review by consensus, with a focus on emergency medicine-focused and -relevant articles, including guidelines. At least two authors reviewed each included article with an additional author added if disagreement occurred (in fact, no disagreement occurred in this article selection process). Initially, 187 articles were identified by manuscript title review; 82 articles were removed from consideration based upon abstract and/or full text review. A total of 105 references were ultimately selected for inclusion and used in the development of this review.
3. Discussion
3.1. Epidemiology, demographics, and prognosis
In both OHCA and IHCA, the outcome of cardiac arrest has worsened during the COVID-19 pandemic with lower survival and optimal neurologic status among survivors. Unfortunately, despite an increased understanding of COVID-19 and its impact on the human body, the general trend from early to later in the pandemic has not shown a significant improvement in survival and/or neurologically intact survival over time for the cardiac arrest victim [24,[33], [34], [35]]. This phenomenon was well-described early in the pandemic in numerous regions of the world, including the United States, Italy, and France [24,33,35,36]. The pooled annual incidence of OHCA increased by as much as 39.9% (p < 0.001); with this increased rate of occurrence, other key metrics significantly worsened, including reduced rates of return of spontaneous circulation (ROSC; OR = 0.65, 95% CI 0.55–0.77), frequency of shockable cardiac arrest rhythms (OR = 0.73, 95% CI 0.60–0.88), survival to hospital admission (OR = 0.65, 95% CI 0.48–0.90), and hospital discharge (OR = 0.52, 95% CI 0.40–0.69) [30].
Beyond the initial wave of the pandemic, the literature reports that each subsequent surge, while there has been an observed increase in cardiac arrest in the out-of-hospital setting, this rise has not been proportional to the magnitude of COVID-19 infection in many areas [37]. This evidence appears to be conflicting, with some authors reporting increases in OHCA incidence (Denver and Lombardy, Italy) while others report either no change or a decrease in subsequent surges (Paris and southern Germany) [24,34,37,38]. This discrepancy likely results from numerous factors, including differences in system capability, geographic variation in COVID-19 burden, and local public measures.
3.1.1. Out-of-hospital cardiac arrest
According to a systematic review by Lim et al., OHCA has increased by 120% since the start of the pandemic, with increased mortality (OR = 0.67, 95% CI 0.49–0.91) [39]. This increase in incidence has been demonstrated worldwide [23,35,40]. When comparing patient characteristics during the COVID-19 pandemic to the prepandemic time, it is clear that epidemiologic patterns have changed. Patients were more likely to be non-White or Black, male, and older [35,41,42]. Other common features of OHCA during the pandemic include a greater share of patients presenting with asystole initially, arrest occurring at home, and mechanism classified as “other”, which would include presumptive COVID-19 infection [39,41]. The proportion of resuscitation attempts did not differ [39]. Before the pandemic, patients were more likely to achieve ROSC and be intubated while mechanical CPR device usage did not differ significantly [39]. Australian authorities reported a reduction in pre-arrival care as well as delays in delivery of several key EMS interventions including time-to-first defibrillation and time-to-first epinephrine [43].
OHCA patients with COVID-19 demonstrated higher 30-day mortality (OR = 3.40, 95% CI 1.31–11.64) compared to those without COVID-19 and lower adjusted 30-day survival (4.7% with COVID-19 versus 9.8% COVID-19 negative) [44]. It is important to note OHCA cases may be underestimated due to limitations in data collection [45] and paucity of viral testing. There are conflicting studies regarding changes in bystander CPR and defibrillator usage, either remaining unchanged or decreasing throughout the pandemic [30,39,41,46].
3.1.2. In-hospital cardiac arrest
As with outpatient cardiac arrest, inpatient experiences revealed a similar increased incidence of IHCA and poorer survival rates [33,47,48]. Among patients with COVID-19, the incidence of IHCA for non-ICU patients was 2.2% and 15.4% for ICU patients [48]; cardiac arrest most often occurred on hospital day 4 [18,26]. For IHCA patients with COVID-19 infection, ROSC varied from 13.2–54%, survival to hospital discharge ranges from 0 to 12%, and good neurologic outcomes 0–0.7% [14,15,17,44,[48], [49], [50], [51]]; when considering inpatient location of cardiac arrest, survival to hospital discharge was worse for non-ICU patients (0.7%) versus ICU patients (9.1%) [48]. As noted in the OHCA population, co-morbid conditions also occurred with greater frequency among inpatients [49]. The majority of IHCA patients presented with a non-shockable rhythm (89%) [18]. Multiple studies have noted an age association with the occurrence of IHCA; in fact, the incidence of cardiac arrest increased relative to age with each successive decade of life, peaking in the seventh decade [18,49].
A study examining patients who died from COVID-19 in the hospital found 50% of those who died were appropriately admitted to a non-ICU level of care [52]. Patients appeared well early on, with 56.1% of patients not requiring any additional oxygen support [52]. Concerningly, before an inpatient rapid response activation or cardiac arrest team was alerted, 76.6% of non-ICU patients had a most recent oxygen saturation of at least 90%; by the time the care team was alerted, however, 43.1% were hypoxic to less than 80% [52]. Rapid deterioration appears to be more frequent in these patients, thus close clinical monitoring, if available, would likely benefit these individuals [52]. Similar findings in the early pandemic with higher rates of IHCA in general wards – compared to pre-pandemic periods – has been reported [17,33,47]. Sparse ICU bed availability is associated with higher case fatality rate from COVID-19 [53].
3.2. Pathophysiology
Several general pathophysiologic scenarios are seen in the COVID-19-related cardiac arrest victim [18,26,27,32,54,55]. COVID-19 can directly cause cardiac arrest through acute respiratory distress syndrome as well as an exaggerated immune response with cytokine storm, cardiovascular injury, and myocarditis [54,56]. In addition, the SARS-CoV-2 virus binds to ACE-2 receptors in the myocardium, leading to cardiomyocyte lysis and subsequent injury [56].
The most readily understood and common process involves viral pneumonia with the development of adult respiratory distress syndrome, progressive hypoxia, and acidosis with resultant cardiac arrest [17,26,30,44,55,57]. A variant of this process includes respiratory failure and associated other organ system malfunction, producing multiorgan failure syndrome and ultimately cardiac arrest [26]. Another pathophysiologic scenario includes primarily cardiac involvement with the development of myocarditis, myocardial injury, and/or myocardial infarction; in any of these myocardial ailments, hypoxia related to cardiac failure, acidosis resulting from poor perfusion, and irritability of the myocardium contribute to the development of cardiac arrest [54,55]. Other acute cardiac events in COVID-19 patients, such as decompensated heart failure and arrhythmias, can result in cardiac arrest [27,54,55]. In addition, certain medications which have been taken for COVID-19, such as hydroxychloroquine or azithromycin, might also increase OHCA risk due to QT prolongation, especially in subjects with pre-existing cardiac disease [54,58]. Moreover, the prothrombotic state that has been reported in patients with COVID-19 can lead to pulmonary emboli [32,56,59,60].
In a study of 40 consecutive post-mortem examinations of patients experiencing fatal cardiac arrest related to COVID-19, three distinct pulmonary pathologic patterns were noted, including acute lung injury (ALI) in 73%, intravascular fibrin and platelet aggregates in 90%, and pulmonary congestion (vascular congestion and hemangiomatosis-like change; VCHL) in 50% [61]. Many patients experienced more than one type of pulmonary pathology; furthermore, a subset of patients did not demonstrate acute lung injury, categorized as non-ALI [61]. When comparing clinical data to post-mortem findings, patients without the ALI pattern had a shorter hospital course prior to cardiac arrest, chest imaging without major consolidation, and no pathologically determined cause of death. These patients potentially experienced a primary cardiogenic process with sudden malignant dysrhythmia, cardiac arrest, and death or possible vascular cause including microthrombi [61].
3.3. Clinical presentation
Patients presenting with OHCA are, by definition, critically ill from the start; little information is typically available to the care team as they initiate resuscitation. When compared to pre-pandemic data, patients experiencing OHCA during the COVID-19 pandemic more often were in non-public locations and were unwitnessed [39,41]. In the subgroup of patients who experienced cardiac arrest after calling for EMS assistance, the most frequent chief complaints were dyspnea, cough, fever, chest pain, altered level of consciousness, and “sick person” [39,41]. Prior to the onset of EMS-witnessed cardiac arrest, these patients frequently demonstrated respiratory distress with sinus tachycardia, tachypnea, and low oxygen saturations. After EMS evaluation, oxygen saturations in the lower 80% range and less, while not predictive of cardiac arrest, were seen frequently in these patients. Non-shockable cardiac arrest rhythms, including asystole and pulseless electrical activity, were encountered much more often [39,41].
This increase in non-shockable rhythms [62] in the out-of-hospital setting could result from several issues, including delayed EMS response times, more frequent unwitnessed events, and less frequent application of pre-arrival care by bystanders; furthermore, a likely increase in non-cardiogenic cardiac arrest physiology (i.e., respiratory mediated with hypoxia, hypercarbia, and/or acidosis) likely also contributes to this higher rate of pulseless electrical activity and asystole.
Among patients with IHCA, more medical information is usually available regarding the peri-arrest period. The location of cardiac arrest is the ICU in most instances, although many studies report an increase in IHCA on non-critical care units; IHCA less often occurred in the emergency department, compared to other inpatient locations. Two particular patterns of cardiac arrest occurrence are noted relative to the duration of illness: early in the hospital course, on days 2 to 4 and later, on days 11 to 14 [14,15,17,50,51]. Patients who experience cardiac arrest early in the hospital course tend to be admitted to non-critical care units and have rather sudden decompensation. In fact, one study demonstrated that patients admitted to non-ICU level of care suffered rapid clinical deterioration, often with a sudden decrease in oxygen saturation, immediately prior to cardiac arrest [52]. Cardiac arrest later in the hospital course more frequently occurs in the ICU; these patients are often already receiving maximal supportive care with mechanical ventilation, vasopressor infusion, and hemodialysis. Either significant multilobar pneumonia or adult respiratory distress syndrome is frequently present; these patients tend to have more gradual, additional decompensation, leading to cardiac arrest. As would be expected among inpatients with multiorgan failure, significant hypoxic, hypercarbic respiratory failure, and non-shockable rhythms are common with pulseless electrical activity predominating [14,15,17,50,51].
3.4. Systems of care – prehospital and hospital considerations
As is widely known, a primary strategy in the management of the OHCA patient is known as the “chain of survival” concept, which emphasizes a system-of-care approach that includes early access to care and consists of five key links: (1) recognition of cardiac arrest and activation of the response system; (2) immediate cardiopulmonary resuscitation; (3) rapid defibrillation of shockable cardiac arrest rhythms; (4) EMS or resuscitation team rapid response; and (5) advanced life support care [7,22,62].
While each link is important, considerable investigation in cardiac arrest resuscitation has demonstrated that the most important links in the chain of survival are the earliest ones — recognition of cardiac arrest, initiation of CPR, and application of an automatic external defibrillator (AED) – all of which are performed largely by lay bystanders in the out-of-hospital setting and non-resuscitation team members in the hospital environment [[63], [64], [65], [66]]. COVID-19 has adversely affected patient outcomes as a function of decreased access to care and the alteration of prehospital- and hospital-based healthcare systems [67]. Even prior to the pandemic, bystanders were reluctant to initiate CPR, apply an AED, or perform other appropriate pre-arrival care; due to fears of contagion during the COVID-19 pandemic, this reluctance has increased in magnitude. Beyond these barriers to pre-arrival care, the COVID-19 pandemic has had many other impacts on cardiac arrest systems of care, including direct and indirect medical effects on patients as well as psychosocial and ethical challenges to responders. Marijon et al. reported a 6% absolute reduction in pre-arrival care by bystanders [36]. A query of the National Emergency Medical Services Information System (NEMSIS) revealed a reduction in pre-arrival care, with some communities reporting up to an 8.9% reduction in bystander-delivered pre-arrival care [43]. A systematic review from the first year of the pandemic noted that the rates of bystander CPR were significantly lower with an odds ratio of 0.52 when compared to the prior year; this reduction in pre-arrival care has continued throughout subsequent phases of the pandemic [30]. Multiple other studies performed across a range of communities around the globe have demonstrated similar findings [14,15,50,51].
The adverse impact on pre-arrival care requires alteration of pre-existing plans and adaptation to local issues, such as available resources, medical needs, and COVID-19 prevalence. For instance, the Paris Fire Brigade initiated a comprehensive plan to protect healthcare personnel and maximize patient outcomes. Modifications to multiple levels and phases of the OHCA response have included the following: (1) emergency communication center dispatcher instructions to responders to open windows and doors at the scene whenever possible to mitigate respiratory aerosols and initiate hands-only CPR; (2) realization that many public access AEDs were not available due to mandatory business or building closures; (3) reduction in the number of responding personnel; (4) EMS crew donning of all personal protective equipment (PPE) prior to initiating the response; (5) use of mechanical CPR devices; and (6) physician-performed endotracheal intubation using video technique. An early additional portion of this plan, which has been curtailed, is a de-activation of smartphone-based applications aimed at community notification of cardiac arrest [68]. Of course, many of these changes are possible in most US-based EMS systems, except physician presence on scene.
Considering the early impact of social isolation and mandatory building closures, a Canadian study compared AED accessibility measured by density of foot traffic for a range of locations with reported AEDs. They noted that 69.9% of public access AEDs were completely inaccessible, 18.8% were partially inaccessible, and 11.3% were unaffected (i.e., accessible). They. noted that recreational parks, retail and recreation locations, and workplaces experienced the greatest reduction in AED accessibility; they also reported that the greatest discrepancies between foot traffic levels and AED accessibility occurred in parks, retail and recreation locations, and transit stations. These investigators identified yet another significant adverse consequence of the COVID-19 pandemic – inaccessibility of AEDs due to voluntary and/or mandated closures of building; furthermore, this inaccessibility not only occurred in areas with markedly reduced presence of persons but also in locales where foot traffic was not reduced [69].
For in-hospital resuscitation, all such patients should be assumed to have COVID-19 until proven otherwise – this statement is universally appropriate for all ED patients and likely appropriate for most hospitalized patients with the noted exception that accurate COVID-19 testing may have been recently performed. It must be stressed that patients with both asymptomatic and symptomatic COVID-19 infection are sources of contagion to the healthcare team [70].
The patient experiencing cardiac arrest with suspected COVID-19 should immediately be placed in an airborne infection isolation room (AIIR) if such a resource is avaialbe, also referred to as a negative pressure room; alternatively, if not possible, a private room with a closed door is strongly encouraged. During the resuscitation of cardiac arrest, healthcare personnel may be required to perform aerosol generating procedures (AGPs) such as bag-mask ventilation, airway suctioning, endotracheal intubation (ETI), and chest compressions (Table 1 ). Performing AGPs in an AIIR reduces cross-contamination among staff and patients outside the room; importantly, resuscitation team personnel can still be exposed to and contaminated with viral particles in these rooms. While many healthcare facilities have developed novel alterations to existing patient care spaces with the ability to convert to negative pressure status, these rooms are still a limited resource. Furthermore, as seen in many instances of IHCA related to COVID-19, [52] sudden deterioration is common, making it difficult to predict the likelihood of cardiac arrest and pre-placement of such patients in an AIIR.
Table 1.
Aerosol-generating procedures during cardiac arrest resuscitation.
|
PPE should be worn by all team members and donned prior to entry into the resuscitation room or immediate area, regardless of patient condition [71,72]. Drawing on the experiences of the Paris Fire Brigade, fire crews would don all PPE prior to initiating the response; the placement of PPE – in this case, gloves, N95 respirators, eye protection, gowns, and overshoes – required approximately one minute [73]. Protocols describing minimum PPE needed to perform resuscitation-related AGPs, as recommended by the Centers for Disease Control and Prevention (CDC) and the American Heart Association's (AHA) updated Advanced Cardiac Life Support (ACLS) guidelines, [71,72,74] have been proposed yet these recommendations are based on low-quality evidence [75]. Healthcare personnel should don the following PPE before entering the resuscitation room or area, regardless of the patient's current condition: respiratory protection [e.g., N95 respirator, P-100 respirator, powered air purifying respirator (PAPR)], eye protection (goggles or full-face shield), long-sleeve gown (covering forearms to the wrist), and gloves. Much of this information is based on experience with other airborne infectious agents, demonstrating that many healthcare personnel have not developed infection during the clinical care of patients involving AGPs when suitably protected [70,75]. While a P-100 respirator or PAPR may provide more protection than a properly fitted N95 respirator, they may negatively impact the provider's ability to communicate with other team members and perform various manual tasks [71,72].
The number of IHCA team members allowed to enter the room and engage in care must be limited. It is also appropriate to limit the number of healthcare personnel in the resuscitation room, primarily to reduce the number of COVID-19-exposed individuals but also to rationally use (and thus conserve) PPE. The actual number of team members in the resuscitation room will vary based upon several factors, including local personnel resource availability, practice patterns, and patient needs. Immediately outside the resuscitation room or area, additional personnel can be stationed, including a pharmacist, nurse serving as a safety officer, and patient care technician for other duties as needed [[70], [71], [72]].
Any equipment or personnel that do not require immediate entry into the patient room should remain in the hallway for prompt access. For example, the code cart with appropriate medications and supplies can remain in the hallway immediately adjacent to the doorway. The safety officer or other assigned personnel can provide rapid access to any requested medications, supplies, or equipment. With this approach, the code cart and its contents do not need to be discarded afterwards due to contamination. Conversely, the monitor-defibrillator and appropriate cables for monitoring and delivery of therapy likely will require placement in the room, adjacent to the patient; after care has been rendered, this device will need to be decontaminated [70].
3.5. Cardiac arrest management
In the approach to cardiac arrest during the pandemic, it should be assumed that the patient has COVID-19 infection with the potential for contagion – this statement includes not only patients with known COVID-19 but also those of either suspected or unknown infection status. The priorities of resuscitation of the patient have not changed; the primary components include appropriate circulation, provision of an adequate airway, and supporting sufficient oxygenation and ventilation (the C-A-B approach with “C” for circulation, “A” for airway, and “B” for breathing). The means and methods of addressing these priorities, however, have changed somewhat. These changes are based not only on patient care needs but also on the mandate to protect team members from COVID-19 infection. Recent guidelines have been published for both OCHA and IHCA [71,72]. Table 2 provides the key points for resuscitation in cardiac arrest in the COVID-19 era.
Table 2.
Considerations in Cardiac Arrest
Contagion reduction
|
Resuscitation
|
3.5.1. Circulation
Interventions aimed at achieving best-possible circulation include chest compressions, defibrillation of shockable rhythms, bolus-dose medications, and intravenous fluid administration. Of these interventions, chest compressions are the essential initial treatment for any patient in cardiac arrest; it is important to note that chest compressions are considered an AGP [72,76]. As has been known for many decades, high-quality compressions with limited interruptions are life-saving and have a significant impact on increasing neurologically intact survival; this observation is still valid during the current pandemic. In fact, it is recommended that chest compressions be started as soon as possible [72]. Mechanical compression devices have been suggested as appropriate for certain cardiac arrest venues, including the resource-limited scenarios and a bridge to extracorporeal membrane oxygenation [72,76]. Such devices would significantly reduce the number of team members in the resuscitation room and thus limit the potential for contagion. [17,70,77] In addition, several simulation studies have demonstrated that the ability to perform CPR and other life-saving procedures is compromised when PPE is in place. It is recommended that additional compressors be available, if possible, due to the enhanced fatigue associated with PPE-performed chest compressions [72,[78], [79], [80]].
A successful strategy aimed at improving oxygenation and ventilation is patient proning, both with and without mechanical ventilation. This approach has been demonstrated to reduce morbidity and mortality in critically ill COVID-19 patients with acute hypoxic respiratory failure [81,82]. Several mechanisms responsible for this improved outcome have been postulated, including improved expansion of lung tissue with increased ability to oxygenate as well as enhanced management of pulmonary secretions. It is not surprising to note that certain critically ill patients placed in the prone position develop cardiac arrest and require chest compressions, among other interventions. The rapid application of chest compressions and defibrillation is critically important, particularly early in cardiac arrest. In turning the cardiac arrest victim to a supine position, delays in treatment are encountered as well as safety issues for the patient (i.e., dislodging endotracheal tube [ETT]) and healthcare personnel exposure to contagion.
There is limited data and recommendations concerning resuscitation for patients in the prone position [76,82,83]. These three formatted reviews did not identify sufficient evidence to make definitive recommendations nor alter existing resuscitation guidelines. Based upon lower quality evidence, however, it is reasonable to initiate resuscitation, including posterior compressions and defibrillation, in the prone position, particularly if repositioning the patient supine would lead to treatment delays, potential dislodgement of the ETT and other invasive devices, and increased risk of infectious exposure for team members. In fact, the Interim Guidance to Healthcare Providers for Basic and Advanced Cardiac Life Support notes that providing chest compressions in the prone position is likely superior to not providing compressions during a prolonged maneuver to return the patient to the supine position; they continue by stating that “for patients in the prone position with an advanced airway, it may be reasonable to provide manual compressions in the prone position until a patient can be safely transitioned to a supine position with a trained team” [72,83].
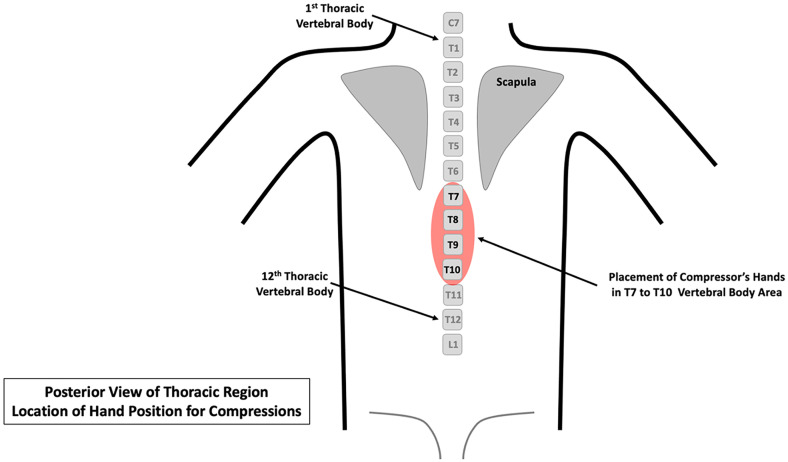
When the patient is in the prone position, compressions are performed on the posterior thoracic spine, at the level of T7 to T10, with a one or two-handed technique (Fig. 1 ); for external landmark positioning, note that vertebral body T7 is located at the inferior tip of the scapula. Counter pressure on the sternum may also be applied, if needed. If initial resuscitation is performed in this position, the quality of chest compressions and patient response to treatment can be determined using end-tidal CO2 monitoring and arterial pressure tracing, if the latter is already in place (note that it is not recommended to place arterial pressure monitoring catheters during active resuscitation); if monitoring values are concerning for less-than-adequate response, then consideration can be made to place the patient in the standard supine position. In general, if deemed necessary for optimal management, such as concerning end-tidal CO2 measurements or constrained ability to monitor endotracheal tube position, the patient can be returned to the standard supine position using the following approach: continue posteriorly delivered compressions until immediately ready to reposition the patient; assemble an adequate number of PPE-protected healthcare personnel with identified tasks (turining, supporting the head and neck, safeguarding the endotracheal tube, managing vascular access, etc.); hold compressions, turn the patient, monitor all tubes and related medical devices; immediately resume chest compressions in standard fashion as well as continue with other appropriate therapies; and confirm adequate positing of the endotracheal tube and other medical devices [72,83].
Fig. 1.
Placement of compressor's hands for posterior compressions when the patient is placed in a prone position. Provide compressions with the hands centered over the T7-T10 vertebral bodies. Note that the inferior tip of the scapula is located at the T7 vertebral body level.
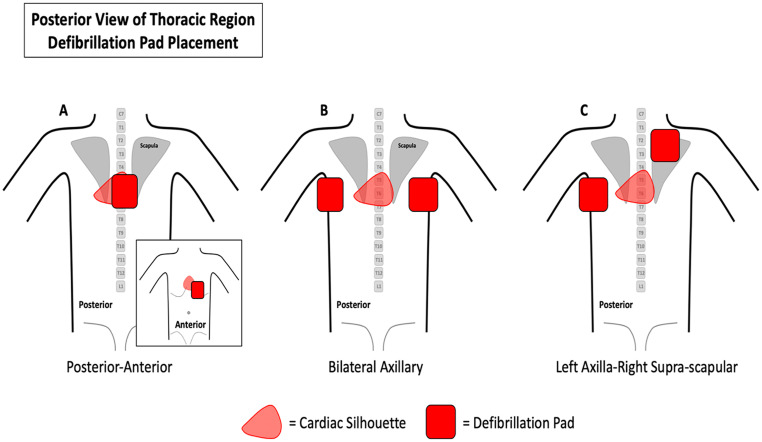
Electrical defibrillation is the treatment-of-choice for ventricular fibrillation and pulseless ventricular tachycardia; in fact, time-to-defibrillation is an important determinant for successful resuscitation. Several investigations from past respiratory viral threats have not demonstrated a clinically significant risk of contagion during defibrillation [84,85]. Importantly, defibrillation is not considered an AGP; in patients with active COVID-19 infection, defibrillation is recommended by the ACLS guidelines [72,86]. Defibrillation can also be performed if the patient is in the prone position with pads in the anterior-posterior position, bi-axillary position (bilateral axillary position or one pad in each axilla), or posterolateral position (left axilla and right supra-scauplar) [83]. Refer to Fig. 2 for placement of defibrillation pads when the patient is in the prone position.
Fig. 2.
Placement of defibrillation pads on the patient while placed in the prone position. A. Posterior-anterior. B. Bilateral axillary. C. Left axilla-right supra-scapular.
The administration of bolus-dose medications during cardiac arrest is not different than pre-pandemic recommendations; the same statement is true for intravenous (IV) fluid administration. One noted exception to this statement is the avoidance of the administration of medications via the endotracheal tube; opening the circuit for medication administration can be considered a source of aerosol generation [72]. With the increased frequency of both PEA and asystole as the initial cardiac arrest rhythm, early administration of bolus dose epinephrine is encouraged. Vascular access should also be performed in standard fashion, appropriate for the cardiac arrest scenario. If a peripheral or central IV is already in place prior to the cardiac arrest, this line can be used for medication administration; caution is advised regarding patency of existing IV lines due to the hypercoagulable state encountered in the COVID-19-infected patient. If no venous access is available at the time of cardiac arrest, an intraosseous (IO) line is strongly recommended; in fact, an IO line is the preferred vascular access device in this situation. An IO line is rapidly and easily placed, even when the team member is in full PPE; in addition, placement of a central or peripheral venous line is more challenging when the team member is in full COVID-19 PPE [[87], [88], [89]].
3.5.2. Oxygenation, ventilation, & airway management
All airway and breathing interventions are considered AGPs to varying degrees and thus pose a contagion risk to the resuscitation team. Such interventions must be performed using appropriate PPE. Management of the airway and breathing status has different priorities depending upon the etiology of the cardiac arrest. Non-cardiogenic events, particularly those events involving compromised respiratory function, have a markedly higher airway management priority as compared to cardiogenic cardiac arrests. This statement has been clearly established prior to the pandemic and remains a standard approach since the advent of COVID-19. In general, earlier application of a definitive airway with oxygenation and ventilation is appropriate for certain non-cardiogenic events.
Upon entering the room following appropriate donning of PPE, application of a 100% non-rebreather face mask connected to high-flow oxygen (i.e.,15 liters) coupled with insertion of an oral airway is recommended. Once additional personnel are donned in appropriate PPE and in the resuscitation room, respiratory management can transition to bag mask ventilations (BMV), attached to an in-line high-efficiency particulate absorbing (HEPA) filter [72]. A tight seal of the face mask is important, preferably using a two-person technique, to ensure best-possible oxygenation and ventilation, as well as to limit contagion risk [72,86]. The HEPA filter is placed in line along the exhalation port of most respiratory devices used in resuscitation, including the bag-mask ventilation, supra-glottic airway, endotracheal tube, and mechanical ventilation. The HEPA filter is a pleated mechanical air filter that can theoretically remove up to 99.97% of particulate matter in the air down to a size of 0.3 μm, including dust, pollen, and various microbes including viral particles. The HEPA filter is recommended by many authorities for such application [72]. Alternatives to the HEPA filter include the low-dead space viral filter or a heat and moisture exchanging filter (HMEF) with at least 99.99% viral filtration efficiency may be placed in the circuit, between ventilation device and the airway.
The next consideration in respiratory management is insertion of an advanced airway, including either a supra-glottic airway (SGA) or an endotracheal tube (ETT), using an in-line HEPA filter. Placement of an advanced airway as early as possible will reduce the risk of aerosolization while maximizing patient care, assuming that other interventions are not adversely impacted by any airway management-associated treatment delays and appropriate resources are available for such. Placement of an advanced airway should occur as soon as clinically feasible, once the resuscitation team is in position and appropriate personnel are available [72]. In fact, numerous authorities recommend that the initiation of airway management in the COVID-19 patient experiencing cardiac arrest should begin with placement of the advanced airway, omitting the earlier steps noted above [72].
The decision regarding which type of advanced airway used must consider several factors, including individual patient needs, resource availability, and team member expertise. If an SGA is selected, it should be placed in conjunction with a HEPA filter. If endotracheal intubation is selected as the advanced airway strategy, video laryngoscopy, if available, − as compared to direct laryngoscopy – is preferred in that the former allows for an increased distance between the patient and the airway management team member [72,[90], [91], [92]]. Furthermore, in an effort to reduce the AGP nature of the chest compression – airway management combination, the AHA recommends that chest compressions be paused during intubation to reduce risk of disease transmission and ensure first-pass intubation success [72]. This represents a significant change from pre-pandemic recommendations to insert an advanced airway with minimal to no interruption in chest compression; an approximate 10-s compression pause is considered appropriate in this setting. As with the SGA strategy, once the ETT is confirmed in correct placement, a HEPA filter should be attached to further limit the risk of viral aerosolization and reduce contagious risk.
If the patient has already been endotracheally intubated and is on mechanical ventilation at the time of cardiac arrest, consider leaving the patient on the ventilator with an in-line HEPA filter; such a strategy maintains the closed circuit and significantly reduces the chance of contagion. If this strategy is pursued, the resuscitation team must adjust the ventilator settings to allow asynchronous ventilations, allowing for the delivery of appropriate tidal volumes and using the following suggestions: maximize the FiO2 to 100%; use either pressure or volume control ventilation with the limitations to either pressure or tidal volume to generate adequate chest rise (4–6 mL/kg ideal body weight); adjust the trigger settings to prevent the ventilator from auto triggering with chest compressions; maintain respiratory rate at 10 breaths/min for adults; adjust the positive end-expiratory pressure setting as needed, considering lung volume expansion and systemic blood pressure; to balance lung volumes and venous return; and maintain a closed circuit with HEPA filter to reduce the opportunity for aerosol generation and contagion [72].
3.6. Provider contagion protection and contagion during resuscitation
The risk of aerosol generation during CPR remains poorly understood [93,94]. In a mannequin and cadaver study, compression-only CPR was shown to disperse nebulized fluorescent marker applied to the airway as an aerosol in the direction of the compressor [95]. Placement of a surgical mask or oxygen mask over the patient's face limited aerosol spread; insertion of a supraglottic airway device connected to an electrostatic airway filter further reduced visible aerosol release. A separate study using similar methodology demonstrated that intubation with a cuffed endotracheal tube connected to a HEPA filter was more effective in preventing aerosol leakage from the mouth and nose during chest compressions than a supraglottic airway device [96]. In a swine cardiac arrest model utilizing an optical particle sizer to characterize aerosol particle concentration and size distribution, no significant difference in aerosol generation was found between a period of ventricular fibrillation without chest compression or ventilation and compression-only CPR prior to defibrillation [97]. Chest compression following defibrillation, however, resulted in significantly greater aerosol generation, particularly of large-size particles.
How these findings translate to real-world risk of SARS-CoV-2 transmission during CPR is unknown, although its primary mode of transmission remains through respiratory droplets and aerosols. In a cross-sectional study of Indian healthcare personnel involved in the care of COVID-19 patients during the early months of the pandemic in 2020, SARS-CoV-2 infection rates were similar between those who participated in CPR at least once and those who did not at all [5/197 (2.53%) vs. 10/196 (5.10%)] [98]. Given the effective implementation of multifaceted infection prevention and control strategies to protect healthcare personnel and mitigate transmission of SARS-CoV-2 in healthcare settings during the pandemic, the overall risk of COVID-19 attributable to CPR is likely low.
Both the CDC and WHO classify CPR an AGP [99,100]. To this effect, it is recommended that healthcare personnel participating in resuscitation don the appropriate PPE first to limit unprotected exposure to respiratory droplets and aerosols [72]. Those not wearing appropriate PPE should be excused immediately from the area to don PPE. Chest compressions and defibrillation when indicated should be initiated as quickly as possible. Mechanical CPR devices may be considered, if available, and the resuscitation team is trained in its proper use. When available and practical, AIIRs and other engineering controls capable of creating a negative pressure environment can provide additional mitigation of aerosols during resuscitation. Placing a face mask on the patient can be a relatively simple intervention to trap aerosols during compression-only CPR, while an advanced airway with HEPA filtration of ventilation exhaust provides significantly greater control over aerosols generated. Finally, COVID-19 vaccination and booster have significantly reduced the risk of infection among healthcare personnel [72,[101], [102], [103], [104]].
3.7. Post-resuscitation management
Once ROSC is achieved and maintained, the patient likely requires additional resuscitation and supportive care. As is true in non-COVID-19 patients, the resuscitation team should assess / re-assess the patient using the airway, breathing, circulation, and disability approach [105]. Airway and breathing must be first addressed. If deficiencies in maintaining a stable airway and/or inadequate oxygenation or ventilation are found, then definitive management must occur as soon as clinically feasible; if the patient was not intubated during the period of active cardiac arrest, endotracheal intubation with mechanical ventilation should follow. Support of appropriate perfusion is the next priority with use of intravenous fluids, blood products, and vasopressor infusion (likely norepinephrine), as indicated by the clinical specifics of the resuscitated patient; in addition, malignant and/or compromising dysrhythmias should be managed in standard fashion. Attention to co-existing issues such as hypoglycemia, CVA, etc. that could not only cause the event but also contribute to continued altered mentation should also be considered at this time. Targeted temperate management can also be considered using the pre-COVID-19 criteria; the avoidance of hyperthermia is strongly encouraged as well [105,106].
4. Conclusions
The COVID-19 pandemic has most certainly adversely impacted the health and safety of the human population. It has not only introduced a new illness with significant morbidity and mortality but also exacerbated many pre-existing medical conditions. Considering cardiac arrest, both out of and in the hospital setting, the COVID-19 pandemic has contributed to a significant increase in its occurrence as well as a simultaneous reduction in neurologically intact survival rates, negatively impacting both those individuals with and without viral infection. While many interventions, including chest compressions, are aerosol-generating procedures, the risk of contagion to healthcare personnel is low, assuming appropriate personal protective equipment is used; vaccination against the COVID-19 virus with appropriate boosting provides further protection against contagion for the healthcare personnel involved in cardiac arrest resuscitation.
CRediT authorship contribution statement
William J. Brady: Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Summer Chavez: Methodology, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Michael Gottlieb: Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Stephen Y. Liang: Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Brandon Carius: Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Alex Koyfman: Resources, Methodology, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft. Brit Long: Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft.
Declaration of Competing Interest
None.
References
- 1.Patil K.D., Halperin H.R., Becker L.B. Cardiac arrest: resuscitation and reperfusion. Circ Res. 2015;116(12):2041–2049. doi: 10.1161/CIRCRESAHA.116.304495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Drennan I.R., Lin S., Sidalak D.E., Morrison L.J. Survival rates in out-of-hospital cardiac arrest patients transported without prehospital return of spontaneous circulation: an observational cohort study. Resuscitation. 2014;85(11):1488–1493. doi: 10.1016/j.resuscitation.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Yan S., Gan Y., Jiang N., et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24(1):61. doi: 10.1186/s13054-020-2773-2. Published 2020 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daya M.R., Schmicker R.H., Zive D.M., et al. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merchant R.M., Berg R.A., Yang L., et al. Hospital variation in survival after in-hospital cardiac arrest. J Am Heart Assoc. 2014;3(1):e000400. doi: 10.1161/JAHA.113.000400. Published 2014 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yannopoulos D., Bartos J.A., Aufderheide T.P., et al. The evolving role of the cardiac catheterization Laboratory in the Management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139(12):e530–e552. doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 8.Bergum D., Nordseth T., Mjølstad O.C., Skogvoll E., Haugen B.O. Causes of in-hospital cardiac arrest - incidences and rate of recognition. Resuscitation. 2015;87:63–68. doi: 10.1016/j.resuscitation.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 13.COVID-19 Map. Johns Hopkins Coronavirus Resource Center; 2022. https://coronavirus.jhu.edu/map.html (accessed Feb 23, 2022) [Google Scholar]
- 14.Thapa S.B., Kakar T.S., Mayer C., Khanal D. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med. 2021;181(2):279–281. doi: 10.1001/jamainternmed.2020.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah P., Smith H., Olarewaju A., et al. Is cardiopulmonary resuscitation futile in coronavirus disease 2019 patients experiencing in-hospital cardiac arrest? Crit Care Med. 2021;49(2):201–208. doi: 10.1097/CCM.0000000000004736. [DOI] [PubMed] [Google Scholar]
- 16.Nene R.V., Amidon N., Tomaszewski C.A., Wardi G., Lafree A. Outcomes for in-hospital cardiac arrest for COVID-19 patients at a rural hospital in Southern California. Am J Emerg Med. 2021;47:244–247. doi: 10.1016/j.ajem.2021.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao F., Xu S., Ma X., et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ippolito M., Catalisano G., Marino C., et al. Mortality after in-hospital cardiac arrest in patients with COVID-19: a systematic review and meta-analysis. Resuscitation. 2021;164:122–129. doi: 10.1016/j.resuscitation.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu-Ray I., Almaddah N.K., Adeboye A., Soos M.P. StatPearls. StatPearls Publishing; Treasure Island (FL): 2022. Cardiac manifestations of coronavirus (COVID-19)http://www.ncbi.nlm.nih.gov/books/NBK556152/ Accessed: Jan. 25, 2022. [Online]. Available: [PubMed] [Google Scholar]
- 20.Wadhera R.K., Shen C., Gondi S., Chen S., Kazi D.S., Yeh R.W. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. 2021;77(2):159–169. doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khot U.N., Reimer A.P., Brown A., et al. Impact of COVID-19 pandemic on critical care transfers for ST-segment-elevation myocardial infarction, stroke, and aortic emergencies. Circ Cardiovasc Qual Outcomes. 2020;13(8) doi: 10.1161/CIRCOUTCOMES.120.006938. [DOI] [PubMed] [Google Scholar]
- 22.Brady W.J., Mattu A., Slovis C.M. Lay responder care for an adult with out-of-hospital cardiac arrest. N Engl J Med. 2019;381(23):2242–2251. doi: 10.1056/NEJMra1802529. [DOI] [PubMed] [Google Scholar]
- 23.Mathew S., Harrison N., Chalek A.D., et al. Effects of the COVID-19 pandemic on out-of-hospital cardiac arrest care in Detroit. Am J Emerg Med. 2021;46:90–96. doi: 10.1016/j.ajem.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldi E., Sechi G.M., Mare C., et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F., Yuan Y., Song Y., Lu X. The effect on the out-of-hospital system of patients with out-of-hospital cardiac arrest during the COVID-19 outbreak in one City in China. Ann Emerg Med. 2020;76(5):687–689. doi: 10.1016/j.annemergmed.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roedl K., Söffker G., Fischer D., et al. Effects of COVID-19 on in-hospital cardiac arrest: incidence, causes, and outcome - a retrospective cohort study. Scand J Trauma Resusc Emerg Med. 2021;29(1) doi: 10.1186/s13049-021-00846-w. Published 2021 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim S.L., Shahidah N., Saffari S.E., et al. Impact of COVID-19 on out-of-hospital cardiac arrest in Singapore. Int J Environ Res Public Health. 2021;18(7) doi: 10.3390/ijerph18073646. Published 2021 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldi E., Caputo M.L., Auricchio A., Vanetta C., Cresta R., Benvenuti C. A quantitative assessment of the contribution of “citizen First Responder” in the adult out-of-hospital chain of survival during COVID-19 pandemic. Resuscitation. 2021;166:41–42. doi: 10.1016/j.resuscitation.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao P. American College of Cardiology Sports and Exercise Cardiology Leadership Council, Friedman E, Chung EH, Levine BD, Isaacs SM. First responder cardiac health amid the COVID-19 pandemic. Resuscitation. 2020;156:120–122. doi: 10.1016/j.resuscitation.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teoh S.E., Masuda Y., Tan D.J.H., et al. Impact of the COVID-19 pandemic on the epidemiology of out-of-hospital cardiac arrest: a systematic review and meta-analysis. Ann Intensive Care. 2021;11(1):169. doi: 10.1186/s13613-021-00957-8. (Published 2021 Dec 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akintoye E., Adegbala O., Egbe A., Olawusi E., Afonso L., Briasoulis A. Association between hospital volume of cardiopulmonary resuscitation for in-hospital cardiac arrest and survival to hospital discharge. Resuscitation. 2020;148:25–31. doi: 10.1016/j.resuscitation.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell O.J.L., Yuriditsky E., Johnson N.J., et al. In-hospital cardiac arrest in patients with coronavirus 2019. Resuscitation. 2021;160:72–78. doi: 10.1016/j.resuscitation.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandroni C., Skrifvars M.B., Nolan J.P. The impact of COVID-19 on the epidemiology, outcome and management of cardiac arrest. Intensive Care Med. 2021;47(5):602–604. doi: 10.1007/s00134-021-06369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland M., Burke J., Hulac S., et al. Excess cardiac arrest in the community during the COVID-19 pandemic. JACC Cardiovasc Interv. 2020;13(16):1968–1969. doi: 10.1016/j.jcin.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldi E., Sechi G.M., Mare C., et al. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41(32):3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijon E., Karam N., Jost D., et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5(8):e437–e443. doi: 10.1016/S2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marijon E., Karam N., Jouven X. Cardiac arrest occurrence during successive waves of the COVID-19 pandemic: direct and indirect consequences. Eur Heart J. 2021;42(11):1107–1109. doi: 10.1093/eurheartj/ehab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber B.C., Brunner S., Schlichtiger J., Kanz K.G., Bogner-Flatz V. Out-of-hospital cardiac arrest incidence during COVID-19 pandemic in southern Germany. Resuscitation. 2020;157:121–122. doi: 10.1016/j.resuscitation.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim Z.J., Ponnapa Reddy M., Afroz A., Billah B., Shekar K., Subramaniam A. Incidence and outcome of out-of-hospital cardiac arrests in the COVID-19 era: a systematic review and meta-analysis. Resuscitation. 2020;157:248–258. doi: 10.1016/j.resuscitation.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fothergill R.T., Smith A.L., Wrigley F., Perkins G.D. Out-of-Hospital Cardiac Arrest in London during the COVID-19 pandemic. Resusc Plus. 2021;5 doi: 10.1016/j.resplu.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan P.S., Girotra S., Tang Y., Al-Araji R., Nallamothu B.K., McNally B. Outcomes for out-of-hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2021;6(3):296–303. doi: 10.1001/jamacardio.2020.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai P.H., Lancet E.A., Weiden M.D., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5(10):1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball J., Nehme Z., Bernard S., Stub D., Stephenson M., Smith K. Collateral damage: hidden impact of the COVID-19 pandemic on the out-of-hospital cardiac arrest system-of-care. Resuscitation. 2020;156:157–163. doi: 10.1016/j.resuscitation.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sultanian P., Lundgren P., Strömsöe A., et al. Cardiac arrest in COVID-19: characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur Heart J. 2021;42(11):1094–1106. doi: 10.1093/eurheartj/ehaa1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubert H., Baert V., Beuscart J.B., Chazard E. Use of out-of-hospital cardiac arrest registries to assess COVID-19 home mortality. BMC Med Res Methodol. 2020;20(1):305. doi: 10.1186/s12874-020-01189-3. (Published 2020 Dec 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masuda Y., Teoh S.E., Yeo J.W., et al. Variation in community and ambulance care processes for out-of-hospital cardiac arrest during the COVID-19 pandemic: a systematic review and meta-analysis. Sci Rep. 2022;12(1):800. doi: 10.1038/s41598-021-04749-9. (Published 2022 Jan 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miles J.A., Mejia M., Rios S., et al. Characteristics and outcomes of in-hospital cardiac arrest events during the COVID-19 pandemic: a single-center experience from a new York City public hospital. Circ Cardiovasc Qual Outcomes. 2020;13(11) doi: 10.1161/CIRCOUTCOMES.120.007303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acharya P., Ranka S., Sethi P., et al. Incidence, predictors, and outcomes of in-hospital cardiac arrest in COVID-19 patients admitted to intensive and non-intensive care units: insights from the AHA COVID-19 CVD registry. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.120.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bielski K., Szarpak A., Jaguszewski M.J., et al. The influence of Covid-19 on out-hospital cardiac arrest survival outcomes: an updated systematic review and meta-analysis. J Clin Med. 2021;10(23):5573. doi: 10.3390/jcm10235573. (Published 2021 Nov 27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayek S.S., Brenner S.K., Azam T.U., et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020;371:m3513. doi: 10.1136/bmj.m3513. (Published 2020 Sep 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheth V., Chishti I., Rothman A., et al. Outcomes of in-hospital cardiac arrest in patients with COVID-19 in New York City. Resuscitation. 2020;155:3–5. doi: 10.1016/j.resuscitation.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jarrett M., Schultz S., Lyall J., et al. Clinical mortality in a large COVID-19 cohort: observational study. J Med Internet Res. 2020;22(9) doi: 10.2196/23565. (Published 2020 Sep 25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer J., Brüggmann D., Klingelhöfer D., et al. Access to intensive care in 14 European countries: a spatial analysis of intensive care need and capacity in the light of COVID-19. Intensive Care Med. 2020;46(11):2026–2034. doi: 10.1007/s00134-020-06229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fried J.A., Ramasubbu K., Bhatt R., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holm A., Jerkeman M., Sultanian P., et al. Cohort study of the characteristics and outcomes in patients with COVID-19 and in-hospital cardiac arrest. BMJ Open. 2021;11(11) doi: 10.1136/bmjopen-2021-054943. (Published 2021 Nov 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page E.M., Ariëns R.A.S. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8. doi: 10.1016/j.thromres.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pimentel M.A.F., Redfern O.C., Hatch R., Young J.D., Tarassenko L., Watkinson P.J. Trajectories of vital signs in patients with COVID-19 [published correction appears in Resuscitation. 2021;162:91-92] Resuscitation. 2020;156:99–106. doi: 10.1016/j.resuscitation.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercuro N.J., Yen C.F., Shim D.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) [published correction appears in JAMA Cardiol. 2020 Sep 1;5(9):1071] JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avruscio G., Camporese G., Campello E., et al. COVID-19 and venous thromboembolism in intensive care or medical Ward. Clin Transl Sci. 2020;13(6):1108–1114. doi: 10.1111/cts.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Michele S., Sun Y., Yilmaz M.M., et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154(6):748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pranata R., Lim M.A., Yonas E., Siswanto B.B., Meyer M. Out-of-hospital cardiac arrest prognosis during the COVID-19 pandemic. Intern Emerg Med. 2020;15(5):875–877. doi: 10.1007/s11739-020-02428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deakin C.D. The chain of survival: not all links are equal. Resuscitation. 2018;126:80–82. doi: 10.1016/j.resuscitation.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Doan T.N., Schultz B.V., Rashford S., Bosley E. Surviving out-of-hospital cardiac arrest: the important role of bystander interventions. Aus Emerg Care. 2020;23(1):47–54. doi: 10.1016/j.auec.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Pollack R.A., Brown S.P., Rea T., et al. Impact of bystander automated external defibrillator use on survival and functional outcomes in shockable observed public cardiac arrests. Circulation. 2018;137(20):2104–2113. doi: 10.1161/CIRCULATIONAHA.117.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moskowitz A., Holmberg M.J., Donnino M.W., Berg K.M. In-hospital cardiac arrest: are we overlooking a key distinction? Curr Opin Crit Care. 2018;24(3):151–157. doi: 10.1097/MCC.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovach C.P., Perman S.M. Impact of the COVID-19 pandemic on cardiac arrest systems of care. Curr Opin Crit Care. 2021;27(3):239–245. doi: 10.1097/MCC.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jost D., Derkenne C., Kedzierewicz R., et al. The need to adapt the rescue chain for out-of-hospital cardiac arrest during the COVID-19 pandemic: experience from the Paris Fire Brigade Basic Life Support and Advanced Life Support teams. Resuscitation. 2020;153:56–57. doi: 10.1016/j.resuscitation.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leung K.H.B., Alam R., Brooks S.C., Chan T.C.Y. Public defibrillator accessibility and mobility trends during the COVID-19 pandemic in Canada. Resuscitation. 2021;162:329–333. doi: 10.1016/j.resuscitation.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramzy M., Montrief T., Gottlieb M., Brady W.J., Singh M., Long B. COVID-19 cardiac arrest management: a review for emergency clinicians. Am J Emerg Med. 2020;38(12):2693–2702. doi: 10.1016/j.ajem.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodloe J.M., Topjian A., Hsu A., et al. Interim guidance for emergency medical services management of out-of-hospital cardiac arrest during the COVID-19 pandemic. Circ Cardiovasc Qual Outcomes. 2021;14(7) doi: 10.1161/CIRCOUTCOMES.120.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atkins D.L., Sasson C., Hsu A., et al. 2022 Interim Guidance to Healthcare Providers for Basic and Advanced Cardiac Life Support in Adults, Children, and Neonates with Suspected or Confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With the Guidelines®-Resuscitation Adult and Pediatric Task Forces of the American Heart Association in Collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, The Society of Critical Care Anesthesiologists, and American Society of Anesthesiologists [published online ahead of print, 2022 Jan 24] Circ Cardiovasc Qual Outcomes. 2022 doi: 10.1161/CIRCOUTCOMES.122.008900. [DOI] [PubMed] [Google Scholar]
- 73.Abrahamson S.D., Canzian S., Brunet F. Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Crit Care. 2006;10(1):R3. doi: 10.1186/cc3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.CDC Recommendations on Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. Centers for Disease Control and Prevention; 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html Published February 11, 2022. Accessed Feb. 19, 2020. [Google Scholar]
- 75.Verbeek J.H., Ijaz S., Mischke C., et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2016;4:CD011621. doi: 10.1002/14651858.CD011621.pub2. Published 2016 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu A., Sasson C., Kudenchuk P.J., et al. 2021 interim guidance to health care providers for basic and advanced cardiac life support in adults, children, and neonates with suspected or confirmed COVID-19. Circ Cardiovasc Qual Outcomes. 2021;14(10) doi: 10.1161/CIRCOUTCOMES.121.008396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeFilippis E.M., Ranard L.S., Berg D.D. Cardiopulmonary resuscitation during the COVID-19 pandemic: a view from trainees on the front line. Circulation. 2020;141(23):1833–1835. doi: 10.1161/CIRCULATIONAHA.120.047260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adler M.D., Krug S., Eiger C., et al. Impact of personal protective equipment on the performance of emergency pediatric tasks. Pediatr Emerg Care. 2021;37(12):e1326–e1330. doi: 10.1097/PEC.0000000000002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J., Lu K.Z., Yi B., Chen Y. Chest compression with personal protective equipment during cardiopulmonary resuscitation: a randomized crossover simulation study. Medicine (Baltimore) 2016;95(14) doi: 10.1097/MD.0000000000003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim T.H., Kim C.H., Shin S.D., Haam S. Influence of personal protective equipment on the performance of life-saving interventions by emergency medical service personnel. SIMULATION. 2016;92(10):893–898. doi: 10.1177/0037549716662322. [DOI] [Google Scholar]
- 81.Ehrmann S., Li J., Ibarra-Estrada M., et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Douma M.J., MacKenzie E., Loch T., et al. Prone cardiopulmonary resuscitation: a scoping and expanded grey literature review for the COVID-19 pandemic. Resuscitation. 2020;155:103–111. doi: 10.1016/j.resuscitation.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moscarelli A., Iozzo P., Ippolito M., et al. Cardiopulmonary resuscitation in prone position: a scoping review. Am J Emerg Med. 2020;38(11):2416–2424. doi: 10.1016/j.ajem.2020.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raboud J., Shigayeva A., McGeer A., et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5(5):e10717. doi: 10.1371/journal.pone.0010717. Published 2010 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Couper K., et al. COVID-19 Infection Risk to Rescuers from Patients in Cardiac Arrest: Systematic Review. 2021. https://costr.ilcor.org/document/covid-19-infection-risk-to-rescuers-from-patients-in-cardiac-arrest-systematic-review Accessed: Jan. 31, 2022. [Online]. Available:
- 87.Suyama J., Knutsen C.C., Northington W.E., Hahn M., Hostler D. IO versus IV access while wearing personal protective equipment in a HazMat scenario. Prehosp Emerg Care. 2007;11(4):467–472. doi: 10.1080/10903120701536982. [DOI] [PubMed] [Google Scholar]
- 88.Castle N., Owen R., Hann M., Clark S., Reeves D., Gurney I. Impact of chemical, biological, radiation, and nuclear personal protective equipment on the performance of low- and high-dexterity airway and vascular access skills. Resuscitation. 2009;80(11):1290–1295. doi: 10.1016/j.resuscitation.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Lamhaut L., Dagron C., Apriotesei R., et al. Comparison of intravenous and intraosseous access by pre-hospital medical emergency personnel with and without CBRN protective equipment. Resuscitation. 2010;81(1):65–68. doi: 10.1016/j.resuscitation.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 90.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75(6):785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song W., Liu Y., Ouyang Y., et al. Recommendations on cardiopulmonary resuscitation strategy and procedure for novel coronavirus pneumonia. Resuscitation. 2020;152:52–55. doi: 10.1016/j.resuscitation.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Couper K., Taylor-Phillips S., Grove A., et al. COVID-19 in cardiac arrest and infection risk to rescuers: a systematic review. Resuscitation. 2020;151:59–66. doi: 10.1016/j.resuscitation.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown E., Chan L.M. Should chest compressions be considered an aerosol-generating procedure? A literature review in response to recent guidelines on personal protective equipment for patients with suspected COVID-19. Clin Med (Lond) 2020;20(5):e154–e159. doi: 10.7861/clinmed.2020-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ott M., Milazzo A., Liebau S., et al. Exploration of strategies to reduce aerosol-spread during chest compressions: a simulation and cadaver model. Resuscitation. 2020;152:192–198. doi: 10.1016/j.resuscitation.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Somri M., Gaitini L., Gat M., Sonallah M., Paz A., Gómez-Ríos M.Á. Cardiopulmonary Resuscitation during the COVID-19 pandemic. Do supraglottic airways protect against aerosol-generation? Resuscitation. 2020;157:123–125. doi: 10.1016/j.resuscitation.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsu C.H., Tiba M.H., Boehman A.L., et al. Aerosol generation during chest compression and defibrillation in a swine cardiac arrest model. Resuscitation. 2021;159:28–34. doi: 10.1016/j.resuscitation.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soni L., Maitra S., Ray B.R., Anand R.K., Subramaniam R., Baidya D.K. Risk of SARS-CoV-2 infection among healthcare providers involved in cardiopulmonary resuscitation in COVID-19 patients. Indian J Crit Care Med. 2021;25(8):920–922. doi: 10.5005/jp-journals-10071-23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clinical Questions about COVID-19: Questions and Answers. 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Infection-Control (accessed Feb 25, 2022)
- 100.World Health Organization Annex to Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-Annex-2021.1 (accessed Feb 25, 2022)
- 101.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pilishvili T., Gierke R., Fleming-Dutra K.E., et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spitzer A., Angel Y., Marudi O., et al. Association of a third Dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA. 2022;327(4):341–349. doi: 10.1001/jama.2021.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paschoud D., Carter C., Notter J. COVID-19 disease: resuscitation. Clin Integrated Care. 2020;3 doi: 10.1016/j.intcar.2020.100023. [DOI] [Google Scholar]
- 106.Berg R.A., Hemphill R., Abella B.S., et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [published correction appears in circulation. 2011 Oct 11;124(15):e402] Circulation. 2010;122(18 Suppl 3):S685–S705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]