Abstract
OBJECTIVE:
To examine the association between surgical volume and survival of women with early-stage cervical cancer who underwent radical hysterectomy.
METHODS:
This is a nationwide multicenter retrospective study examining consecutive women with clinical stage IB1-IIB cervical cancer who underwent radical hysterectomy and pelvic lymphadenectomy from 2004 to 2008 (N=5,964). The surgical volume per site over the 5-year period was defined as low-volume (fewer than 32 surgeries, 46 [39.7%] institutions, n=649 [10.9%]), mid-volume (32–104 surgeries, 60 [51.7%] institutions, n=3,662 [61.4%]), and high-volume (105 surgeries or more, 10 [8.6%] institutions, n=1,653 [27.7%]). Surgical volume-specific survival was examined with multivariable analysis and propensity score matching.
RESULTS:
The median number of surgeries per site was 44 (interquartile range, 17–65). The 5-year disease-free survival rates among stage IB1-IIB disease were 77.2%, 79.9%, and 84.5% for low-, mid-, and high-volume groups, respectively. On multivariable analysis, women in high-volume centers had a decreased risk of recurrence (adjusted hazard ratio [HR] 0.69, 95% CI 0.58–0.82, P<.001) and all-cause mortality (adjusted HR 0.73, 95% CI 0.59–0.90, P=.003) compared with those in mid-volume centers. Specifically, women in high-volume centers had a decreased risk of local recurrence (adjusted HR 0.62, 95% CI 0.49–0.78, P<.001) but not distant recurrence (adjusted HR 0.85, 95% CI 0.67–1.06, P=.142) compared with those in mid-volume centers. Among 1,700 women with clinical stage IB1 disease treated with surgery alone, surgery at high-volume centers was associated with a decreased risk of recurrence (adjusted HR 0.45, 95% CI 0.25–0.79, P=.006) and all-cause mortality (adjusted HR 0.29, 95% CI 0.11–0.76, P=.013) compared with surgery at mid-volume centers on multivariable analysis. After propensity score matching, surgery at high-volume centers remained an independent prognostic factor for decreased recurrence (adjusted HR 0.69, 95% CI 0.57–0.84, P<.001) and all-cause mortality (adjusted HR 0.75, 95% CI 0.59–0.95, P=.016) compared with surgery at mid- and low-volume centers on multivariable analysis.
CONCLUSION:
Hospital volume for radical hysterectomy may be a prognostic factor for early-stage cervical cancer. Surgery at high-volume centers is associated with decreased local recurrence risk and improved survival.
Cervical cancer globally remains the most common female malignancy, with an estimated 527,000 women receiving a new diagnosis in 2012.1 In Japan, the vast majority of invasive cervical cancers are early-stage disease where the surgery-based approach is the most common treatment of choice for those with stage I-II disease.2 Even in stage IIB disease, which represents ~25% of cervical cancer cases, primary surgical treatment is performed in 30–50%.2,3
Surgery for stage IB-II cervical cancer consists of radical hysterectomy and pelvic lymphadenectomy.4 Radical hysterectomy is amongst the most complex of pelvic surgeries, and various clinico-pathologic factors are associated with performance and morbidity.4 One such factor may be surgical volume.5 The effects of surgical volume on surgical outcome have been well-described in gynecologic procedures, and when performed by high-volume surgeons are associated with improved perioperative outcomes.6 Moreover, high surgical volume and improved oncologic outcomes have been reported in malignancies other than cervical cancer (Onda T, Satoh T, Saito T, Katsumata T, Nakanishi T, Takehara K, et al. Comparison of survival between upfront primary debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomized trial: JCOG0602 [abstract]. J Clin Oncol 2018;36:5500.).7–10 Despite this, there is limited data regarding the association between surgical volume and oncologic outcomes of women who undergo radical hysterectomy for early-stage cervical cancer.
Learning the surgical skills needed for the performance of a radical hysterectomy requires additional gynecology oncology subspecialty training. A recent study suggests that possible learning-curve effects of surgical skills for radical hysterectomy may be a prognostic factor for early-stage cervical cancer.11 These clearly imply that surgical performance for radical hysterectomy affects survival. Thus, it is hypothesized that a high surgical volume is associated with improved cervical cancer survival. The objective of the study was to examine the association between surgical volume and survival of women with early-stage cervical cancer who underwent radical hysterectomy.
METHODS
This is a nation-wide retrospective observational study conducted in Japanese Gynecologic Oncology Group (JGOG)–designated institutions.12–14 The study concept and participation call was initially announced to all JGOG-designated institutions (182 sites), and 116 (63.7%) sites voluntarily participated in the study. Institutional Review Board approval was obtained at Tottori University, which served as the host institution. Participating JGOG institutions obtained their own approvals at each site as indicated. Eligible patients were consecutive women with clinical stage IB-IIB cervical cancer who underwent type III radical hysterectomy and pelvic lymphadenectomy between January 2004 and December 2008. The data acquisition period was set between October 2012 and February 2013. The STROBE guidelines were consulted to outline this observational cohort study.
Data collection was executed at each study center by using a universal data entry form for collecting clinical, tumor, treatment, and survival information from archived medical records. On the completion of data collection by clinicians participating in the study, the anonymous de-identified data sheet was transferred to the host institution. The data was then compiled into a master data spreadsheet by research staff. The principal investigators reviewed the accuracy, consistency, and quality of the dataset, and at their discretion, missing and outlying data were excluded from the analysis.
Clinical demographics included patient age, calendar year of surgery, and clinical stage per the 2014 International Federation of Gynecology and Obstetrics system (stage IB1, IB2, IIA, and IIB).15 Surgical-pathologic factors included histologic subtype (squamous cell carcinoma, adenocarcinoma, adenosquamous, and other), tumor size (more than 4 cm vs 4 cm or less), parametrial tumor involvement (yes vs no), deep stromal invasion (outer half vs inner half), lympho-vascular space invasion (yes vs no), uterine corpus tumor invasion (yes vs no), malignant cells in peritoneal cytology (yes vs no), and ovarian metastasis (yes vs no). Lymph node metastasis (yes vs no) was assessed in both the pelvic and para-aortic chains. Among staged cases, the number of harvested lymph nodes was collected.
Treatment type included use of neoadjuvant chemotherapy (yes vs no) and adjuvant therapy (radiotherapy, chemotherapy, and both). Surgical volume per institution during the 5-year study period was also collected. Survival outcomes included follow-up time, presence of recurrence and anatomical site, vital status, and cause of death. Disease-free survival, overall survival, and recurrence patterns were examined for analysis.
The cutoff of surgical volume was examined and defined by the minimum P-value method.16 The minimum P-value method is widely used to determine thresholds of dichotomous outcomes. We used this approach to identify the cutoff for surgical volume related to disease-free survival via an unadjusted Cox proportional hazard regression model. The P-value results with corresponding hazard ratio (HR) were plotted for each increment of radical hysterectomy case performed, and the surgical volume exhibiting the smallest P-value was defined for high-volume centers. In addition, we modified this analytic approach, and the first surgical volume exhibiting statistical significance was used to define low-volume centers. Institutions between low- and high-volume centers were grouped as mid-volume centers.
Disease-free survival was defined as the time interval between surgery and first recurrence or death from disease. Overall survival was defined as the time interval between surgery and death (all-cause). Patients were censored if there was no survival event as above. Among recurrent cases, the anatomical location of recurrence was assessed as: local recurrence (vaginal cuff, pelvis, or both) and distant recurrence (any site other than local recurrence).
The primary objective of this analysis was to examine the association of surgical volume and clinico-pathologic characteristics of women with stage IB1-IIB cervical cancer who underwent radical hysterectomy. The secondary objective was to estimate the association of surgical volume and survival outcome. The performance of high-volume centers was compared with mid-volume centers.
Multiple group comparisons were assessed with the one-way analysis of variance test, Kruskal-Wallis H test, or chi-square test as appropriate. The Kaplan-Meier method was used to construct survival curves, and differences were assessed with the log-rank test. Cox proportional hazard regression models were used to assess the association of surgical volume and survival on multivariable analysis. We used various adjustment models to examine this association to assess the durability and observe the interaction in each layer. We designed the stepwise-adjustment models based on clinically relevant variables: pretreatment factors alone (model 1), surgical-pathologic factors to model 1 (model 2), and treatment factors to model 2 (model 3). The magnitude of statistical significance was expressed with HR and 95% CI. The proportional hazard assumption was tested and showed no interaction with time.
Propensity score matching was also used to adjust background differences between the groups. The propensity score for surgical volume (high-volume vs mid- and low-volume) was computed by a multivariable logistic regression analysis. Covariates for pretreatment, surgical-pathologic, and treatment factors were entered in the propensity score model. An automated algorithm was used for one-to-one propensity score matching, and the optimal caliper width for estimating differences was equal to 0.2 of the SD of the logit of the propensity score, resulting in a propensity score difference cutoff of 0.017 in this study.17 In the postmatching assessment, standardized difference was assessed to evaluate frequency distributions between the two groups, and a value 0.10 or less was considered to indicate good balance.
Various sensitivity analyses were performed to assess the robustness of our analytic approach. First, women with clinical stage IB1 disease who underwent radical hysterectomy and pelvic lymphadenectomy alone without neoadjuvant or adjuvant therapy were examined. This is based on the rationale that this surgical treatment-only group most likely reflects the effects of surgical performance on survival. Women with node-positive cases were then examined as this group carries the highest risk of recurrence. Next, women with clinical stage IIB cases were examined as these are likely the most complex surgery for tumor removal. The interaction between surgical volume and adjuvant therapy for survival was also examined. This is based on the rationale that, although recent studies have demonstrated comparable effectiveness of adjuvant therapy (radiotherapy vs chemotherapy), the effects of surgical volume remain unknown (Weng D, Wang H, Zhu C, Cui B, Wang C, Li K, et al. Randomized trial of paclitaxel and cisplatin versus concurrent chemoradiotherapy in early stages cervical carcinoma after radical surgery: A Chinese Gynecologic Oncology Group Study (CSEM-002) [abstract]. Gynecol Oncol 2018;149:30.).18 Association of surgical volume and survival was also examined by fitting the extent of lymphadenectomy by means of sampled node counts as well as duration of neoadjuvant chemotherapy among received.
Over-adjustment was examined by the ratio of event-of-interest per variable in the final model, and a ratio of more than 10 was considered to indicate the absence of over-adjustment. All statistical analyses were based on two-sided hypothesis and a P<.05 was considered statistically significant. Statistical Package for Social Sciences 24.0 was used for all the analyses.
RESULTS
Among 6,003 cases in the dataset, 39 outdated cases were excluded and the remaining 5,964 cases of radical hysterectomy performed between 2004 and 2008 were examined for analysis. The median number of surgeries per site was 44 (interquartile range, 17–65) over the 5-year study period. P-values with corresponding HR are plotted per surgical volume (Fig. 1). Surgical volume that reached the first statistical significance of P<.05 was 32 cases. Surgical volume that exhibited the smallest P-value was 105 cases (P=7.76×10−06). Thus, surgical volume in this study was defined as follows: low-volume, fewer than 32 cases (46 [39.7%] institutions, n=649 [10.9%]); mid-volume 32–104 cases (60 [51.7%] institutions n=3,662 [61.4%]); and high-volume 105 cases or more (10 [8.6%] institutions, n=1,653 [27.7%]) (Appendix 1, available online at http://links.lww.com/AOG/B367).
Fig. 1.
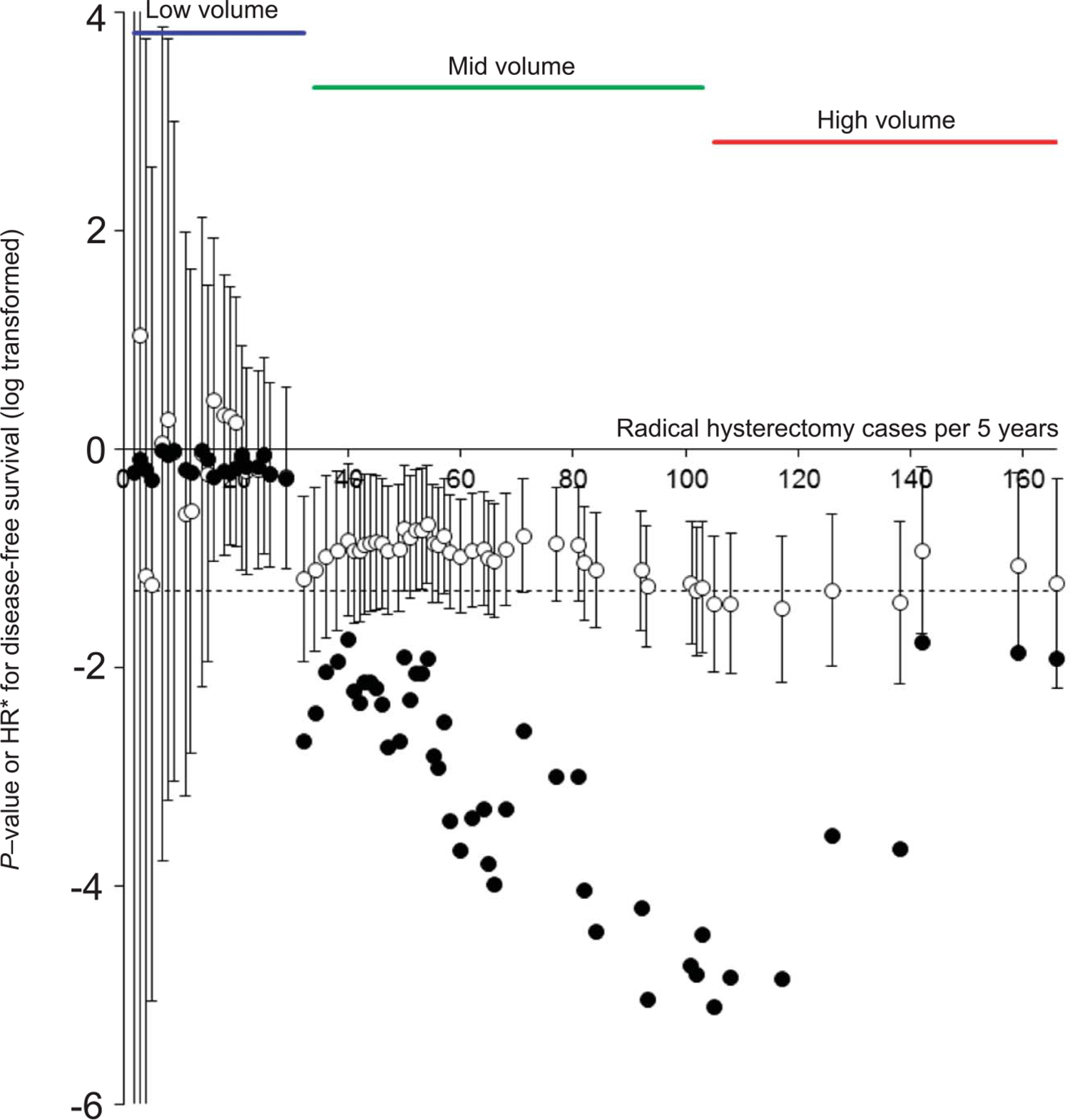
Definition of the study criteria for surgical volume. Unadjusted hazard ratio (HR) with 95% CI and P-value was determined for each radical hysterectomy case cutoff point, and results are displayed with log scale. Cutoff of 32 cases met the first statistically significant value. Cutoff of 105 cases had the smallest P-value. Based on this, the low-volume group was defined as radical hysterectomy below 32 cases, the mid-volume group was defined as 32–104 cases, and the high-volume group was defined as 105 cases or more over the 5-year study period. Open circles note HR (95% CI). Closed circles note P-value. Horizontal dashed line indicates P=.05. *HR results are 10-fold scale.
Clinico-pathologic characteristics are shown in Table 1. Women in the high-volume group were younger compared with the women in the other two groups. There were increasing trends of surgical volume in the mid- and low-volume centers in recent years. Women in the high-volume centers were more likely to have lower stage disease, whereas those in the mid- and low-volume centers were more likely to have higher stage disease. Women in the high-volume group were more likely to undergo para-aortic lymphadenectomy than other groups; and had the largest number of sampled lymph nodes among the three groups (all, P<.05).
Table 1.
Patient Demographics (N=5,964)
| Characteristic | Surgical Volume |
P | ||
|---|---|---|---|---|
| High (n=1,653) | Mid (n=3,662) | Low (n=649) | ||
|
| ||||
| Age (y) | 47.2±11.7 | 48.0±12.0 | 49.5±12.1 | <.001 |
| Year | <.001 | |||
| 2004 | 352 (21.3) | 581 (15.9) | 132 (20.3) | |
| 2005 | 323 (19.5) | 751 (20.5) | 103 (15.9) | |
| 2006 | 329 (19.9) | 751 (20.5) | 114 (17.6) | |
| 2007 | 327 (19.8) | 835 (22.8) | 137 (21.1) | |
| 2008 | 322 (19.5) | 744 (20.3) | 163 (25.1) | |
| Clinical stage | .003 | |||
| IB1 | 931 (56.3) | 1,906 (52.0) | 348 (53.6) | |
| IB2 | 270 (16.3) | 544 (14.9) | 86 (13.3) | |
| IIA | 141 (8.5) | 390 (10.6) | 72 (11.1) | |
| IIB | 311 (18.8) | 822 (22.4) | 143 (22.0) | |
| Histology | .064 | |||
| Squamous | 1,070 (64.7) | 2,382 (65.0) | 448 (69.0) | |
| Adenocarcinoma | 399 (24.1) | 943 (25.8) | 147 (22.7) | |
| Adenosquamous | 163 (9.9) | 289 (7.9) | 44 (6.8) | |
| Others | 21 (1.3) | 48 (1.3) | 10 (1.5) | |
| Parametrial involvement | .521 | |||
| No | 1,320 (79.9) | 2,970 (81.1) | 527 (81.2) | |
| Yes | 333 (20.1) | 690 (18.9) | 122 (18.8) | |
| Node metastasis (pelvic) | .122 | |||
| No | 1,191 (72.4) | 2,723 (74.9) | 469 (72.9) | |
| Yes | 454 (27.6) | 911 (25.1) | 174 (27.1) | |
| Node metastasis (para-aortic) | .004 | |||
| No | 218 (14.2) | 552 (16.0) | 67 (10.7) | |
| Yes | 39 (2.5) | 71 (2.1) | 10 (1.6) | |
| Not examined* | 1,283 (83.3) | 2,829 (82.0) | 551 (87.7) | |
| Sampled lymph nodes | <.001 | |||
| Pelvic | 42 (32–53) | 35 (26–47) | 40 (28–52) | |
| Para-aortic | 9 (5–15) | 8 (3–14) | 6 (4–12) | .036 |
| Deep stromal invasion | .001 | |||
| No | 621 (43.0) | 1,708 (48.7) | 307 (48.3) | |
| Yes | 822 (57.0) | 1,801 (51.3) | 329 (51.7) | |
| Tumor size (cm) | .809 | |||
| 4 or less | 1,056 (70.0) | 2,377 (69.4) | 421 (68.9) | |
| More than 4 | 453 (30.0) | 1,047 (30.6) | 193 (31.4) | |
| LVSI | .020 | |||
| No | 676 (42.4) | 1,662 (46.6) | 275 (44.4) | |
| Yes | 918 (57.6) | 1,907 (53.4) | 344 (55.6) | <.001 |
| Uterine corpus invasion | ||||
| No | 1,305 (83.4) | 3,157 (87.3) | 561 (87.9) | |
| Yes | 260 (16.6) | 459 (12.7) | 77 (12.1) | |
| Ovarian metastasis | .735 | |||
| No | 1,469 (98.6) | 3,538 (98.9) | 620 (98.7) | |
| Yes | 21 (1.4) | 41 (1.1) | 8 (1.3) | |
| Peritoneal cytology | <.001 | |||
| No malignant cells | 463 (28.0) | 748 (20.5) | 146 (22.5) | |
| Malignant cells | 33 (2.0) | 55 (1.5) | 2 (0.3) | |
| Not examined* | 1,157 (70.0) | 2,837 (77.9) | 501 (77.2) | |
| Neoadjuvant chemotherapy | .002 | |||
| No | 1,356 (83.1) | 2,998 (82.4) | 500 (77.2) | |
| Yes | 275 (16.9) | 639 (17.6) | 148 (22.8) | |
| Adjuvant therapy | .003 | |||
| None | 678 (41.7) | 1,313 (37.1) | 253 (39.7) | |
| Radiotherapy alone | 295 (18.2) | 642 (18.2) | 101 (15.9) | |
| CCRT | 341 (21.0) | 830 (23.5) | 136 (21.4) | |
| Chemotherapy alone | 302 (18.6) | 697 (19.7) | 140 (22.0) | |
| Combined† | 9 (0.6) | 54 (1.5) | 7 (1.1) | |
LVSI, lymphovascular space invasion; and CCRT, concurrent chemoradiotherapy.
Data are mean±SD, n (% per column), or median (interquartile range) unless otherwise specified.
One-way analysis of variance test, Kruskal-Wallis test, or χ2 test was used for P-values.
Bold indicates significant P-value.
Total number may not be 5,964 owing to missing data.
Histologically assessed.
Both systemic chemotherapy and radiotherapy.
Tumors in the low-volume group were less likely to have deep stromal invasion, lympho-vascular space invasion, uterine corpus invasion, and malignant cells in peritoneal cytology than other groups. Women in the low-volume centers were more likely to receive neoadjuvant chemotherapy before radical hysterectomy compared with the other two groups. Women in the high-volume group were least likely to receive adjuvant therapy. Among those who received adjuvant therapy, women in the low-volume centers were more likely to receive chemotherapy but less likely to receive radiotherapy (all, P<.05).
Median follow-up of censored cases was 5.4 years (interquartile range, 4.4–6.7). There were 1,162 recurrences and 750 deaths recorded. Among recurrent cases, local recurrence and distant recurrence were seen in 654 and 636 cases, respectively. The 5-year disease-free survival rate among stage IB1-IIB disease was 77.2% for the low-volume group, 79.9% for the mid-volume group, and 84.5% for the high-volume group, respectively (absolute difference 7.3%; Fig. 2A).
Fig. 2.
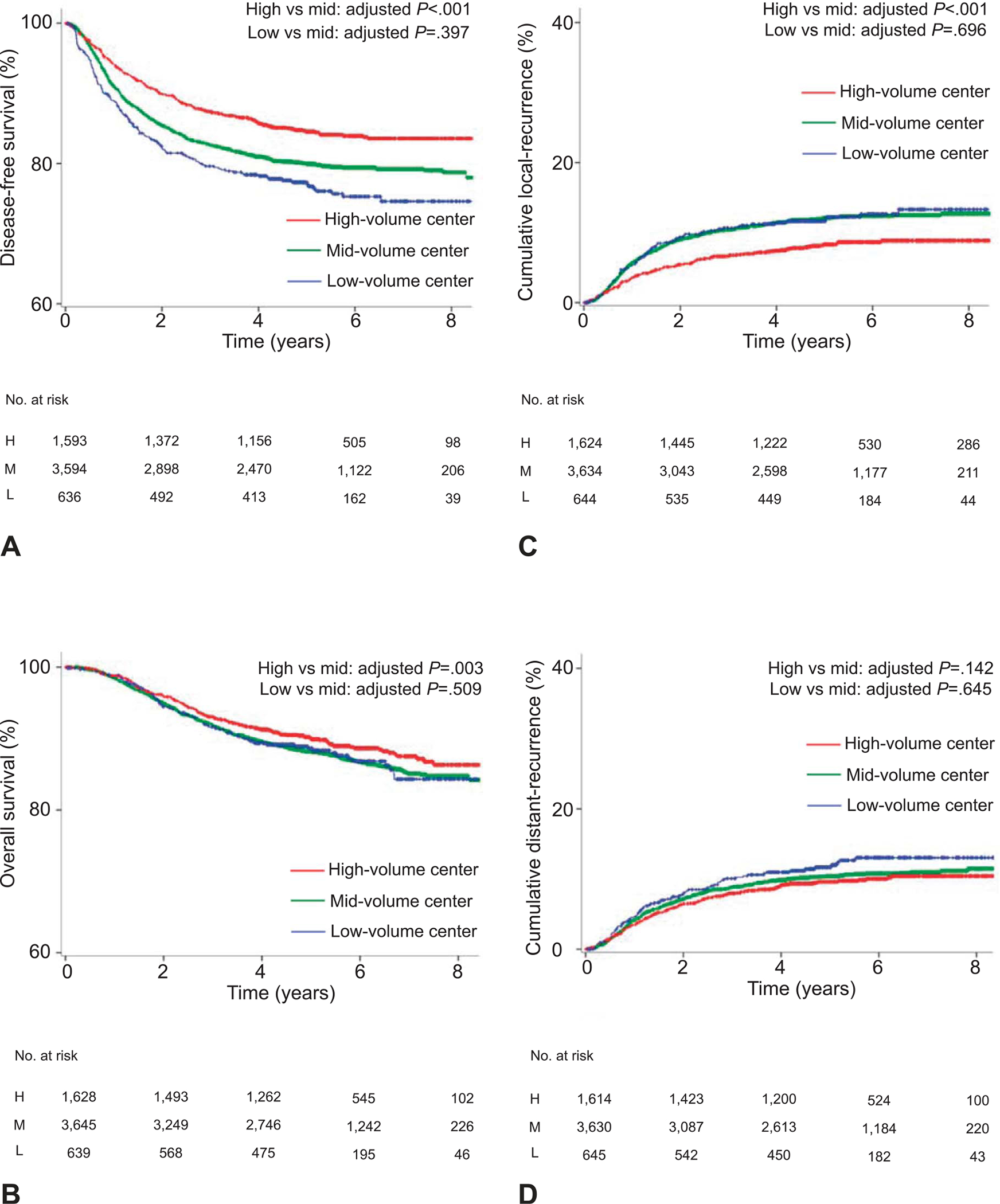
Survival and recurrence based on surgical volume (whole cohort). (A) Disease-free survival, (B) overall survival, (C) cumulative local recurrence, and (D) cumulative distant recurrence are shown based on surgical volume for radical hysterectomy. The y-axis is truncated to 60–100% or 0–40%. Results of multivariable analysis are shown in Table 2. H, high; M, mid; L, low.
On multivariable analysis controlling for patient, surgical-pathologic, and treatment factors (Table 2), women in high-volume centers had a decreased risk of recurrence (adjusted HR 0.69, 95% CI 0.58–0.82, P<.001) and all-cause mortality (adjusted HR 0.73, 95% CI 0.59–0.90, P=.003; Fig. 2B) compared with those in mid-volume centers. This association was also observed in other adjustment models (Appendices 2–4, available online at http://links.lww.com/AOG/B367). Survival outcomes were similar between the low-volume and mid-volume centers (all, P>.05). When the extent of lymphadenectomy by sampled node counts (Appendix 5, available online at http://links.lww.com/AOG/B367) or duration of neoadjuvant chemotherapy among those received (Appendix 6, available online at http://links.lww.com/AOG/B367) was fitted in the analysis, the association remained significant.
Table 2.
Association of Surgical Volume and Survival in Cervical Cancer (Adjusting Model)
| Characteristic | Disease-Free Survival |
Overall Survival |
Local Recurrence |
Distant Recurrence |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
|
| ||||||||
| All cases (prematching) | ||||||||
| Mid-volume | 1 | 1 | 1 | 1 | ||||
| High-volume | 0.69 (0.58–0.82) | <.001 | 0.73 (0.59–0.90) | .003 | 0.62 (0.49–0.78) | <.001 | 0.85 (0.67–1.06) | .142 |
| Low-volume | 1.09 (0.89–1.33) | .397 | 0.91 (0.69–1.20) | .509 | 0.95 (0.72–1.24) | .696 | 0.93 (0.70–1.25) | .645 |
| All cases (postmatching) | ||||||||
| Mid- and low-volume | 1 | 1 | 1 | 1 | ||||
| High-volume | 0.69 (0.57–0.84) | <.001 | 0.75 (0.59–0.95) | .016 | 0.65 (0.50–0.85) | .001 | 0.78 (0.61–1.02) | .066 |
| Stage IB1 disease* | ||||||||
| Mid-volume | 1 | 1 | 1 | 1 | ||||
| High-volume | 0.45 (0.25–0.79) | .006 | 0.29 (0.11–0.76) | .013 | 0.37 (0.19–0.73) | .004 | 0.54 (0.21–1.39) | .200 |
| Low-volume | 0.99 (0.54–1.84) | .982 | 0.31 (0.07–1.36) | .121 | 0.95 (0.49–1.87) | .890 | 0.56 (0.13–2.45) | .439 |
| Stage IIB disease | ||||||||
| Mid-volume | 1 | 1 | 1 | 1 | ||||
| High-volume | 0.72 (0.53–0.96) | .027 | 0.77 (0.55–1.08) | .133 | 0.66 (0.45–0.98) | .038 | 0.85 (0.58–1.26) | .423 |
| Low-volume | 0.89 (0.63–1.26) | .496 | 0.88 (0.58–1.34) | .549 | 0.73 (0.45–1.19) | .207 | 0.82 (0.51–1.34) | .433 |
| Node-positive case | ||||||||
| Mid-volume | 1 | 1 | 1 | 1 | ||||
| High-volume | 0.76 (0.60–0.97) | .025 | 0.76 (0.57–0.99) | .044 | 0.66 (0.47–0.92) | .013 | 0.88 (0.66–1.18) | .394 |
| Low-volume | 0.82 (0.62–1.11) | .197 | 0.82 (0.58–1.16) | .258 | 0.80 (0.53–1.20) | .279 | 0.76 (0.52–1.13) | .175 |
HR, hazard ratio.
Association for surgical volume and survival outcome was adjusted for pretreatment factors, surgical-pathologic results, and treatment types (adjusting model 3). Pretreatment factors: age (continuous), year of diagnosis (2004, 2005, 2006, 2007, and 2008), and histology (squamous vs nonsquamous). Surgical-pathologic factors: pelvic lymph node metastasis (yes vs no), para-aortic lymph node metastasis (yes, no, or not examined), parametrial involvement (yes vs no), deep stromal invasion (yes vs no), lympho-vascular space invasion (yes vs no), tumor size (more than 4 cm vs 4 cm or less), uterine corpus invasion (yes vs no), ovarian metastasis (yes vs no), and malignant peritoneal cytology (yes, no, or not examined). Treatment types: neoadjuvant chemotherapy (yes vs no) and adjuvant therapy (radiation-based, chemotherapy-based, or none). Cox proportional hazard regression models were used for analysis. Proportional hazard assumption was tested and showed no interaction with time.
Bold indicates significant P-value.
Clinical stage IB1 disease without preoperative or postoperative chemotherapy or radiotherapy.
Recurrence patterns were examined per surgical volume (Fig. 2C–D). Using multivariable models controlling for patient, surgical-pathologic, and treatment factors (Table 2), women in high-volume centers had a decreased risk of local recurrence (adjusted HR 0.62, 95% CI 0.49–0.78, P<.001) compared with those in mid-volume centers. Distant recurrence in the high-volume group was similar to the mid-volume group (adjusted HR 0.85, 95% CI 0.67–1.06, P=.142).
After propensity score matching (n=3,296), clinico-pathologic characteristics were well-balanced between the high-volume group and the mid- and low-volume groups (all, standardized difference 0.10 or less; Table 3 and Appendix 7, available online at http://links.lww.com/AOG/B367). On multivariable analysis controlling for patient, surgical-pathologic, and treatment factors (Table 2), surgery at high-volume centers remained an independent prognostic factor for decreased recurrence (adjusted HR 0.69, 95% CI 0.57–0.84, P<.001; Fig. 3A) and all-cause mortality (adjusted HR 0.75, 95% CI 0.59–0.95, P=.016; Fig. 3B) compared with surgery at mid- and low-volume centers. This association was also observed in other adjustment models (Appendices 2–4, http://links.lww.com/AOG/B367).
Table 3.
Patient Demographics After Propensity Score Matching (n=3,296)
| Characteristic | Surgical Volume |
P | Standardized Difference | |
|---|---|---|---|---|
| High (n=1,648) | Mid and Low (n=1,648) | |||
|
| ||||
| Age (y) | 47.2±11.7 | 47.2±12.4 | .930 | .003 |
| Year | .300 | .033 | ||
| 2004 | 349 (21.2) | 311 (18.9) | ||
| 2005 | 323 (19.6) | 318 (19.3) | ||
| 2006 | 328 (19.9) | 366 (22.2) | ||
| 2007 | 327 (19.8) | 343 (20.8) | ||
| 2008 | 321 (19.5) | 310 (18.8) | ||
| Clinical stage | .337 | .042 | ||
| IB1 | 931 (56.5) | 883 (53.6) | ||
| IB2 | 266 (16.1) | 297 (18.0) | ||
| IIA | 141 (8.6) | 142 (8.6) | ||
| IIB | 310 (18.8) | 326 (19.8) | ||
| Histology | .898 | .015 | ||
| Squamous | 1,066 (64.7) | 1,069 (64.9) | ||
| Adenocarcinoma | 399 (24.2) | 408 (24.8) | ||
| Adenosquamous | 162 (9.8) | 153 (9.3) | ||
| Others | 21 (1.3) | 18 (1.1) | ||
| Parametrial involvement | .733 | .059 | ||
| No | 1,317 (79.9) | 1,324 (80.4) | ||
| Yes | 331 (20.1) | 323 (19.6) | ||
| Node metastasis (pelvic) | .166 | .049 | ||
| No | 1,186 (72.3) | 1,210 (74.5) | ||
| Yes | 454 (27.7) | 415 (25.5) | ||
| Node metastasis (para-aortic) | .246 | .042 | ||
| No | 217 (14.1) | 241 (15.9) | ||
| Yes | 39 (2.5) | 30 (2.0) | ||
| Not examined* | 1,279 (83.3) | 1,243 (82.1) | ||
| Deep stromal invasion | .662 | .012 | ||
| No | 621 (43.2) | 683 (44.0) | ||
| Yes | 817 (56.8) | 870 (56.0) | ||
| Tumor size (cm) | .104 | .016 | ||
| 4 or less | 1,055 (70.1) | 1,015 (67.4) | ||
| More than 4 | 449 (29.9) | 491 (32.6) | ||
| LVSI | .404 | .030 | ||
| No | 676 (42.5) | 647 (41.1) | ||
| Yes | 913 (57.5) | 928 (58.9) | ||
| Uterine corpus invasion | .175 | .048 | ||
| No | 1,304 (83.6) | 1,379 (85.3) | ||
| Yes | 256 (16.4) | 237 (14.7) | ||
| Ovarian metastasis | .926 | .003 | ||
| No | 1,464 (98.6) | 1,559 (98.5) | ||
| Yes | 21 (1.4) | 23 (1.5) | ||
| Peritoneal cytology | .775 | .015 | ||
| No malignant cells | 458 (27.8) | 436 (26.7) | ||
| Malignant cells | 33 (2.0) | 33 (2.0) | ||
| Not examined* | 1,157 (70.2) | 1,165 (71.3) | ||
| Neoadjuvant chemotherapy | .385 | .025 | ||
| No | 1,353 (83.2) | 1,340 (82.1) | ||
| Yes | 273 (16.8) | 293 (17.9) | ||
| Adjuvant therapy | .630 | .030 | ||
| None | 675 (41.7) | 640 (40.9) | ||
| Radiotherapy alone | 294 (18.1) | 282 (18.0) | ||
| CCRT | 340 (21.0) | 352 (22.5) | ||
| Chemotherapy alone | 302 (18.6) | 277 (17.7) | ||
| Combined† | 9 (0.6) | 14 (0.9) | ||
LVSI, lymphovascular space invasion; CCRT, concurrent chemoradiotherapy.
Data are mean±SD or n (% per column) unless otherwise specified.
Binary logistic regression test was used for P-value.
A standardized difference of 0.10 or less indicates a good balance between the two groups.
Histologically assessed.
Both systemic chemotherapy and radiotherapy. Total number may not be 3,296 owing to missing data.
Fig. 3.
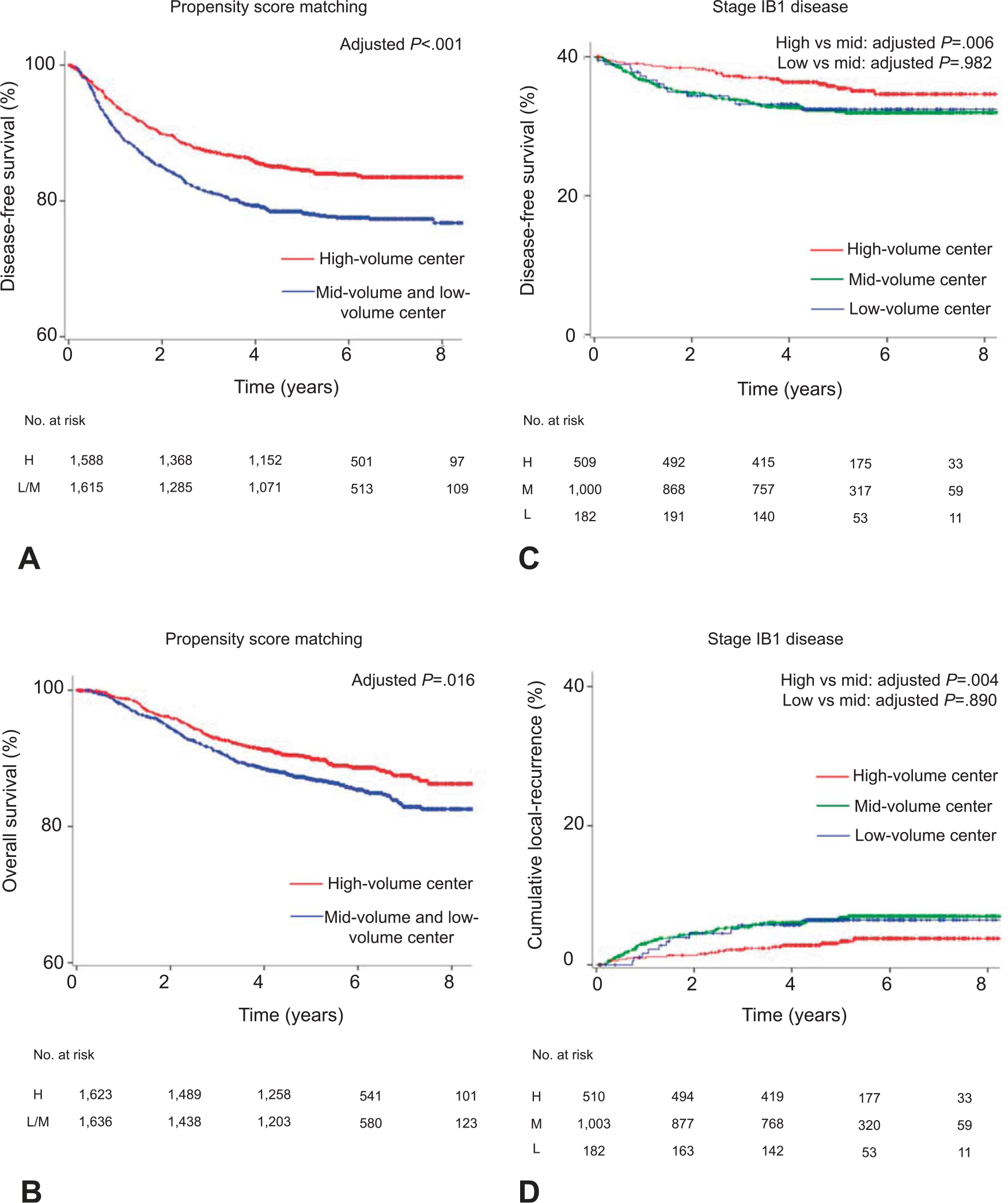
Survival and recurrence based on surgical volume (sensitivity analysis). Results of propensity score matching for (A) disease-free survival and (B) overall survival are shown based on surgical volume for radical hysterectomy. Results for clinical stage IB1 disease (surgery alone) for (C) disease-free survival and (D) cumulative local recurrence are shown based on surgical volume for radical hysterectomy. The y-axis is truncated to 60–100% or 0–40%. Results of multivariable analysis are shown in Table 2. H, high; LM, low to mid; M, mid; L, low.
Among 1,700 (28.5%) women with clinical stage IB1 disease who received primary radical hysterectomy and pelvic lymphadenectomy alone without neoadjuvant or adjuvant therapy, surgery at high-volume centers was associated with a decreased risk of recurrence (adjusted HR 0.45, 95% CI 0.25–0.79, P=.006; Fig. 3C) and all-cause mortality (adjusted HR 0.29, 95% CI 0.11–0.76, P=.013) compared with surgery at mid-volume centers on multivariable analysis (Table 2 and Appendices 2–4, http://links.lww.com/AOG/B367). Specifically, surgery at a high-volume center was associated with decreased risks of local recurrence compared with surgery at a mid-volume center (adjusted HR 0.37, 95% CI 0.19–0. 73, P=.004; Fig. 3D).
Among 1,276 (21.4%) women with clinical stage IIB disease, the high-volume group had decreased risk of recurrence (adjusted HR 0.72, 95% CI 0.53–0.96), particularly local recurrence (adjusted HR 0.66, 95% CI 0.45–0.98), compared with the mid-volume group (both, P<.05; Table 2). Among 1,539 (25.8%) women with node-positive disease, surgery at high-volume centers was associated with decreased risk of recurrence (adjusted HR 0.72, 95% CI 0.60–0.97), all-cause mortality (adjusted HR 0.76, 95% CI 0.57–0.99), and local recurrence (adjusted HR 0.66, 95% CI 0.47–0.92) compared with surgery at mid-volume centers (all, P<.05; Table 2).
The interaction between surgical volume and adjuvant therapy for node-positive cases was examined (Appendix 8, available online at http://links.lww.com/AOG/B367). In the high-volume centers, women who received chemotherapy had a survival and recurrence pattern similar to those who received radiotherapy on multivariable analysis (all, P>.05). Conversely, in the mid- and low-volume centers, women who received chemotherapy had higher risks of local recurrence (adjusted HR 1.66, 95% CI 1.14–2. 43) but lower risks of distant recurrence (adjusted HR 0.66, 95% CI 0.45–0.98) compared with those who received radiotherapy (both, P<.05).
DISCUSSION
Key findings of the study are that the hospital surgical volume for radical hysterectomy may be a prognostic factor for stage IB1-IIB cervical cancer and that surgery at high-volume centers, representing less than 10% of institutions but covering more than 25% of radical hysterectomy cases, is associated with improved survival by ~30%. A previous study reported that radical hysterectomy performed by high-volume surgeons is associated with fewer postoperative complications and improved index admission outcome for cervical cancer but did not examine the association between hospital surgical volume and oncologic outcome.5
Our study externally validates the studies from other gynecologic malignancies in that surgery at high-volume centers is associated with improved survival in women with advanced-stage ovarian cancer (Onda et al. J Clin Oncol 2018;36:5500.).9 Of note, these studies found that surgical volume of 20–21 cases per year or more per institution is associated with improved survival. This was similar to the cutoff observed in this study (average 21 cases per year or more). Similar trends have also been observed in endometrial cancer, demonstrating that surgery at a high-volume center is associated with decreased complications.10
Our results and others clearly imply the importance of surgical performance in cervical cancer treatment. Generally, surgical performance correlates to perioperative outcome.19 This concept most likely applies to cervical cancer surgery and survival, given the complexity of surgical procedure in the deep pelvis for local tumor control. Indeed, our study showed that surgical volume particularly affects local tumor control, and high-volume centers had lower local recurrence risk compared with mid- and low-volume centers. Further study is warranted to examine the association between a surgeon’s surgical skills and volume for radical hysterectomy and cervical cancer survival.
We observed various differences in clinio-pathologic factors across the groups. Markedly, use of chemotherapy was high in the low-volume centers; this may reflect the low use of radiotherapy in the low-volume centers. We do not know the exact causality for this association. However, it may be that the accessibility of radiotherapy resources is limited in certain centers, resulting in different treatment choices. Favorable surgical-pathologic factors in low-volume centers are most likely a result of the effects of neoadjuvant chemotherapy.
We acknowledge several limitations in this study. First, this is a retrospective study likely missing confounding factors for analysis. For instance, we only examined surgical volume per institution, but not per surgeon. Details of radical hysterectomy procedures were not recorded in this study, and objective evaluation of the radicality of these surgeries was not assessable in this study. Likewise, preoperative decision making was not reflected in this database. As clinical stage significantly differs across the groups, it may be that there is a selection-bias affecting survival. For instance, in the high-surgical volume group stage IIB disease was less frequently seen as compared with other groups. However, we corroborated these differences with propensity score matching. Moreover, we performed a sensitivity analysis for substages including stage IB1 disease that reflects solely surgical cohort. All sensitivity analyses showed the robustness of our results for improved survival in the high-volume centers.
Second, we analyzed only the oncologic outcome, as perioperative complications as well as patient-reported outcomes were not available in this dataset. As vesico-ureteral and lymphatic complications, as well as quality-of-life are salient outcomes related to radical hysterectomy, lack of this information made us unable to perform composite endpoint analysis in this study. Third, although the study sites cover the vast majority of JGOG-participating sites, they do not completely cover all institutions in Japan. Fourth, recent analyses found that the institution’s accreditation status for gynecologic oncology affects survival in cervical cancer but this study did not include this information.20 As the subspecialty board system for gynecologic oncology was not launched until 2007 and a core curriculum-based fellowship training program remains lacking in Japan, the effect of subspecialty training on surgical performance was unable to be determined in this study.
Fifth, the route of radical hysterectomy either with laparotomy compared with minimally-invasive surgery was not available in this dataset. Recent studies have shown that minimally-invasive radical hysterectomy for early-stage cervical cancer is associated with increased risks of recurrence and mortality compared with laparotomy.11,21 Although a lack of information regarding the surgical approach is a drawback in this study, minimally-invasive radical hysterectomy is a relatively new procedure in Japan, and there would have been scant cases performed between 2004 and 2008, placing minimum effect on our analysis and results. Similarly, there might have been surgical innovations during the study period that may affect the results of this study but were also not captured in the analysis.
Sixth, performance status, comorbidities, quality of care, and surveillance approach such as use of routine radiologic imaging during the surveillance period were not available in this dataset, but all have been associated with survival. Seventh, this study is conducted in Japan, and reproducibility and generalizability in different populations is unknown. Eighth, postrecurrence information is not available in this database. Thus, it is unknown whether patients were followed and treated in the same or a different institution after recurrence, possibly leading to misclassification. Ninth, as women with clinical stage IIB disease generally undergo definitive chemoradiotherapy in the United States,22 inclusion of stage IIB disease makes the results of our study less applicable to U.S. surgeons.
Last and most importantly, there may be a methodologic flaw for defining the surgical volume in this study. We used a common approach with the minimum P-value method. Although this methodologic approach has been widely used in the cutoff analysis elsewhere, lack of validation is a weakness of the study. However, the recurrence risk was also lowest at the same surgical volume determined per the minimum P-value method (cutoff for 105 cases, HR 0.72, 95% CI 0.63–0.83), serving as the internal validation to support our methodologic approach. To contrive the approach, we have also examined the arbitrary cutoffs for surgical volume in a solely surgical cohort with clinical stage IB1 disease (Table 4). The results demonstrated that the highest surgical volume group had significantly lower local recurrence risk compared with the mid surgical volume group (HR 0.46, 95% CI 0.23–0.93).
Table 4.
Association of Surgical Volume and Local Recurrence Risk (Stage IB1 Disease, n=1,700)
| Surgeries Over5 y | n | HR (95% CI) vs 20–29 Cases | P |
|---|---|---|---|
|
| |||
| Fewer than 10 | 237 | 0.92 (0.47–1.76) | .790 |
| 10–19 | 441 | 0.65 (0.36–1.19) | .165 |
| 20–29 | 313 | 1 | |
| 30–49 | 360 | 0.88 (0.49–1.58) | .875 |
| 50 or more | 349 | 0.46 (0.23–0.93) | .030 |
HR, hazard ratio.
One thousand seven hundred women with clinical stage IB1 cervical cancer who underwent primary radical hysterectomy without neoadjuvant therapy or postoperative therapy were examined. Surgical volume for radical hysterectomy per institution during the study period (5 years) was arbitrarily grouped every 10–20 cases. Association for surgical volume and cumulative local recurrence risk was examined with a Cox proportional hazard regression model.
Bold indicates significant P-value.
Until a further study validates our findings, we caution against the use of the proposed cutoffs for surgical volume to define high-volume center as 21 cases per year or more. Our study only examined the surgical volume in the span of five calendar years, and not every institution remained in the assigned surgical volume group throughout the study period of five years (Table 5). Certain time spans would be necessary to assess the surgical volume given inter-year variability.
Table 5.
Proportion of Institutions That Crossed Over the Groups
| Intragroup Crossover | Surgical Volume |
||
|---|---|---|---|
| Low | Mid | High | |
|
| |||
| No | 28 (61) | 22 (36.7) | 4 (40) |
| Yes | 18 (39) | 38 (63.3) | 6 (60) |
| Total | 46 sites | 60 sites | 10 sites |
Data are n (%) unless otherwise specified.
Each institution, grouped as low-volume, mid-volume, or high-volume based on the 5-year surgical volume, was assessed for surgical volume in each calendar year for 5 years during study window of 2004–2008. Based on the cutoff for surgical cases over the 5-year period (Fig. 1), we arbitrarily used the annual cutoff of surgical volume as low-volume (6 surgeries or less per year), mid-volume (7–20 surgeries per year), and high-volume (21 surgeries per year or more). Intergroup variability was assessed in each given calendar year. Institutions that remained in the assigned group based on the 5-year surgical volume definition were allocated as no intergroup crossover; institutions that changed the case number to different a category were allocated as intergroup crossover.
There are multiple clinical implications of this study. First, awareness and recognition of radical hysterectomy as complex pelvic surgery requiring additional training is necessary. More importantly, an objective assessment of surgical procedures, standardized training of the required surgical skills, and treatment at centralized institutions for the surgical treatment of women with early-stage cervical cancer may lead to improved outcomes. This would be particularly applicable in areas where there is a decreasing number of radical hysterectomies performed,23 or when the minimally-invasive approach is being considered. To this end, reforming the existing system to construct a more concentrated treatment structure would certainly require national- and society-based efforts, but would be worth the challenges, given the likely improvements in patient care and outcome.
Supplementary Material
Acknowledgments
The authors thank all the JGOG institutions that participated in this study, the JGOG Cervical Cancer Committee members for their administrative work on the study, and Dr. Brendan H. Grubbs, MD, for his scientific input.
Footnotes
Financial Disclosure
Dr. Matsuo received honorarium from Chugai, compensation from Springer for a book editorial, and meeting expense fee from VBL therapeutics. The other authors did not report any potential conflicts of interest.
Presented at the 50th annual meeting on Women’s Cancer, March 16–19, 2019, Honolulu, Hawaii.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Katabuchi H. Annual report of the committee on gynecologic oncology, Japan Society of Obstetrics and Gynecology: patient annual report for 2013 and treatment annual report for 2008. J Obstet Gynaecol Res 2016;42:1069–79. [DOI] [PubMed] [Google Scholar]
- 3.Mikami M, Aoki Y, Sakamoto M, Shimada M, Takeshima N, Fujiwara H, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (bulky tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 2014;24:1333–40. [DOI] [PubMed] [Google Scholar]
- 4.Sideri M Surgery for cervical neoplasia. In: Morrow CP, editor. Morrow’s gynecologic cancer surgery. 2nd ed. Encinitas (CA): South Coast Medical Publishing; 2013. p. 513–698. [Google Scholar]
- 5.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. The influence of surgical volume on morbidity and mortality of radical hysterectomy for cervical cancer. Am J Obstet Gynecol 2011;205:225 e1–7. [DOI] [PubMed] [Google Scholar]
- 6.Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol 2016;215:21–33. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
- 8.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA 2000;284:3028–35. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol 2010;118:262–7. [DOI] [PubMed] [Google Scholar]
- 10.Wright JD, Ruiz MP, Chen L, Gabor LR, Tergas AI, St Clair CM, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol 2018;132:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med 2018;379:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo K, Shimada M, Yamaguchi S, Kanao H, Nakanishi T, Saito T, et al. Identifying a candidate population for ovarian conservation in young women with clinical stage IB-IIB cervical cancer. Int J Cancer 2018;142:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Shimada M, Yamaguchi S, Kigawa J, Tokunaga H, Tabata T, et al. Neoadjuvant chemotherapy with Taxane and Platinum followed by radical hysterectomy for stage IB2-IIB cervical cancer: impact of histology type on survival. J Clin Med 2019;8:pii. E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo K, Shimada M, Yokota H, Satoh T, Katabuchi H, Kodama S, et al. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget 2018;8:106866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet 2014;125:97–8. [DOI] [PubMed] [Google Scholar]
- 16.Seaman SR, Muller-Myhsok B. Rapid simulation of P values for product methods and multiple-testing adjustment in association studies. Am J Hum Genet 2005;76:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer 2017;141:1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Finks JF, O’Reilly A, Oerline M, Carlin AM, Nunn AR, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434–42. [DOI] [PubMed] [Google Scholar]
- 20.Mikami M, Shida M, Shibata T, Katabuchi H, Kigawa J, Aoki D, et al. Impact of institutional accreditation by the Japan Society of Gynecologic Oncology on the treatment and survival of women with cervical cancer. J Gynecol Oncol 2018;29:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–904. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network clinical practice guideline in oncology. Cervical cancer. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Retrieved February 4, 2019.
- 23.Uppal S, Rebecca Liu J, Kevin Reynolds R, Rice LW, Spencer RJ. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012–2015). Gynecol Oncol 2019;152:133–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


