Abstract
We tested the combined activity of vancomycin and seven β-lactam antibiotics against Staphylococcus aureus clinical strain Mu3, which displays heterogeneous resistance to vancomycin. When combined with vancomycin, four of the seven tested β-lactams exhibited an additive effect at or near their MICs, while all showed an antagonistic effect at lower, sub-MIC levels. This study implicated the unpredictable nature of combination therapy of β-lactams and vancomycin against S. aureus with reduced susceptibility to vancomycin.
Mu3 (ATCC 700698) is a methicillin-resistant Staphylococcus aureus (MRSA) strain with heterogeneous resistance to vancomycin, designated heterogeneously vancomycin-resistant Staphylococcus aureus (hetero-VRSA) (8, 12, 13). Here VRSA is equivalent to vancomycin-intermediate S. aureus (22); the difference in meaning between “intermediate” and “resistant” derives from the NCCLS definition of a MIC of 8 μg/ml as intermediate, while in other locations, the breakpoints are lower (e.g., “resistant” is used for MICs of ≥8 μg/ml in Great Britain and ≥4 μg/ml in Japan). We define a hetero-VRSA strain as an S. aureus strain that satisfies all of the following criteria. (i) Its vancomycin MIC is less than 8 μg/ml when determined by NCCLS-based broth dilution methods. (ii) It contains subpopulations of cells resistant to higher concentrations of vancomycin, including 4 μg of vancomycin/ml. (iii) Mutant strains with increased vancomycin resistance (a MIC of ≥8 μg/ml) can be obtained from the strain by one-step vancomycin-selection procedure with a frequency of 1 in 1,000,000 or greater (12).
To screen clinical S. aureus isolates for hetero-VRSA strains, we proposed a plating efficiency test on brain heart infusion (BHI) agar containing 4 μg of vancomycin/ml (12). Strains yielding countable numbers of colonies on the plate by plating, about 107 CFU, are considered candidates for hetero-VRSA. Confirmation of a heterogeneous susceptible pattern by subsequent population analysis and by the derivation test of resistant mutants (with a MIC of ≥8 μg/ml) is required for the final identification of hetero-VRSA (12).
Researchers earlier noticed a property of Mu3, an antagonistic phenomenon between vancomycin and β-lactam antibiotics, when they were searching for an effective combination therapy against infection caused by Mu3-like MRSA strains (11). The antagonism was demonstrated as a thick growth of Mu3 cells around the paper disks that were impregnated with β-lactam antibiotics and placed on the above screening agar (11). In the present study, we adopted a broth dilution method to better quantify the antagonistic phenomenon between vancomycin and β-lactam antibiotics observed on the screening agar plate.
The 1.0-ml BHI broth (Difco, Detroit, Mich.) containing various concentrations of vancomycin (0, 0.5, 1, 2, 4, and 8 μg/ml) and β-lactam antibiotics (0 to 1,024 μg/ml) was inoculated with 107 CFU of Mu3. Tested β-lactams were oxacillin, ampicillin, cefoxitin, cefmetazole (Sigma Chemical Co., St. Louis, Mo.); penicillin G (Meiji Pharmacy Co., Tokyo, Japan); piperacillin (Toyama Pharmacy Co., Toyama, Japan); and imipenem (Banyu Pharmacy Co., Tokyo, Japan). The optical density at 578 nm (OD578) of the cultures after a 24-h incubation at 37°C was measured using a U-3200 spectrophotometer (Hitachi Inc., Tokyo, Japan), and expressed as “OD curves” after smoothing using Kaleidagraph (version 3.0.5; Synergy Software, Reading, Pa.). Correlation coefficients were calculated with a simple regression analysis program (StatView version 4.11; Abacus Concepts, Inc, Berkeley, Calif.).
To test the effect of β-lactam on the cell wall synthesis, test tubes holding 5 ml each of BHI broth with 4 μg of vancomycin/ml plus varied concentrations of β-lactam antibiotics ranging from 0 to 1,000 μg/ml were prepared. To them were added Mu3 cells to a final cell density of 3 × 10 7 CFU/ml and 8 μl of [14C]GlcNAc (1.48 MBq/ml; Amersham Life Science, Little Chalfont, Buckinghamshire, England). The tube preparations were then incubated at 37°C with shaking. A 0.5-ml portion of the culture was taken from each test tube after 0, 1, 2, and 3 h of incubation and was subjected to the measurement of incorporated radioactivity as described previously (8).
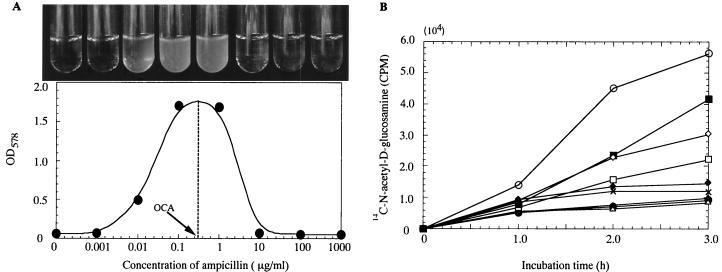
Figure 1A shows that addition of ampicillin in the concentration range of 0.01 to 1 μg/ml helped Mu3 cells grow in an otherwise inhibitory concentration of vancomycin (4 μg/ml). There was an optimal ampicillin concentration within the range of antagonism that elicited maximum cell growth beyond an OD578 of 1.7. The optimal concentration for antagonism (OCA) was thus defined from the OD curve (with a procedure illustrated in Fig. 1A) as the concentration of β-lactam that allows cells to grow to the highest opacity in the presence of inhibitory concentrations of vancomycin. On the other hand, a slight additive effect was observed at higher ampicillin concentrations of 8 and 16 μg/ml, which corresponded to one-fourth and one-half of the MIC against Mu3 (Table 1). Antagonism similar to that demonstrated with ampicillin in Fig. 1A was observed with all seven tested β-lactams, although the range of concentrations eliciting antagonism and the OCA varied considerably among them (Table 1). The additive effect demonstrated with ampicillin was observed with four of the seven tested β-lactams at concentrations near their MICs. Table 1 summarizes the ranges of concentrations eliciting either antagonism or an additive effect for each antibiotic and shows that the OCA of each antibiotic was considerably less than the MIC of the antibiotic against Mu3. On the other hand, the OCA for β-lactam with Mu3 was very close to the MIC of each β-lactam against the methicillin-susceptible S. aureus type strain FDA 209P (ATCC 6538P): there was a significant correlation between the OCA for Mu3 and the MIC for FDA 209P (correlation coefficient = 0.994, P < 0.0001).
FIG. 1.
The effect of ampicillin on the growth and peptidoglycan synthesis of Mu3 in the presence of vancomycin. (A) Growth of Mu3 cells in 4 μg of vancomycin/ml and various concentrations of ampicillin. Turbidity of the culture was monitored after 24-h incubation at 37°C. The concentration of a β-lactam that makes the cell grow to maximum opacity in 4 μg of vancomycin/ml is defined as the OCA of the β-lactam. (B) Ampicillin reverses vancomycin-mediated suppression of peptidoglycan synthesis of Mu3. Uptake of [14C]N-acetyl-d-glucosamine by Mu3 cells was measured in the broth containing 4 μg of vancomycin/ml with and without various concentrations of ampicillin. Symbols: empty circles, no antibiotic; solid circles, 4 μg of vancomycin/ml alone; other symbols, 4 μg of vancomycin/ml plus ampicillin. Ampicillin concentrations in micrograms per milliliter were 0.001 (open triangles), 0.01 (solid triangles), 0.1 (empty squares), 1 (solid squares), 10 (empty diamonds), 100 (solid diamonds), and 1,000 (crosses). CPM, counts per minute.
TABLE 1.
β-Lactam in combination with vancomycin elicits both additive and antagonistic effects against Mu3, depending on the concentration
| β-Lactam | Properties of additive effect
|
Properties of antagonistic effect
|
MIC (μg/ml) againsta:
|
||||
|---|---|---|---|---|---|---|---|
| Range (μg/ml) | Minimum FIC (vancomycin concn)b | Range (μg/ml) | Maximum FIC (vancomycin concn)b | OCAc | Mu3 | FDA 209P | |
| Penicillin G | —d | 0.016–1 | 2.03 (8) | 0.4 | 32 | 0.25 | |
| Ampicillin | 8–16 | 0.75 (2) | 0.125–4 | 2.125 (8) | 0.3 | 32 | 0.5 |
| Oxacillin | 512 | 1.0 (2) | 0.03–4 | 2.004 (8) | 1.0 | 1,024 | 1 |
| Piperacillin | — | 0.016–32 | 2.25 (8) | 3.2 | 128 | 4 | |
| Imipenem | 128 | 1.0 (2) | 0.002–0.5 | 2.002 (8) | 0.01 | 256 | 0.016 |
| Cefoxitin | — | 0.016–32 | 2.03 (8) | 1.0 | 1,024 | 1 | |
| Cefmetazole | 128 | 1.0 (2) | 0.125–8 | 2.03 (8) | 0.3 | 256 | 0.5 |
MIC was determined as the concentration which inhibited cell growth in BHI broth without vancomycin. At the MIC, the OD values were less than 0.008 for all β-lactam antibiotics.
The minimum or maximum FIC and the vancomycin concentration (in micrograms per milliliter) of the culture that recorded the FIC are given. The FICs were calculated as (MIC of vancomycin in combination with a β-lactam)/(MIC of vancomycin alone) + (MIC of a β-lactam in combination with vancomycin)/(MIC of a β-lactam alone).
OCA was defined as the concentration of β-lactam that allowed maximum growth of Mu3 culture in the presence of the MIC of vancomycin (4 μg/ml).
—, indifferent.
Figure 1B shows the uptake of N-acetyl-d-1-14C-glucosamine ([14C]GlcNAc) by Mu3 cells, a major precursor nutrient of cell wall peptidoglycan synthesis. Vancomycin at 4 μg/ml suppressed GlcNAc uptake to less than 17% of that with the drug-free control throughout the measured period. In the copresence of ampicillin, however, there was a significant recovery of uptake at the range of ampicillin concentration between 0.1 and 10 μg/ml, and maximum recovery was observed at 1 μg/ml. These values coincided well with the antagonism-eliciting concentrations of ampicillin (Fig. 1A). The reversal of vancomycin-mediated suppression was also demonstrated within the range of antagonistic concentrations for each of the seven β-lactam antibiotics (not shown).
We and others have reported isolation of Mu3-like hetero-VRSA strains from clinical specimens (6, 9, 12, 23, 24) which are sometimes associated with infections that were refractory to vancomycin therapy (9, 12, 23, 24). Whether such cases could be or should be treated with vancomycin plus a β-lactam combination is a focus of argument (9). Besides Mu3, we have tested 20 such hetero-VRSA clinical isolates obtained from 10 hospitals in Japan for the antagonism between selected β-lactam antibiotics and vancomycin: all showed antagonism OD curves and OCAs similar to those demonstrated with Mu3 (N. Aritaka, unpublished observation).
Currently, the mechanism for β-lactam-elicited antagonism or the reversal of vancomycin-mediated suppression of cell wall synthesis is unknown. These observations may indicate existence of a novel sub-MIC effect of β-lactam antibiotics, inducing activation of cell wall synthesis of S. aureus. It has been demonstrated recently that β-lactams can induce expression of certain cell wall synthesis-associated genes, such as pbp2, in S. aureus (17). Recently, we have also found that β-lactams induce increased transcription of a response regulator gene, vraR, which is considered to be at least partially responsible for the increased vancomycin resistance in Mu3 (16). Therefore, it seems plausible to postulate that β-lactams induce activation of cell wall synthesis of Mu3 cells. If we consider that the induction is triggered upon saturation by β-lactams of the intrinsic set of S. aureus penicillin-binding proteins, the reason why OCA values are close to the MICs of β-lactams for S. aureus type strain FDA 209P would be explained. An alternative and equally attractive explanation for the antagonism is that β-lactams act by reducing cross-linking of peptidoglycan of Mu3. Reduced cross-linking increases the number of d-alanyl-d-alanine residues in the cell wall, which serve as “false targets” of vancomycin (11). More false targets trap and consume more vancomycin molecules within the cell wall layers, preventing efficient access of vancomycin molecules to their “real target,” lipid II, on the cytoplasmic membrane.
There have been many contradictory observations with regard to the combination effect of vancomycin and β-lactam against S. aureus clinical isolates, including additive and/or synergistic effects (2, 4, 15, 18, 19, 20, 21) and antagonistic effects (7, 9, 14; H. Hanaki, S. Ohkawa, Y. Inaba, T. Hashimoto, and K. Hiramatsu, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-132, 1998; this study). With Mu3, both additive and antagonistic effects were seen at high and low concentrations of β-lactams, respectively. However, the additive concentrations (≥128 μg/ml) (Table 1) are likely difficult to achieve in patients for most of the tested β-lactam antibiotics. The exception was ampicillin, which is known to have relatively high antimicrobial activity against MRSA among extant β-lactam antibiotics as long as β-lactamase is countered (1, 3). (Ampicillin per se has potent activity against Mu3, because it does not produce penicillinase.) In terms of clinical application, however, both the additive and antagonistic ranges of concentration of ampicillin are achievable in patients for various time periods, which makes the prediction of the clinical effect of the combination difficult. We are concerned, therefore, that β-lactam antibiotics may not provide a significant advantage in combination with vancomycin against Mu3-like hetero-VRSA strains.
Acknowledgments
This work was supported by the Core University Program under the Japan Society for the Promotion of Science (JSPS), coordinated by the University of Tokyo, Graduate School of Medicine, and Universiti Sains Malaysia, School of Medical Sciences; by Specially Designated Research Promotion of Monbusho; by a Grant for International Health Cooperation Research (11-C) from the Ministry of Health and Welfare; and also by a nonrestricted research grant from Merck & Co., Inc., Rahway, N.J.
REFERENCES
- 1.Asada K, Inaba Y, Tateda-Suzuki E, Kuwahara-Arai K, Ito T, Hiramatsu K. Evolution and resistance expression of MRSA. Evaluation of β-lactam antibiotics against a set of isogenic strains with different types of phenotypic expression. Acta Biochim Pol. 1995;42:517–524. [PubMed] [Google Scholar]
- 2.Barr J G, Smyth E T, Hogg G M. In vitro antimicrobial activity of imipenem in combination with vancomycin or teicoplanin against Staphylococcus aureus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1990;9:804–809. doi: 10.1007/BF01967378. [DOI] [PubMed] [Google Scholar]
- 3.Chambers H F, Sachdeva M. Binding of β-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 4.Climo W M, Patron R L, Archer G L. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob Agents Chemother. 1999;43:1747–1753. doi: 10.1128/aac.43.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgopapadakou N H, Liu F Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980;18:148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerin F, Buu-Hoi A, Mainardi J L, Kac G, Colardelle N, Vaupre S, Gutmann L, Podglajen I. Outbreak of methicillin-resistant Staphylococcus aureus with reduced susceptibility to glycopeptides in a Parisian hospital. J Clin Microbiol. 2000;38:2985–2988. doi: 10.1128/jcm.38.8.2985-2988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanaki H, Inaba Y, Sasaki K, Hiramatsu K. A novel method of detecting Staphylococcus aureus heterogeneously resistant to vancomycin (hetero-VRSA) Jpn J Antibiot. 1998;51:521–530. . (In Japanese.) [PubMed] [Google Scholar]
- 8.Hanaki H, Kuwabara-Arai K, Boyle-Vavra S, Daum R S, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Haraga I, Nomura S, Nagayama A. The effects of vancomycin and β-lactam antibiotics against vancomycin low-level or intermediately resistant Staphylococcus aureus. N Engl J Med. 1999;341:1624–1625. doi: 10.1056/NEJM199911183412117. [DOI] [PubMed] [Google Scholar]
- 10.Hartman B J, Tomasz A. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K. Vancomycin resistance in staphylococci. Drug Resist Updates. 1998;1:135–150. doi: 10.1016/s1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Howe R A, Wootton M, Bennett P M, MacGowan A P, Walsh T R. Interactions between methicillin and vancomycin in methicillin-resistant Staphylococcus aureus strains displaying different phenotypes of vancomycin susceptibility. J Clin Microbiol. 1999;37:3068–3071. doi: 10.1128/jcm.37.9.3068-3071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. Effect of combination of oxacillin and nonβ-lactam antibiotics on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1994;33:1155–1163. doi: 10.1093/jac/33.6.1155. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda M, Kuwabara-Arai K, Hiramatsu K. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem Biophys Res Commun. 2000;269:485–490. doi: 10.1006/bbrc.2000.2277. [DOI] [PubMed] [Google Scholar]
- 17.Murakami H, Matsumaru H, Kanamori M, Hayashi H, Ohta T. Cell wall-affecting antibiotics induce expression of a novel gene, drp35, in Staphylococcus aureus. Biochem Biophys Res Commun. 1999;264:348–351. doi: 10.1006/bbrc.1999.1388. [DOI] [PubMed] [Google Scholar]
- 18.Seibert G, Isert D, Klesel N, Limbert M, Markus A, Schrinner E. The in vitro antibacterial activity of a combination of cefpirome or cefoperazone with vancomycin against enterococci and Staphylococcus aureus. J Antimicrob Chemother. 1992;29:25–30. doi: 10.1093/jac/29.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- 19.Sieradzki K, Roberts R B, Haber S W, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 20.Simon C, Simon M. In vitro activity of flomoxef and cefazolin in combination with vancomycin. Infection. 1991;19:276–278. doi: 10.1007/BF01645539. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura A, Jono K, Kono T, Higaside E. The effect of combination of cefotiam and other antibiotics on methicillin-resistant Staphylococcus aureus in vitro. J Antimicrob Chemother. 1991;28:707–717. doi: 10.1093/jac/28.5.707. [DOI] [PubMed] [Google Scholar]
- 22.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O'Hara C M, McAllister S K, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong S S, Ho P L, Woo P C, Yuen K Y. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin Infect Dis. 1999;29:760–767. doi: 10.1086/520429. [DOI] [PubMed] [Google Scholar]
- 24.Wong S S, Ng T, Yam W, Tsang D N, Woo P C, Fung S K, Yune K. Bacteremia due to Staphylococcus aureus with reduced susceptibility to vancomycin. Diagn Microbiol Infect Dis. 2000;36:261–268. doi: 10.1016/s0732-8893(99)00141-8. [DOI] [PubMed] [Google Scholar]