Abstract
Background
Besides antistaphylococcal beta-lactams and source control, there are limited validated antimicrobial salvage options in patients with prolonged methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, including infective endocarditis (IE).
Methods
MSSA IE cases treated with ertapenem (ETP) plus cefazolin (CZ) were compared with matched IE cases treated with standard beta-lactam monotherapy. The bactericidal activity of ETP plus CZ was also compared with nafcillin (NAF), CZ, and ETP alone using an in vitro MSSA biofilm model.
Results
The median duration of bacteremia experienced by patients (n = 12) while on CZ or NAF was 4 days (range 1–16 days) compared with 1 day (range 1–3 days) for patients (n = 5) treated with ETP + CZ (P = .01, Mann-Whitney U test). Cefazolin and NAF alone or in combination did not achieve biofilm eradication at clinically relevant concentrations. However, the addition of ETP to CZ led to bactericidal eradication within biofilms at standard dosing.
Conclusions
Ertapenem reduces CZ concentrations required to eradicate MSSA biofilms to those achievable in vivo by standard dosing, translating into shorter bacteremia duration in patients with MSSA endocarditis. Larger studies are needed to investigate ETP plus CZ therapy in the treatment of biofilm-related MSSA infections such as endocarditis.
Keywords: cefazolin, ertapenem, endocarditis, Staphylococcus aureus
Ertapenem reduces cefazolin concentrations required to eradicate methicillin-susceptible Staphylococcus aureus(MSSA) biofilms to those achievable in vivo by standard dosing, translating into shorter bacteremia duration in patients with MSSA endocarditis.
Staphylococcus aureus is one of the most common pathogens causing bacteremia and is a leading cause of bacterial endocarditis, particularly in developed countries [1, 2]. Although persistent methicillin-susceptible S aureus (MSSA) bacteremia (≥3 days) is a predictor of mortality [3], minimal data exist on enhanced pharmacotherapy to facilitate bacteremia clearance [4]. We have recently shown that ertapenem (ETP) plus cefazolin (CZ) was successful in clearing refractory MSSA bacteremia [5, 6] and validated the enhanced potency of this combination in a rat model of MSSA endocarditis [6]. However, the high degree of bactericidal activity in vivo was not predicted by in vitro studies showing only additive/borderline synergy [6]. Therefore, further explanation of ETP plus CZ potency against MSSA is needed.
Given that core pathophysiological mechanisms in S aureus endocarditis involve bacterial adherence, colonization, and survival within a biofilm matrix of fibrin, platelets, and inflammatory cells adhered to the endocardium [7], we hypothesized that ETP plus CZ therapy may exhibit antistaphylococcal activity within biofilms. The purposes of this study were as follows: (1) to examine the microbiological outcomes of MSSA endocarditis treated with ETP plus CZ, and determine whether benefits were seen in shortening bacteremia duration when compared with clinically matched cases treated with conventional beta-lactam monotherapy (ie, nafcillin [NAF], cefazolin); (2) to assess the biofilm-producing phenotypes of the MSSA endocarditis cases treated with ETP plus CZ using a physiologically relevant in vitro assay; and (3) to evaluate minimum biofilm eradication concentration (MBEC) of ETP and CZ in comparison to standard antistaphylococcal beta-lactam antibiotics against MSSA endocarditis isolates grown as biofilms.
METHODS
Clinical Cases
A retrospective matched case control study was conducted at Sharp Memorial Hospital (San Diego, CA) and Sharp Grossmont Hospital (La Mesa, CA) that compared the duration of bacteremia in patients with endocarditis treated with standard of care (SOC) monotherapy (controls) to those treated with ETP + CZ (study cases). Right- or left-sided endocarditis was determined by echocardiographic findings. A subset of the study cases (isolates 3 and 4) was included in a previously published noncomparative case series [6], but in this study we focus exclusively on those patients with infective endocarditis. Each study case was matched to 2–3 control cases receiving SOC based on age (within 10 years) and renal function (creatinine clearance, >50 mL/min; <50 mL/min, no dialysis; ongoing dialysis). Electronic medical records were reviewed to gather relevant clinical data such as vital signs, demographics, laboratory data (eg, white blood cell count, C-reactive protein, renal function, blood culture, and echocardiogram results), and need for intensive care unit support. This information was used to compute Charlson Comorbidity Index and Pitt Bacteremia Scores for comparative statistics between the groups. Of note, none of the controls or study cases were deemed in need of surgical source control to achieve bacteremia clearance.
Patient Consent Statement
The research protocol was approved by the Sharp Institutional Review Board (IRB). An IRB exemption was granted for the retrospective chart review; therefore, written consent was not obtained.
Bacterial Isolates
A total of 10 clinical MSSA bloodstream isolates were studied from patients with (n = 5) and without (n = 5) endocarditis. The 5 endocarditis isolates (3, 4, 12, 13, 15) were obtained from the 5 study patients from our retrospective matched case control study. Isolates 1–6 were derived from previously published cases of patients with endocarditis (isolates 3 and 4) and without (isolates 1, 2, 5, and 6) treated with ETP + CZ therapy [6].
Antibiotics and In Vitro Susceptibility Assays
All antibiotics were purchased from the Sharp Memorial Hospital Pharmacy (San Diego, CA) and supplied as vials available for clinical use and administration to patients. The MSSA isolates were evaluated for in vitro susceptibility to ETP, CZ, and NAF under both standard (105 colony-forming units [CFU]/mL) and high-inoculum (107 CFU/mL) conditions using Roswell Park Memorial Institute (RPMI) physiological cell culture media based on Clinical and Laboratory Standards Institute guidelines. Minimum bactericidal concentration (MBC) values were determined as the lowest antibiotic concentration that resulted in no growth in the well.
Endocarditis Biofilm Model Assay
To mimic biofilms that occur in vivo during endocarditis, we developed a novel robust in vitro biofilm assay using collagen-coated plates and media with the addition of plasma (RPMI 1640 + 20% pooled human plasma). A previously published cohort of MSSA bloodstream isolates [8] was used to validate the endocarditis biofilm assay as a discriminatory marker of endocarditis. In brief, we coated MBEC Assay peg lids (Innovotech, Alberta, Canada) with collagen (5 μg/cm2; Gibco Collagen I, rat tail; Thermo Fisher Scientific, Waltham, MA). The base wells were filled with RPMI 1640 medium (Gibco, Thermo Fisher Scientific) with 20% pooled human plasma from healthy donors (sodium citrate anticoagulant; Innovative Research, Novi, MI), inoculated with 1 × 106 CFU/mL of each isolate, and incubated for 24 hours at 37°C with gentle shaking. Biofilms formed on pegs were gently washed 3 times with phosphate-buffered saline (PBS) to remove nonadherent bacteria and stained with 0.1% crystal violet (CV). Plates were incubated with CV for 15 minutes and subsequently washed 3 times gently with PBS to remove unincorporated stain, and the remaining stain was solubilized with 100% ethanol. Absorbance (570 nm) was measured on a microtiter plate reader (Synergy 2; BioTek Instruments, Inc., Winooski, VT). Higher absorbance values corresponded to more stain uptake and, therefore, a higher formation of biofilm biomass.
Minimum Biofilm Eradication Concentration
Biofilm eradication experiments were performed in the endocarditis biofilm model assay described above with some modifications. After biofilms were established on the pegs for 24 hours, the peg lid containing the established biofilms was removed, washed 3 times with PBS, and transferred to another 96-well plate containing serial dilutions of each antibiotic (ETP, CZ, or NAF) and incubated for an additional 24 hours at 37°C with gentle shaking. The lid was then removed from the plate containing antibiotic, washed 3 times gently with PBS to remove any residual antibiotic, placed into a new 96-well base plate containing fresh RPMI + 5% plasma and 200 µg/mL proteinase-K to disperse any remaining biofilms on the pegs into the fresh media in the base well, and incubated for 24 hours at 37°C. The MBEC values were determined as the lowest test concentration that resulted in no growth in the well.
Antibiotic Checkerboard Assay
Antibiotic synergy was determined using the endocarditis biofilm model as described above with minor modifications. Established biofilms were challenged with a combination of ETP + CZ, ETP + NAF, or NAF + CZ. The antibiotic plate was set up as a checkerboard to determine the antibiotic concentrations that resulted in complete eradication of the biofilm; a well with no antibiotic served as a negative control. Serial 2-fold dilutions of one antibiotic were added vertically along the numbered columns (1–11 on the long side of the 96-well plate), and serial 2-fold dilutions of the second antibiotic were added horizontally along the lettered rows (A–G on the short side of the 96-well plate). The checkerboard assay was incubated for 24 hours at 37°C with gentle shaking. The lid was then removed from the checkerboard plate, washed 3 times gently with PBS to remove any residual antibiotic, placed in a new 96-well plate containing fresh RPMI + 5% plasma and 200 µg/mL proteinase-K to disperse any remaining biofilms on the pegs into the fresh media in the base well, and incubated for 24 hours at 37°C. Combination MBEC values were determined as the lowest test concentration that resulted in no growth in the well. Synergy was calculated as the fractional inhibitory concentration (FIC) index value as follows: FIC = FIC (antibiotic 1) + FIC (antibiotic 2), where FIC (antibiotic 1) is the MBEC of antibiotic 1 in combination divided by the MBEC of antibiotic 1 alone. The combination was considered synergistic for FIC values ≤0.5, additive for FIC values >0.50 to ≤1.0, indifferent for FIC values >1 to ≤4, and antagonistic for FIC values >4.
Biofilm Imaging and Bacterial Density Determination
Biofilms were grown as above on collagen-coated, 24-well, tissue culture plates for 24 hours before washing 3 times gently with PBS and replacing with fresh RPMI + 5% plasma. Established biofilms were left untreated or treated with each antibiotic alone or in combination at the maximum serum concentration per the Johns Hopkins Antibiotic Guide [9]. Treatments were carried out for 24 hours before determination of bacterial load. Posttreatment biofilms were washed 3 times with warm PBS to remove any remaining antibiotic, incubated with PBS containing 200 μg/mL proteinase-K for 15 minutes to disperse biofilms, and dispersed biofilms were enumerated for bacterial concentration (CFU/mL) by plating of serial dilutions and counting colonies at 24 hours. In addition, biofilms were fixed with 4% paraformaldehyde and stained with Invitrogen LIVE/DEAD BacLight Bacterial Viability Kit (Thermo Fisher Scientific) per manufacturers’ instructions. Images were collected on an Olympus IX73 inverted scope with DP80 camera using appropriate fluorescent channels (Olympus Corporation, Shinjuku, Tokyo, Japan).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism, version 9.2.1. P < .05 were considered significant.
RESULTS
Ertapenem Plus Cefazolin Resulted in Shorter Duration of Methicillin-Susceptible Staphylococcus aureus Bacteremia in Patients With Endocarditis
Five patients with MSSA endocarditis treated with ETP + CZ were matched to 12 control patients treated with standard beta-lactam monotherapy. Study and control patients had median (mean) ages of 34 (41) and 29 (36) years, respectively. Mean Pitt bacteremia scores were 1.8 for both groups, and Charlson comorbidity indices were 1.6 and 1.4 for study and control groups, respectively.
All study patients had persistent MSSA bacteremia and ETP + CZ was used as a salvage regimen, with a median of 6 days of prior therapy (range 3–16 days). After switching to ETP + CZ, the median time for bacteremia clearance was 1 day (range 1–3 days). The control group had a median bacteremia duration of 4 days (range 1–11 days). Combining the study (n = 5) and control patients (n = 12), median duration of bacteremia while on nafcillin or cefazolin was 4 days (range 1–16 days) compared with 1 day (range 1–3 days) on ETP + CZ (P = .01, Mann-Whitney U test) as shown in Figure 1.
Figure 1.
Total bacteremia days on standard versus ertapenem plus cefazolin (ETP + CZ) therapy. Duration of methicillin-susceptible Staphylococcus aureus bacteremia in 17 patients with endocarditis (12 control patients + 5 study patients before salvage with ETP + CZ) while on standard therapy (cefazolin or nafcillin), compared with the 5 cases while on ETP + CZ salvage therapy. The 5 study patients are denoted as open squares in the standard therapy column. Median values are denoted by the horizontal bar. Median of bacteremia days was significantly lower with ETP + CZ than standard therapy (1 day vs 4 days, P = .01, Mann-Whitney U test).
Higher Biofilm Production From Methicillin-Susceptible Staphylococcus aureus Causing Endocarditis
A physiologic assay of biofilm production was applied to 10 MSSA bacteremia isolates differentiated by the presence or absence of endocarditis. Pilot studies utilizing previously characterized clinical MSSA bloodstream isolates showed more rapid aggregation of MSSA from cases of endocarditis in pooled human plasma than those not associated with endocarditis. Application of the endocarditis biofilm model against 10 MSSA from ETP + CZ-treated patients found all isolates could form biofilms. However, MSSA from cases of endocarditis had significantly increased biofilm formation compared to those without endocarditis (P < .008) (Figure 2).
Figure 2.
Biofilm production of methicillin-susceptible Staphylococcus aureus (MSSA) clinical isolates from patients with and without endocarditis. A total of 10 MSSA clinical isolates were grown as biofilms in a simulated endocarditis model. Higher absorbance values correspond to a higher formation of biofilm biomass. The MSSA isolates from patients (n = 5) with a positive transesophageal echocardiography (TEE) indicative of endocarditis had more biofilm production compared with those with a negative TEE or no endocarditis (n = 5, P < .008). Lines represent the mean biofilm biomass. Statistical analysis was performed using an unpaired t test.
Ertapenem Plus Cefazolin Synergy Against Methicillin-Susceptible Staphylococcus aureus in Biofilm
Minimum bactericidal concentrations were determined for cefazolin, ertapenem, and nafcillin using standard and high inocula (105 and 107 CFU/mL, respectively) across 10 clinical MSSA bloodstream isolates (Table 1). Considerable reductions of bactericidal activities were seen for all antibiotics at higher inoculum against all strains, with increases in MBC. This represents a well established rationale for the importance of surgical source control in high-inoculum infections, such as S aureus endocarditis, and highlights the challenges of antimicrobial monotherapy in stabilizing such infections.
Table 1.
Minimum Bactericidal Concentrations of Antibiotics Under Standard (105 CFU/mL) and High Inocula (107 CFU/mL) for 10 MSSA Bloodstream Isolatesa
| MBC (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Ertapenem | Cefazolin | Nafcillin | ||||
| Isolate | 105 | 107 | 105 | 107 | 105 | 107 |
| 1 | 2 | 32 | 2 | 4 | 0.5 | 8 |
| 2 | 0.125 | 32 | 0.125 | 4 | 0.5 | 8 |
| 3 | 0.25 | 64 | 0.25 | 32 | 0.25 | 16 |
| 4 | 0.25 | 32 | 0.125 | 16 | 0.125 | 8 |
| 5 | 0.125 | 32 | 0.125 | 8 | 0.125 | 8 |
| 6 | 0.5 | 32 | 0.25 | 16 | 0.5 | 16 |
| 12 | 2 | 32 | 2 | 8 | 2 | 4 |
| 13 | 1 | 32 | 1 | 16 | 1 | 16 |
| 14 | 0.125 | 16 | 0.0625 | 8 | 0.125 | 8 |
| 15 | 2 | 32 | 2 | 8 | 2 | 8 |
Abbreviations: CFU, colony-forming unit; MBC, minimum bactericidal concentration; MSSA, methicillin-susceptible Staphylococcus aureus.
Minimum bactericidal concentration (MBC) values were determined as the lowest antibiotic concentration that resulted in no growth in the well. The 5 endocarditis isolates are highlighted.
To determine whether ETP enhanced the activity of CZ in biofilms, we first calculated the minimum bacterial eradicating concentration (MBEC) of each antibiotic against each isolate alone. We then performed a checkerboard assay to determine whether ETP lowered the MBEC of CZ or NAF. Ertapenem in combination with CZ showed synergy with an FIC ≤0.50 against all isolates tested in the endocarditis biofilm model described above (Table 2). Ertapenem in combination with NAF had an additive effect (FIC >0.50 to ≤1.0) against 9 of the MSSA isolates. The NAF and CZ combination showed additivity against 4 isolates and no interaction (FIC >1 to ≤4) against the other 6 MSSA isolates. No combination was antagonistic (FIC >4) against the MSSA isolates.
Table 2.
Minimum Biofilm Eradicating Concentrations of Antibiotics Alone and in Combination for 10 MSSA Isolatesa
| Isolate | Ertapenem | Cefazolin | Nafcillin | Ertapenem + Cefazolin | Ertapenem + Nafcillin | Cefazolin + Nafcillin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ertapenem | Cefazolin | FIC | Ertapenem | Nafcillin | FIC | Cefazolin | Nafcillin | FIC | ||||
| 1 | 1024 | 2048 | 2048 | 64 | 32 | 0.08 | 512 | 128 | 0.6 | 1024 | 128 | 0.6 |
| 2 | 1024 | 2048 | 2048 | 256 | 32 | 0.27 | 512 | 256 | 0.6 | 1024 | 512 | 0.8 |
| 3 | 2048 | 2048 | 2048 | 128 | 64 | 0.09 | 1024 | 512 | 0.8 | 2048 | 1024 | 1.5 |
| 4 | 2048 | 2048 | 2048 | 128 | 64 | 0.09 | 1024 | 1024 | 1.0 | 2048 | 2048 | 2.0 |
| 5 | 2048 | 2048 | 2048 | 256 | 64 | 0.16 | 1024 | 1024 | 1.0 | 2048 | 2048 | 2.0 |
| 6 | 1024 | 2048 | 2048 | 256 | 32 | 0.27 | 1024 | 1024 | 1.5 | 2048 | 2048 | 2.0 |
| 12 | 2048 | 2048 | 2048 | 256 | 64 | 0.16 | 1024 | 128 | 0.6 | 2048 | 128 | 1.1 |
| 13 | 2048 | 2048 | 2048 | 128 | 64 | 0.09 | 256 | 256 | 0.3 | 512 | 512 | 0.5 |
| 14 | 2048 | 2048 | 2048 | 256 | 64 | 0.16 | 1024 | 256 | 0.6 | 2048 | 512 | 1.3 |
| 15 | 2048 | 2048 | 2048 | 256 | 64 | 0.16 | 1024 | 64 | 0.5 | 2048 | 64 | 1.0 |
Abbreviations: FIC, fractional inhibitory concentration; MSSA, methicillin-susceptible Staphylococcus aureus.
Fractional inhibitory concentration indices were interpreted as follows: synergy, FIC of ≤0.50; additivity, FIC of >0.50 to ≤1.0; no interaction (indifference), FIC of >1 to ≤4; antagonism, FIC of >4. The 5 endocarditis isolates are highlighted.
To place these MBEC values in the context of antibiotic concentrations in vivo with standard dosing, we present pharmacokinetic curves of ertapenem (Figure 3A), cefazolin (Figure 3B), and nafcillin (Figure 3C) superimposed with the average minimum inhibitory concentration (MIC), MBC, and MBEC of the MSSA isolates used in this study. Examination of Figure 3B demonstrates that only the addition of ETP to CZ lowered the MBEC of CZ to concentrations achieved with standard CZ dosing.
Figure 3.
Pharmacokinetics of ertapenem (A), cefazolin (B), and nafcillin (C) against methicillin-susceptible Staphylococcus aureus (MSSA) clinical isolates. Antibiotic concentrations (µg/mL) over time were superimposed with the average minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum biofilm eradication concentration (MBEC) of the MSSA isolates used in this study. Antibiotic concentrations were based off the maximum serum concentration and half-life from the Johns Hopkins Antibiotic Guide [9]. The addition of ertapenem to cefazolin lowered the MBEC of cefazolin to physiologically achievable concentrations (B). The individual MBC and MBEC data for each MSSA isolate are presented in Tables 1 and 2, respectively.
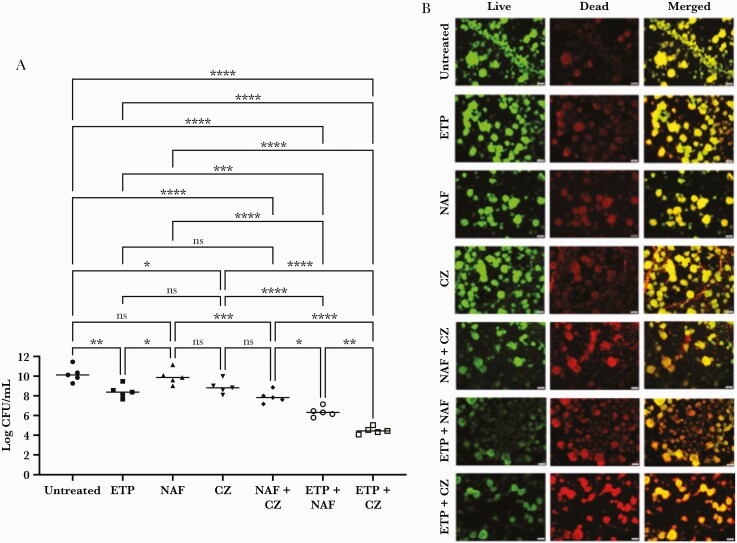
The activities of ETP, CZ, and NAF alone and in combination on preformed biofilms were assessed against the MSSA isolates from the 5 endocarditis cases shown in Figure 2. Cefazolin and NAF were not significantly different in their antistaphylococcal activities within biofilms (Figure 4A). Ertapenem monotherapy did show surprising activity within biofilms against MSSA, significantly exceeding that of NAF. The addition of ETP to either NAF or CZ resulted in greater activity than either agent alone. Ertapenem plus CZ showed the greatest activity, achieving 5 log10 reduction compared with untreated and 2 log10 lower CFU than ETP + NAF, the second-most active combination (Figure 4A).
Figure 4.
Synergistic killing of methicillin-susceptible Staphylococcus aureus (MSSA) biofilms by ertapenem plus cefazolin (ETP + CZ). (A) Preformed MSSA biofilms were treated with ETP, nafcillin (NAF), and CZ alone and in combination. Ertapenem plus CZ was the most bactericidal against MSSA biofilms (P < .0001), followed by ETP + NAF (P < .0001). Bars represent the mean ± standard deviation of the 5 MSSA endocarditis isolates from Figure 2. Statistical analyses were performed using a one-way analysis of covariance with 1 MSSA isolate covariate, and Tukey post hoc test. (B) Representative images of the MSSA isolate with the greatest biofilm production (isolate 4) were obtained before and after treatment with ETP (155 µg/mL), NAF (49 µg/mL), and CZ (188 µg/mL) alone or in combination. Antibiotic concentrations were based off the maximum serum concentration from the Johns Hopkins Antibiotic Guide [9]. Live cells are green, dead cells are red, and the merged images are yellow. Ertapenem plus CZ demonstrated the greatest killing against this MSSA biofilm isolate. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001. CFU, colony-forming units; ns, not significant.
The MSSA isolate with the greatest biofilm production (isolate 4) was used to demonstrate this effect microscopically (Figure 4B). This effect is appreciated by the greatest number of dead cells (red) seen in the merged images of ETP + CZ.
DISCUSSION
Building on previous findings that ETP + CZ was an effective salvage regimen in persistent MSSA bacteremia [5, 6], ETP + CZ rapidly cleared bacteremia (within 3 days) in high-risk patients with evidence of MSSA endocarditis by echocardiography. Some of these patients achieved rapid bacteremia clearance despite very large cardiac vegetations, as previously described [6]. Duration of bacteremia while on ETP + CZ therapy was significantly shorter than on NAF or CZ monotherapy.
The initial rationale for using this regimen was to target complementary penicillin-binding proteins (PBPs) involved in staphylococcal cell wall synthesis by ETP and CZ [10], analogous to the ampicillin plus ceftriaxone strategy used in Enterococcus faecalis endocarditis [11]. However, additional mechanisms are likely involved given the modest synergy observed in vitro. One possibility for the profound effect in vivo may be the sensitization of MSSA exposed to both antibiotics to the innate immune system. Compared to either drug alone, MSSA exposed to sub-MIC concentrations of both CZ and ETP are more effectively killed by the human host defense peptide cathelicidin LL-37 or neutrophils [5].
Given the relevance of bacterial biofilms in the pathophysiology of endocarditis, in this study we tested the hypothesis that ETP plus CZ possesses potent activity against MSSA within biofilms. Results showed that MSSA from patients with confirmed endocarditis had higher biofilm production using a physiologic biofilm assay. In addition, we confirmed that indeed ETP plus CZ exhibits enhanced activity against MSSA within these physiologic biofilms in vitro. In particular, the addition of ETP lowers the drug exposure required for cefazolin to achieve bactericidal activity within biofilms to physiologically achievable concentrations by standard dosing. Cefazolin monotherapy did not eradicate MSSA within biofilms at clinically achievable concentrations. In addition, single beta-lactam antibiotics appear to have decreased bactericidal activity under high inocula conditions (Table 1), which appears to be overcome with the combination of ETP plus CZ. This speaks to the overall need for combination antibiotics in situations of high bacterial inocula such as endocarditis.
The mechanism of this effect is unclear but may involve quorum sensing effects induced by ETP. In addition, S aureus exposed to ETP may drive immune-mediated clearance through augmented stimulation of interleukin (IL)-1β responses. Indeed, previously published studies are suggestive of this hypothesis given that (1) failure to induce IL-1β in the serum of patients with S aureus bacteremia has been associated with prolonged time to clearance [8], (2) the observation that alpha-toxin in S aureus is a potent inducer of IL-1β [12, 13], (3) alpha toxin expression reduces virulence in S aureus endocarditis [14], and (4) carbapenem antibiotics with proclivity towards PBP1 binding have been shown to induce expression sarA (a positive regulator of alpha-toxin) and reduce rot (a negative regulator of alpha-toxin) [15]. Ertapenem may be inducing IL-1β expression in the host via its interaction with PBP1 influencing alpha-toxin expression. Our group recently explored this hypothesis and found that ETP + CZ induce higher IL-1β expression in peripheral blood mononuclear cells than either agent alone [16]. It is interesting to note that this induction occurs even in the absence of S aureus, suggesting a possible secondary effect of ETP on host immune cells independent of the effects on bacteria [16].
CONCLUSIONS
In summary, ETP + CZ resulted in a shorter MSSA bacteremia duration than NAF or CZ alone in a small clinical sample of confirmed cases of MSSA endocarditis. A significant limitation is that this assessment excluded the lead time of bacteremia while on SOC monotherapy before ETP plus CZ salvage. Nevertheless, the immediate clearance on ETP + CZ therapy was notable and warrants a more thorough evaluation in larger prospective studies. Two recent studies report the effective use of ETP plus CZ in refractory MSSA bacteremia cases, including sterilization of left ventricular device infections with successful bridging to heart transplant [17, 18]. These results suggest that not only are MSSA from endocarditis patients enriched for their biofilm formation phenotypes in a physiologic biofilm model, but that ETP significantly enhances the activity of CZ within biofilms. Larger prospective randomized studies will be needed to determine any comprehensive clinical benefits of early use of ETP plus CZ in patients with MSSA bacteremia that have documented endocarditis or are otherwise at high-risk. The medical need for more potent antimicrobial therapies is greatest for patients with MSSA left-sided endocarditis, who may benefit in preparation for cardiac surgery by reducing preoperative bacterial burden on infected valves requiring repair or replacement. Indeed, a sterile intraoperative valve culture is associated with better clinical outcomes in patients with endocarditis [19]. We hope that large multicenter, prospective, randomized studies will be performed to obtain a more objective head-to-head comparison of ETP + CZ versus SOC monotherapy in patients with MSSA endocarditis.
Acknowledgments
Disclaimer. The views expressed here do not necessarily reflect the views of the Foundation.
Financial support. This research was funded by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) Grant K08 AI151253-01 (to E. R. U.), NIH/NIAID Grant U01-AI124316-01 (to V. N. and G. S.), and NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54-HD090259 (to V. N. and G. S.). Further funding was provided in part by the Robert Wood Johnson Foundation (to E. R. U.).
Potential conflicts of interest. G. S. has received speaking honoraria and consulting fees from Allergan/Abbvie and Paratek. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009; 48:S231–7. [DOI] [PubMed] [Google Scholar]
- 2. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 3. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB.. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakoulas G, Olson J, Yim J, et al. Cefazolin and ertapenem, a synergistic combination used to clear persistent Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2016; 60:6609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ulloa ER, Singh KV, Geriak M, et al. Cefazolin and ertapenem salvage therapy rapidly clears persistent methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2019. doi: 10.1093/cid/ciz995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Domenico EG, Rimoldi SG, Cavallo I, et al. Microbial biofilm correlates with an increased antibiotic tolerance and poor therapeutic outcome in infective endocarditis. BMC Microbiol 2019; 19:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johns Hopkins Guides ABX, diabetes, HIV, and psychiatry. Available at: https://www.hopkinsguides.com/hopkins/. Accessed 16 February 2022.
- 10. Chambers HF, Sachdeva M.. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis 1990; 161:1170–6. [DOI] [PubMed] [Google Scholar]
- 11. Pericas JM, Cervera C, del Rio A, et al. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 2014; 20:O1075–83. [DOI] [PubMed] [Google Scholar]
- 12. Craven RR, Gao X, Allen IC, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 2009; 4:e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kebaier C, Chamberland RR, Allen IC, et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 2012; 205:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL.. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 1997; 65:4652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dumitrescu O, Choudhury P, Boisset S, et al. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:3261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smelter D, Hayney M, Sakoulas G, Rose W.. Is the success of cefazolin plus ertapenem in methicillin-susceptible Staphylococcus aureus bacteremia based on release of interleukin 1-beta? Antimicrob Agents Chemother 2022;66:e0216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardenas-Alvarez JL, Suarez J, Motoa G, et al. Cefazolin plus ertapenem and heart transplantation as salvage therapy for refractory LVAD infection due to methicillin-susceptible Staphylococcus aureus: a case series. J Card Surg 2021; 36:4786–8. [DOI] [PubMed] [Google Scholar]
- 18. Akers SM, Kinney K, Butcher MI, Moise A.. Clearance of persistent Staphylococcus aureus bacteremia in a preterm neonate with the use of combination cefazolin and ertapenem. J Pediatr Pharmacol Ther 2020; 25:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renzulli A, Carozza A, Marra C, et al. Are blood and valve cultures predictive for long-term outcome following surgery for infective endocarditis? Eur J Cardiothorac Surg 2000; 17:228–33. [DOI] [PubMed] [Google Scholar]