Abstract
Background
Age-related immunosenescence may impair the immune response to vaccination in older adults. Adjuvanted influenza vaccines are designed to overcome immune senescence in older adults. This study estimated the relative vaccine effectiveness (rVE) of MF59-adjuvanted trivalent inactivated influenza vaccine (aIIV3) vs egg-derived quadrivalent inactivated influenza vaccine (IIV4e) and high-dose trivalent inactivated influenza vaccine (HD-IIV3) in preventing influenza-related medical encounters in the 2019–2020 US season.
Methods
This retrospective cohort study used electronic medical records linked to pharmacy and medical claims data. The study population included adults age ≥65 years with a record of aIIV3, IIV4e, or HD-IIV3 vaccination. A doubly robust inverse probability of treatment weighting model was used to derive adjusted odds ratios (ORs). rVE was calculated by (1 – ORadjusted)*100 and was determined overall and separately for age subgroups. An exploratory analysis evaluated the outcome separately in inpatient and outpatient settings.
Results
Subjects received aIIV3 (n = 936 508), IIV3e (n = 651 034), and HD-IIV3 (n = 1 813 819), and influenza-related medical encounters were recorded in 0.5%, 0.9%, and 0.7% of each cohort, respectively. Overall, the rVE of aIIV3 was 27.5% (95% CI, 24.4% to 30.5%) vs IIV4e and 13.9% (95% CI, 10.7% to 17.0%) vs HD-IIV3. aIIV3 had a more favorable rVE in inpatient and outpatient settings. Findings remained consistent across age subgroups and during alternative seasonal dates.
Conclusions
Adults age ≥65 years vaccinated with aIIV3 had fewer influenza-related medical encounters compared with IIV4e or HD-IIV3 during the 2019–2020 US influenza season.
Keywords: adjuvanted influenza vaccine, high-dose influenza vaccine, older adults, quadrivalent inactivated influenza vaccine, relative vaccine effectiveness
Adults age 65 years and older are at increased risk of death and complications from respiratory virus infections [1]. Seasonal epidemics of influenza viruses cause substantial morbidity and mortality, and each year the highest rates of influenza-associated deaths and hospitalizations are reported among older adults in the United States and elsewhere [2, 3]. For this reason, adults age ≥65 years are considered a high-priority vaccination group by the US Advisory Committee on Immunization Practices (ACIP) [4].
Standard egg-grown inactivated influenza vaccines with 15 mcg per antigen may elicit diminished immune responses in older adults as compared with younger adult age groups due to age-related immunosenescence [5, 6]. To help address this phenomenon, an MF59-adjuvanted egg-grown trivalent inactivated influenza vaccine (aIIV3; Fluad, Seqirus USA Inc., Summit, NJ, USA) with 15 mcg per antigen has been developed that elicits a greater immune response, including production of cross-reactive antibodies, and has demonstrated greater effectiveness than unadjuvanted influenza vaccines in adults age ≥65 years [5, 7–17]. In addition, a high-dose egg-grown trivalent inactivated influenza vaccine (HD-IIV3; Fluzone High-Dose, Sanofi Pasteur Inc., Swiftwater, PA, USA) with 60 mcg per antigen has also been shown to have greater efficacy and effectiveness than lower-dose vaccines in older adults [18–20]. Both the adjuvanted and enhanced vaccines are currently licensed and available for use in the United States, United Kingdom, Canada, Europe, Australia, and other countries worldwide.
In a prior study during the 2017–2018 and 2018–2019 US influenza seasons, we evaluated the effectiveness of aIIV3 relative to alternative standards of care for persons age ≥65 years—namely egg-derived quadrivalent inactivated influenza vaccine (IIV4e) and HD-IIV3 [17]. Given the variability of seasonal influenza epidemiology and to understand the consistency of the effectiveness of aIIV3, we sought to conduct a similar analysis in the 2019–2020 influenza season.
METHODS
Study Design
The primary objective of this retrospective cohort study was to estimate the adjusted relative vaccine effectiveness (rVE) of aIIV3 vs IIV4e and of aIIV3 vs HD-IIV3 in the prevention of influenza-related medical encounters in adult subjects ≥65 years of age. A secondary objective was to determine rVE in older adult subgroups (≥65 to ≤74 years, ≥75 to ≤84 years, and ≥85 years). The primary analysis study period was from August 1, 2019, through March 7, 2020. This aligns with the Centers for Disease Control and Prevention (CDC) influenza surveillance season, defined as epidemiologic weeks 40 through 20 of the subsequent year, though we truncated the end of the study period to avoid potential bias arising from the co-circulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United States in March 2020. The study was designed, implemented, and reported in accordance with Good Pharmacoepidemiological Practice, applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. Study results have been reported according to the Reporting of Studies Conducted using Observational Routinely Collected Health Data (RECORD) recommendations [21].
Data Sources
We conducted the analysis using a data set linking patient-level electronic medical records (EMRs) from primary care and specialty clinics with open and closed claims data, where available. Three national EMR systems form the basis of the integrated data set: Veradigm Health Insights Ambulatory database, which comprises Allscripts Touchworks and Allscripts PRO (Chicago, IL, USA), as well as Practice Fusion, Inc. (San Francisco, CA, USA), and a variety of medical practices of different sizes, as well as integrated delivery networks. The Komodo Healthcare Map (Komodo Health Inc., New York, NY, USA) consists of anonymized patient-level US pharmacy and medical claims data and includes both open and closed claims. Data from open claims can be captured within days of a health care encounter and are sourced from practice management systems, billing systems, and claims clearinghouses, whereas data from closed claims have longer lag times and are sourced from insurance providers and payers. The integrated data set includes data since 2014 for roughly 123 million individuals with representation from all 50 US states and provides comprehensive pharmaceutical, demographic, diagnostic, and health care utilization information on patients. Before linkage, each individual data set underwent de-identification and privacy certification to verify it met the minimum Protected Health Information (PHI) data requirements and was evaluated and certified for Health Insurance Portability and Accountability Act (HIPAA) compliance by a third-party statistician (see the Supplementary Data for de-identification and linkage details). As this study was a noninterventional retrospective study using a certified HIPAA-compliant de-identified database, the study does not fall within the regulatory definition of research involving human subjects as outlined by the Code of Federal Regulations (policy 46.102(f)), and approval for this analysis by an institutional review board was not necessary.
Exposure Ascertainment
The exposure of interest was aIIV3, which was compared with both IIV4e and HD-IIV3. The date of recorded immunization was considered the index date. Current Procedural Terminology (CPT) codes, codes for vaccines administered (CVX), and national drug codes (NDCs) (Supplementary Table 1) were used to identify vaccinated subjects from both EMRs and claims data. In addition, we identified patients receiving nonadjuvanted, standard-dose, trivalent influenza vaccine (IIV3e).
During the 2019–2020 Northern Hemisphere season, the World Health Organization and US Food and Drug Administration recommended that trivalent vaccines include an A/Brisbane/02/2018 (H1N1)pdm09-like virus, an A/Kansas/14/2017 (H3N2)-like virus, and a B/Colorado/06/2017-like virus (B/Victoria lineage), while quadrivalent vaccines should include a B/Phuket/3073/2013-like virus (B/Yamagata lineage) [22].
Study Population
The study population included US residents ≥65 years of age at the time of vaccination with a record of receiving aIIV3, IIV4e, HD-IIV3, or IIV3e between August 1, 2019, and January 31, 2020 (vaccination intake period). Formulations of IIV4e were first distributed in the United States in 2013–2014 and gradually replaced IIV3e. Individuals receiving IIV3e were excluded from the analysis population due to limited sample size. Subjects needed to have activity in the Veradigm EMR as well as the claims database within the 12 months before the index date to be included in the analysis. Patients were excluded if they had received >1 influenza vaccination, had a record of influenza vaccination outside of the vaccination intake period, or had an influenza-related medical encounter (IRME) during the 2019–2020 season before being fully vaccinated (to allow for development of vaccine-specific influenza immunity, subjects were considered fully vaccinated 14 days after the index date). Subjects who had an influenza-related medical encounter before the start of the influenza season (ie, before September 29, 2019) and those with missing sex or geographic information were also excluded from the analysis.
Outcome Ascertainment
The outcome was an influenza-related medical encounter ascertained using International Classification of Diseases (ICD) codes specific to the diagnosis of influenza disease (Supplementary Table 2) [23]. Inpatient influenza-related medical encounters were evaluated separately when recorded as the admitting diagnosis vs a diagnosis in any position within the medical claim. The admitting diagnosis is the initial working diagnosis for which an individual was admitted, whereas any diagnosis includes secondary diagnoses, that is, conditions that coexisted at the time of admission or developed subsequently. Influenza-related medical encounters recorded during an emergency room (ER) visit were classified as inpatient.
Covariates
Covariates were identified per protocol and were ascertained from EMRs and claims in the 12 months before the index date (pre-index period). These included age, sex (male, female), race (Black, White, not reported, other), ethnicity (Hispanic, Non-Hispanic, not reported), US geographic region (Northeast, Midwest, South, West, other), index week, frailty index (a summary score for activities of daily living [24]) (Supplementary Table 3), individual comorbidities included in the Charlson comorbidity index [25, 26] (Supplementary Table 4), and 2 variables used as proxies for health care–seeking behavior: number of outpatient visits in the pre-index period and number of inpatient admissions in the pre-index period.
Statistical Methods
Differences in baseline covariates between the exposure groups (aIIV3, IIV4e, and HD-IIV3) were assessed using standardized mean differences (SMDs), with a value of ≤0.1 indicating a negligible difference. Inverse probability of treatment weighting (IPTW) was implemented to adjust for covariate imbalance between cohorts [27]. In the IPTW method, weights were assigned to individuals based on the inverse of their probability of receiving the treatment, as estimated by propensity scores. First, propensity scores were calculated for each exposure cohort using a multivariable logit model adjusted for all covariates listed above. Propensity scores were then used to create stabilized IPTWs. Weights were truncated at the 99th percentile to attenuate any extreme variability from outlier patients. Adjusted odds ratios (ORs) were estimated using a doubly robust approach. Final adjusted ORs were estimated using a multivariable logistic regression model (including all variables in the propensity score model) in the IPTW-weighted cohort [28]. rVE was calculated as 100*(1 – ORadjusted) and is reported with 95% CIs. Analyses were repeated for each age subgroup and location of influenza-related medical encounter (inpatient and outpatient). Weights were redrawn for each age subgroup. Categorical variables with missing or null values were classified as “not reported/unknown”; missing or out-of-range values were not imputed. Analyses were conducted using SQL and SAS (version 9.4).
The robustness of study assumptions was assessed with 4 sensitivity analyses. First, to improve the specificity of outcome case definitions, the moving epidemic method (MEM) was used to evaluate rVE during a period of highest incidence of laboratory-confirmed influenza (as reported by the CDC): December 8, 2019, through March 7, 2020 [29]. Second, to account for the impact of potential co-circulation of SARS-CoV-2 with influenza on rVE estimates, a sensitivity analysis was conducted with an earlier study period cutoff date (September 29, 2019, through February 15, 2020). A third sensitivity analysis was based on a hypothetical complete influenza season (September 29, 2019, through May 16, 2020). Lastly, urinary tract infections (UTIs) were evaluated as a negative control outcome as they are not expected to be prevented or otherwise affected by the influenza vaccine and can be used to assess balance among cohorts as well as indicate residual bias in effect estimates. UTI-related visits were defined as an ICD-10 diagnosis of N39.0 in any position. A Cox regression model was used to evaluate UTIs to factor in seasonal variability in the frequency of UTIs [30–32].
RESULTS
Study Subjects
A total of 3 553 040 individuals were included in the study, of whom 936 508 (26.4%) had a record of receiving aIIV3, 651 034 (18.3%) IIV4e, and 1 813 819 (51.0%) HD-IIV3 during the vaccine intake period (Table 1). In addition, 151 679 (4.3%) subjects received IIV3e during the same period (Table 1). Table 2 lists the demographic characteristics of each cohort used in the rVE analysis (see Supplementary Table 5 for characteristics of the IIV3e cohort, including numbers of subjects with conditions included in the Charlson comorbidity index). The mean age (±SD) of aIIV3, IIV4e, and HD-IIV3 recipients was similar (75.0 ± 6.7, 74.2 ± 7.1, and 75.2 ± 6.9 years, respectively). Frailty index scores were as follows: aIIV3 group, 0.09 ± 0.09; IIV4e group, 0.10 ± 0.12; HD-IIV3 group, 0.10 ± 0.11. The same was true of Charlson comorbidity index scores: aIIV3, 1.4 ± 1.8; IIV4e, 1.7 ± 2.0; HD-IIV3, 1.6 ± 1.9. In all groups, most subjects were White and female; <15% were Hispanic. SMD differences between groups were generally between –0.05 and 0.05 (Table 2). An exception was region, where 56% of aIIV3 recipients resided in the South compared with ~36% of the other cohorts, and only 11% of aIIV3 recipients resided in the West, compared with 21%–23% of the other cohorts. Recipients of aIIV3 also had fewer baseline outpatient and inpatient visits (Table 2). Chronic pulmonary disease, diabetes, renal disease, and peripheral vascular disease were the most common medical conditions, with comparable rates across vaccine cohorts (Supplementary Table 4). There were no differences in the frequency of missing data between exposure groups (Table 1; Supplementary Table 4).
Table 1.
Subject Selection in the 2018–2019 Influenza Season
| Selection Criterion | No. (%) |
|---|---|
| 1. Received an influenza vaccine between August 1, 2019, and January 31, 2020 | 12 283 735 (100) |
| 2. Does not have ≥1 influenza immunization during the influenza season | 11 667 407 (95.0) |
| 3. ≥65 y of age at time of immunization | 4 674 721 (38.1) |
| 4. Does not have an influenza-related medical encounter before becoming fully vaccinated or before the influenza season | 4 670 971 (38.0) |
| 5. Has a transcript record in the Veradigm EMR ≥1 y before immunization date | 3 835 271 (31.2) |
| 6. Has activity in Komodo claims ≥1 y before immunization date | 3 587 238 (29.2) |
| 7. Does not have missing or conflicting data for age, gender, or geographic region | 3 553 040 (28.9) |
| aIIV3 recipients | 936 508 (26.4) |
| HD-IIV3 recipients | 1 813 819 (51.0) |
| IIV4e recipients | 651 034 (18.3) |
| IIV3e recipients | 151 679 (4.3) |
Abbreviations: aIIV3, adjuvanted inactivated quadrivalent influenza vaccine; EMR, electronic medical record; HD-IIV3, high-dose inactivated trivalent influenza vaccine; IIV4e, egg-based quadrivalent inactivated influenza vaccine.
Table 2.
Subject Demographics at Baseline
| Characteristic | aIIV3 (n = 936 508) |
IIV4e (n = 651 034) |
HD-IIV3 (n = 1 813 819) |
SMD aIIV3 vs IIV4e |
SMD aIIV3 vs HD-IIV3 |
|---|---|---|---|---|---|
| Mean age ± SD, y | 75.0 ± 6.7 | 74.2 ± 7.1 | 75.2 ± 6.9 | 0.05 | –0.02 |
| ≥65–≤74 y, No. (%) | 489 964 (52.3) | 372 868 (57.3) | 941 558 (51.9) | ||
| ≥75–≤84 y, No. (%) | 330 701 (35.3) | 197 129 (30.3) | 626 502 (34.5) | ||
| ≥85 y, No. (%) | 115 843 (12.4) | 81 037 (12.4) | 245 759 (13.5) | ||
| Female sex, No. (%) | 547 251 (58.4) | 381 138 (58.5) | 1 054 163 (58.1) | 0.00 | 0.00 |
| Race and ethnicity, No. (%) | |||||
| Black or African American | 35 506 (3.8) | 36 969 (5.7) | 86 709 (4.8) | –0.01 | –0.01 |
| White | 567 270 (60.6) | 337 374 (51.8) | 1 085 710 (59.9) | 0.03 | –0.01 |
| Other | 27 356 (2.9) | 49 050 (7.5) | 69 601 (3.8) | –0.02 | –0.02 |
| Not reported | 306 376 (32.7) | 227 641 (35.0) | 571 799 (31.5) | –0.01 | 0.03 |
| Hispanic | 32 336 (3.5) | 44 582 (6.8) | 59 507 (3.3) | –0.02 | 0.00 |
| Non-Hispanic | 795 072 (84.9) | 562 204 (86.4) | 1 534 000 (84.6) | ||
| Not reported | 109 100 (11.6) | 44 248 (6.8) | 220 312 (12.1) | ||
| Geographic region, No. (%) | |||||
| Northeast | 157 186 (16.8) | 143 583 (22.1) | 378 938 (20.9) | –0.02 | 0.00 |
| Midwest | 147 719 (15.8) | 116 805 (17.9) | 412 178 (22.7) | –0.01 | 0.00 |
| South | 526 419 (56.2) | 237 428 (36.5) | 645 854 (35.6) | 0.06 | 0.07 |
| West | 103 519 (11.1) | 150 226 (23.1) | 374 108 (20.6) | –0.03 | –0.08 |
| Other | 1665 (0.2) | 2992 (0.5) | 2741 (0.2) | 0.00 | 0.01 |
| Charlson comorbidity index ± SD | 1.4 ± 1.8 | 1.7 ± 2.0 | 1.6 ± 1.9 | –0.04 | –0.07 |
| Frailty index,a mean ± SD | 0.09 ± 0.09 | 0.10 ± 0.12 | 0.10 ± 0.11 | –0.04 | –0.05 |
| <5%, No. (%) | 274 365 (29.3) | 209 392 (32.2) | 553 025 (30.5) | 0.01 | –0.02 |
| ≥5%–<20%, No. (%) | 596 195 (63.7) | 375 066 (57.6) | 1 100 748 (60.7) | ||
| ≥20%, No. (%) | 65 948 (7.0) | 66 576 (10.2) | 160 046 (8.8) | ||
| Outpatient visits, mean ± SD | 1.0 ± 2.3 | 1.7 ± 3.2 | 1.7 ± 3.1 | –0.08 | –0.28 |
| All-cause hospitalizations, No. (%) | |||||
| 0 | 692 124 (73.9) | 427 514 (65.7) | 1 235 213 (68.1) | –0.04 | –0.06 |
| 1 | 153 869 (16.4) | 125 311 (19.2) | 333 474 (18.4) | ||
| ≥2 | 90 515 (9.7) | 98 209 (15.1) | 245 132 (13.5) | ||
| Place of service (data source), No. (%) | |||||
| Pharmacy-only claims | 672 751 (71.8) | 101 203 (15.5) | 586 152 (32.3) | ||
| Medical claims | 221 589 (23.7) | 449 819 (69.1) | 988 481 (54.5) | ||
| EMR-only claims | 42 168 (4.5) | 100 012 (15.4) | 239 186 (13.2) | ||
| No. recorded immunizations | |||||
| August 2019 | 63 579 (6.8) | 15 004 (2.3) | 17 416 (1.0) | ||
| September 2019 | 262 942 (28.1) | 153 998 (23.7) | 457 618 (25.2) | ||
| October 2019 | 423 696 (45.2) | 322 338 (49.5) | 829 140 (45.7) | ||
| November 2019 | 131 012 (14.0) | 96 013 (14.7) | 328 815 (18.1) | ||
| December 2019 | 40 248 (4.3) | 38 250 (5.9) | 123 955 (6.8) | ||
| January 2020 | 15 031 (1.6) | 25 431 (3.9) | 56 875 (3.1) | ||
Abbreviations: ADL, activities of daily living; eIIV3, egg-derived inactivated quadrivalent influenza vaccine; EMR, electronic medical record; SMD, standardized mean difference.
Frailty was approximated using a summary score for ADL [
] to represent an operational definition of frailty in claims data using ADL dependency as a proxy outcome (Supplementary Table 3).
Overall Influenza-Related Medical Encounters
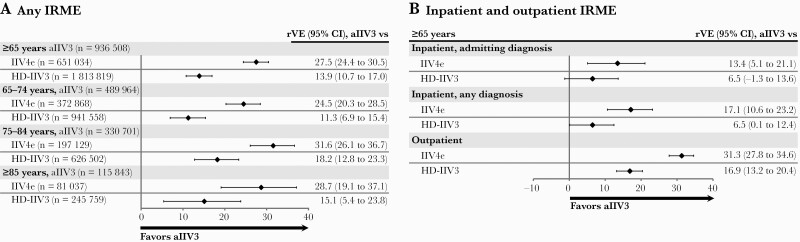
Influenza-related medical encounters were recorded in recipients of aIIV3 (4991, 0.5%), IIV4e (5585, 0.9%), and HD-IIV3 (12 154, 0.7%). Among all individuals age ≥65 years, the rVE adjusted using the doubly robust model was 27.5% (95% CI, 24.4% to 30.5%) vs IIV4e and 13.9% (95% CI, 10.7% to 17.0%) vs HD-IIV3 (Figure 1A; see Supplementary Figure 1 for unadjusted rVE analyses). Findings were consistent within each age group (Figure 1A).
Figure 1.
Relative vaccine effectiveness of aIIV3 compared with IIV4e and HD-IIV3 among individuals age ≥65 years in the 2019–2020 US influenza season using doubly robust IPTW adjustment methodology. A, Any influenza-related medical encounter. B, Inpatient (admitting diagnosis and any diagnosis) and outpatient influenza-related medical encounters. Abbreviations: aIIV3, adjuvanted trivalent inactivated influenza vaccine; HD-IIV3, high-dose inactivated influenza vaccine; IIV4e, egg-derived quadrivalent inactivated influenza vaccine; IPTW, inverse probability of treatment weights; IRME, influenza-related medical encounter; rVE, relative vaccine effectiveness.
Inpatient and Outpatient Influenza-Related Medical Encounters
Inpatient influenza-related medical encounters were recorded for 1500 (0.2%) aIIV3, 1638 (0.3%) IIV4e, and 3583 (0.2%) HD-IIV3 recipients, and 3491 (0.4%), 3947 (0.6%), and 8571 (0.5%) individuals from these cohorts had an outpatient influenza-related medical encounter. The rVE for an inpatient encounter (admitting diagnosis on the claim) was 13.4% (95% CI, 5.1% to 21.1%) vs IIV4e and 6.5% (95% CI, –1.3% to 13.6%) vs HD-IIV3. For a diagnosis in any diagnosis position on the claim, the rVE was 17.1% (95% CI, 10.6% to 23.2%) vs IIV4e and 6.5% (95% CI, 0.1% to 12.4%) vs HD-IIV3. Outpatient rVEs were 31.3% (95% CI, 27.8% to 34.6%) vs IIV4e and 16.9% (95% CI, 13.2% to 20.4%) vs HD-IIV3 (Figure 1B).
Sensitivity Analyses
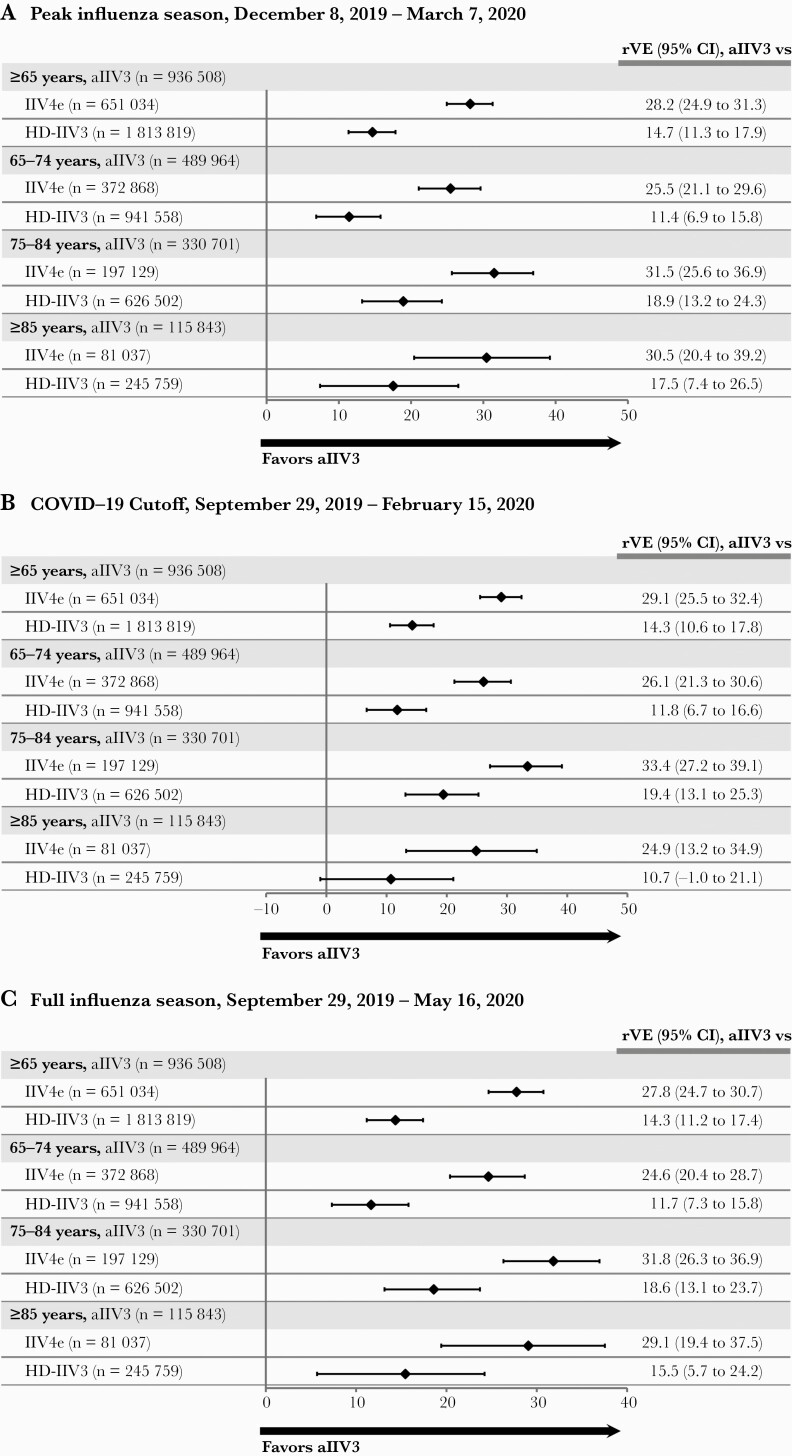
The rVE during the period of highest influenza activity (December 8, 2019, to March 7, 2020) (Supplementary Figure 2) generated slightly higher point estimates than in the main period, at 28.2% (95% CI, 24.9% to 31.3%) vs IIV4e and 14.7% (95% CI, 11.3% to 17.9%) vs HD-IIV3, with consistent trends observed when stratified by age group (Figure 2A). The pattern of rVEs remained consistent in the analyses that applied stricter criteria to account for SARS-COV-2 impact (Figure 2B and C), with positive rVE estimates and confidence intervals for all comparisons in all age groups except the rVE for aIIV3 vs HD-IIV3 in the ≥85 age group (10.7%; 95% CI, –1.0% to 21.1%). In the negative control analysis, the covariate-adjusted incidence of UTIs was 5.1% in the aIIV3 cohort and 6.0% in the IIV4e cohort, with a hazard ratio (HR) of 1.02 (95% CI, 1.00 to 1.03). When compared with HD-IIV3, the adjusted incidence was 5.0% in the aIIV3 cohort and 5.0% in the HD-IIV3 cohort, with an HR of 0.99 (95% CI, 0.98 to 1.00).
Figure 2.
Sensitivity analyses determining rVE aIIV3 compared with IIV4e and HD-IIV3 among individuals age ≥65 years in the 2019–2020 US influenza season using doubly robust IPTW adjustment methodology. A, Restricted season with peak influenza activity between December 8, 2019, and March 7, 2020. B, COVID-19 onset cutoff analysis, September 29, 2019, through February 15, 2020. C, Full influenza season analysis, September 29, 2019, through May 16, 2020. Abbreviations: aIIV3, adjuvanted trivalent inactivated influenza vaccine; COVID-19, coronavirus disease 2019; HD-IIV3, high-dose inactivated influenza vaccine; IIV4e, egg-derived quadrivalent inactivated influenza vaccine; IPTW, inverse probability of treatment weights; rVE, relative vaccine effectiveness.
DISCUSSION
In this study cohort of >3 million influenza vaccine recipients in the 2019–2020 flu season, aIIV3 was more effective than IIV4e and HD-IIV3 in preventing influenza-related medical encounters in adults ≥65 years of age. The adjusted rVE of aIIV3 in preventing any influenza-related medical encounter was 27.5% (95% CI, 24.4% to 30.5%) vs IIV4e and 13.9% (95% CI, 10.7% to 17.0%) vs HD-IIV3. Similar trends were observed in subgroup analyses by age. Confidence intervals increased with age, most likely due to decreasing sample size. Relatively high point estimates and narrow confidence intervals for the outpatient visits likely drove the overall point estimates, particularly for the aIIV3 vs HD-IIV3 comparison. The rVE values for inpatient (admitting and any diagnosis) and outpatient influenza-related medical encounters were significant in the comparison between aIIV3 and IIV4e. However, the inpatient rVE for aIIV3 vs HD-IIV3 was not statistically significant when influenza was the admitting diagnosis, although it was significant when influenza was reported during any diagnosis.
Overall, in the United States, the burden of influenza in the 2019–2020 season included 400 000 influenza-related hospitalizations and 22 000 deaths [33]. Influenza virus activity was lower than in prior influenza seasons and consisted of 2 waves, first of influenza B, followed by A(H1N1) in the overall population. Influenza A(H1N1) was the predominant strain in the older adult population and accounted for 73% of all circulating viruses [33, 34]. Absolute vaccine effectiveness was estimated to be 39% (95% CI, 32% to 44%) overall and 39% (95% CI, 9% to 59%) in adults age ≥65 years by the US Centers for Disease Control and Prevention [35].
Both high-dose and adjuvanted influenza vaccines have been developed to overcome immunosenescence in older adults. MF59 has been found to increase both the magnitude and the breadth of the immune response as compared with traditional inactivated influenza vaccines [36]. This may explain the greater effectiveness over comparators across all age groups. In the case of the aIIV3 vs IIV4e comparison, the advantages of the fourth antigen in the quadrivalent vaccine may have been outweighed by the immunogenicity boost from the MF59 adjuvant. As HD-IIV3 also increases the magnitude of the immune response [37], the additional breadth of protection against variant strains as seen with the adjutant may account for the benefits of aIIV3 against HD-IIV3 observed in this study.
Our results add to a body of literature that shows that adjuvanted vaccines are at least as effective as, and possibly more effective than, high-dose vaccines [10, 17, 38]. In previous studies conducted using the same databases, we found improved effectiveness of aIIV3 relative to HD-IIV3 in the 2017–2018 and 2018–2019 seasons [17]. Other studies evaluating aIIV3 vs HD-IIV3 differed with respect to study population, setting, influenza case definition, and methodological aspects. In particular, researchers have previously demonstrated that the vaccine effectiveness of aIIV3 and HD-IIV3 did not differ significantly, but both vaccines were significantly more effective than IIV4e [38]. As larger numbers of people receive aIIV3, we may gain a more comprehensive understanding of the effectiveness of aIIV3 in real-world settings.
Our study had several strengths. The use of a database integrating EMR and claims data permitted evaluation of a large cohort of older adults, providing robust statistical power to detect effects. The variety and completeness of data permitted adjustment of well-established confounders. Additionally, retrospectively ascertaining covariate information from the integrated database in the same manner for all vaccine cohorts limited information bias. Furthermore, we applied a doubly robust adjustment methodology in all analyses. Conclusions from the main analysis were supported by sensitivity analyses. The negative control analysis showed no differences between the 3 cohorts in UTI rates, and effect estimates were nonsignificant, suggesting that the doubly robust adjustment adequately addressed confounding by the included measured confounders.
A key limitation of this study is the reliance on diagnostic codes for influenza disease rather than a laboratory-confirmed influenza diagnosis. However, results were consistent when limited to a period of high incidence of CDC-reported laboratory-confirmed influenza (Figure 2A) [34]. Moreover, incidence rates of CDC-reported, laboratory-confirmed influenza showed a similar trend when compared with the incidence of influenza-related medical encounters in the study cohort (Supplementary Figure 2). While this is not a validation of the outcome definition per se, it does support the use of these diagnostic codes for the identification of influenza disease. Another limitation of this study was that older adults will likely be covered by Medicare, which makes the results more generalizable to the overall US older adult population. The type of health care insurance coverage received was not evaluated and may impact health care–seeking behavior. Finally, residual confounding is possible in all observational research and is particularly prominent in studies using routinely collected data, as these data are not specifically collected for research purposes.
CONCLUSIONS
In this analysis of a large integrated EMR and medical claims database, aIIV3 was associated with significantly fewer influenza-related medical encounters than IIV4e and HD-IIV3 in adults ≥65 years of age during the 2019–2020 influenza season in the United States. These findings are consistent with previous study results and lend further support to the use of aIIV3 to prevent influenza illness in older individuals [5, 7, 9–13, 17, 39–41].
Supplementary Material
Acknowledgments
Consultants C. Gordon Beck and Amanda M. Justice provided editorial support in the preparation of this manuscript, which was funded by Seqirus Inc.
Financial support. This work was supported by Seqirus Inc.
Potential conflicts of interest. M.I. and C.B. are employees of Seqirus Inc. J.A.M. was an employee of Seqirus during study conduct. J.R.O. reports that his institution has received vaccine research grants from the Bill and Melinda Gates Foundation, GlaxoSmithKline, Pfizer, PATH, WHO, and National Institutes of Health. He has received honoraria and travel expenses from Pfizer and Seqirus to serve on scientific advisory boards. He serves on a data and safety monitoring board and has been an independent data monitor for non-influenza-related research conducted by Pharmaron. M.B., L.F., and D.O. are paid employees of Veradigm, which received a research contract to conduct this study with and on behalf of Seqirus Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. M.I., C.B., and J.A.M. were involved in study conception, design, and conceptual frameworks. L.F., D.O., and M.B. were involved in the analysis. J.P.B., and J.R.O. provided regular feedback on each of these steps. All authors were involved in the interpretation of data. M.I. and C.B. were involved in drafting the manuscript, and L.F., D.B., M.B., J.A.M., J.P.B., and J.O. revised the paper critically. All authors made substantive intellectual contributions to the development of this manuscript and approved the final version.
Patient consent. This retrospective cohort study does not include factors necessitating patient consent.
References
- 1. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coleman BL, Fadel SA, Fitzpatrick T, Thomas SM.. Risk factors for serious outcomes associated with influenza illness in high- versus low- and middle-income countries: systematic literature review and meta-analysis. Influenza Other Respir Viruses 2018; 12:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB.. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019-20 influenza season. MMWR Recomm Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 influenza season. MMWR Recomm Rep 2020; 69:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coleman BL, Sanderson R, Haag MDM, McGovern I.. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses 2021; 15:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med 2017; 5:200–11. [DOI] [PubMed] [Google Scholar]
- 7. Van Buynder PG, Konrad S, Van Buynder JL, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31:6122–8. [DOI] [PubMed] [Google Scholar]
- 8. Frey SE, Reyes MR, Reynales H, et al. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027–34. [DOI] [PubMed] [Google Scholar]
- 9. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017-2018. J Infect Dis 2019; 220:1255–64. [DOI] [PubMed] [Google Scholar]
- 10. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J Infect Dis 2020; 222:278–87. [DOI] [PubMed] [Google Scholar]
- 11. Lapi F, Marconi E, Simonetti M, et al. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Exp Rev Vaccines 2019; 18:663–70. [DOI] [PubMed] [Google Scholar]
- 12. Pebody R, Whitaker H, Zhao H, et al. Protection provided by influenza vaccine against influenza-related hospitalisation in ≥65 year olds: early experience of introduction of a newly licensed adjuvanted vaccine in England in 2018/19. Vaccine 2020; 38:173–9. [DOI] [PubMed] [Google Scholar]
- 13. Bella A, Gesualdo F, Orsi A, et al. Effectiveness of the trivalent MF59 adjuvated influenza vaccine in preventing hospitalization due to influenza B and A(H1N1)pdm09 viruses in the elderly in Italy, 2017-2018 season. Exp Rev Vaccines 2019; 18:671–9. [DOI] [PubMed] [Google Scholar]
- 14. Ansaldi F, Zancolli M, Durando P, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010; 28:4123–9. [DOI] [PubMed] [Google Scholar]
- 15. Ansaldi F, Bacilieri S, Durando P, et al. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008; 26:1525–9. [DOI] [PubMed] [Google Scholar]
- 16. Scheifele DW, McNeil SA, Ward BJ, et al. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults: results of a randomized, controlled comparison. Hum Vaccin Immunother 2013; 9:2460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boikos C, Fischer L, O’Brien D, Vasey J, Sylvester GC, Mansi JA.. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in US adults ≥65 years during the 2017-2018 and 2018-2019 influenza seasons. Clin Infect Dis 2021; 73:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilkinson K, Wei Y, Szwajcer A, et al. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine 2017; 35:2775–80. [DOI] [PubMed] [Google Scholar]
- 19. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Exp Rev Vaccines 2018; 17:435–43. [DOI] [PubMed] [Google Scholar]
- 20. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 21. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X, Blanton L, Elal AIA, et al. Update: influenza activity in the United States during the 2018-19 season and composition of the 2019-20 influenza vaccine. MMWR Morb Mortal Wkly Rep 2019; 68:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armed Forces Health Surveillance Center (AFHSC). AFHSC Standard Case Definitions: Influenza-Like Illness. Defense Health Agency; 2015. [Google Scholar]
- 24. Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015; 24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 26. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA.. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57:1288–94. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M.. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vega T, Lozano JE, Meerhoff T, et al. Influenza surveillance in Europe: establishing epidemic thresholds by the moving epidemic method. Influenza Other Respir Viruses 2013; 7:546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson JE. Seasonality of symptomatic bacterial urinary infections in women. J Epidemiol Community Health 1983; 37:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosello A, Pouwels KB, Domenech DECM, et al. Seasonality of urinary tract infections in the United Kingdom in different age groups: longitudinal analysis of The Health Improvement Network (THIN). Epidemiol Infect 2018; 146:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM.. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998-2011. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States—2019-2020 influenza season. 2021. Available at: https://www.cdc.gov/flu/about/burden/2019-2020.html. Accessed 30 August 2021.
- 34. Centers for Disease Control and Prevention. FluView summary ending on September 26, 2020. 2020. Available at: https://www.cdc.gov/flu/weekly/weeklyarchives2019-2020/Week39.htm. Accessed 25 March 2021.
- 35. Centers for Disease Control and Prevention. US flu VE data for 2019-2020. 2020. Available at: https://www.cdc.gov/flu/vaccines-work/2019-2020.html. Accessed 23 August 2021.
- 36. O’Hagan D, Ott GS, De Gregorio E, Seubert A.. The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine 2012; 30:4341–8. [DOI] [PubMed] [Google Scholar]
- 37. Diaco M, Chang LJ, Seet B, et al. Introductory paper: high-dose influenza vaccine. Vaccine 2021; 39(Suppl 1):A1–5. [DOI] [PubMed] [Google Scholar]
- 38. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among U.S. Medicare beneficiaries ages 65 years and older during the 2019-20 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed] [Google Scholar]
- 39. Mannino S, Villa M, Apolone G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol 2012; 176:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gravenstein S, Davidson HE, Mcconeghy K, et al. A cluster-randomized trial of adjuvanted trivalent influenza vaccine vs. standard dose in U.S. nursing homes. Paper presented at: IDWeek; 3–7 October 2018; San Francisco, CA. [Google Scholar]
- 41. Yang J, Zhang J, Han T, et al. Effectiveness, immunogenicity, and safety of influenza vaccines with MF59 adjuvant in healthy people of different age groups: a systematic review and meta-analysis. Medicine 2020; 99:e19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.