Dear Editor
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mainly affects the respiratory system. In fact, fever, cough, and dyspnea are the symptoms most commonly experienced during the acute coronavirus disease 2019 (COVID-19) phase [1]. Further, according to a meta-analysis published in the Eur J Internal Medicine, fatigue and dyspnea are also the most prevalent post-COVID symptoms [2]. Another meta-analysis specifically evaluating post-COVID fatigue reported a prevalence rate of 42% the first six months after the infection [3]. The presence of post-COVID fatigue is associated with worse quality of life [4]. Damage in lung function is proposed as a hypothesis for the presence of respiratory post-COVID; however, a recent study has observed that lung function and exercise capacity are almost normal 10-months after the infection albeit the presence of post-COVID symptoms [5].
Most studies investigating post-COVID fatigue and dyspnea are cross-sectional since they assessed the presence of these symptoms at one follow-up period [2], [3], [4], [5]. Two longitudinally assessing symptoms during the first year after infection reported different results. The first one observed a decrease of post-COVID fatigue and dyspnea from 6- to 12-months after infection [6] whereas the second one reported an increase of these post-COVID symptoms from 5- to 12-months after discharge [7]. Understanding the evolution of post-COVID fatigue and dyspnea could have implications for optimizing patient care and public health outcomes. This letter to the editor presents the trajectory recovery curve of dyspnea and fatigue in hospitalized COVID-19 survivors by using exponential model bar plots. Mosaic plots were also used to determine the prevalence of dyspnea and fatigue at hospital admission (T0) and during the first year after hospitalization (T1 and T2) and reporting the roller coaster presence of “de novo” symptoms at each follow-up period [8].
The LONG-COVID-EXP-CM is a multicenter cohort study including individuals with a diagnosis of SARS-CoV-2 (ICD-10 code) by RT-PCR technique and radiological findings hospitalized during the first wave of the pandemic (from March 10 to May 31, 2020) in five hospitals of Madrid (Spain). From all patients hospitalized during the first wave, a sample of 400 individuals from each hospital was randomly selected. The Ethics Committees of all involved hospitals approved the study (HCSC20/495E, HSO25112020, HUFA 20/126, HUIL/092-20, HUF/EC1517). Informed consent was obtained from all participants.
Patients were scheduled for a telephone interview conducted by trained healthcare professionals at two follow-up periods with a 5-month period in between. Patients were systematically asked about self-reported fatigue and dyspnea symptoms developed after hospitalization. Clinical features (i.e., age, gender, height, weight, medical comorbidities) and hospitalization data (e.g., COVID-19 symptoms at hospital admission, days at the hospital, intensive care unit admission) were collected from hospital medical records. Patients were excluded if they had received any treatment for post-COVID symptoms.
Mosaic plots were created with Python's library statsmodels 0.11.1 while Matplotlib 3.3.4 was used for the bar plots. The exponential curves were fitted to the data according to the formula , where represents the modeled prevalence of the symptom (dyspnea or fatigue) at a time (in months), and and are the parameters of the model.
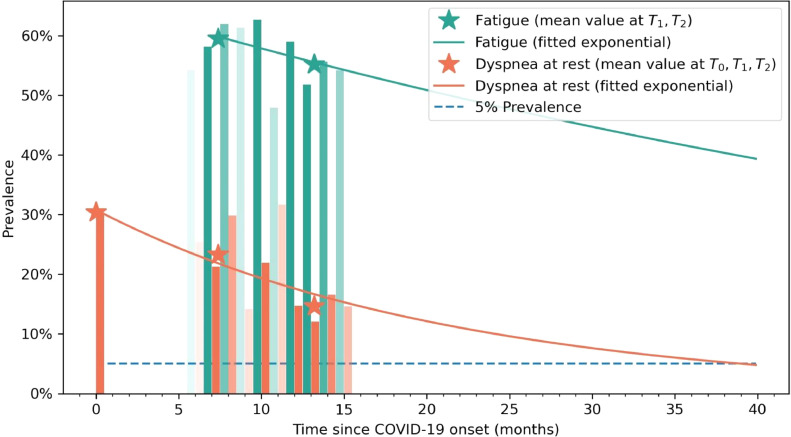
From 2,000 patients randomly selected and invited to participate, six refused to participate, 11 were not contacted, and 14 had deceased after hospital discharge. Finally, 1,969 (46.5% women, age: 61, SD: 16 years) were included at baseline (T0). Fatigue could not be collected at hospital admission (T0) to avoid confusion with generalized viral-induced myalgias. Patients were also assessed at T1 (mean: 8.4, range 6 to 10) and T2 (mean: 13.2, range 11 to 15) months after hospital discharge. A total of 1,593 (80.9%) completed all assessments. The fitted exponential curves showed a decrease tendency of dyspnea and fatigue symptoms (Fig. 1 ). The vertical bars represent the percentage of patients that have dyspnea (orange) or fatigue (green) at any time (opacity approximately indicates the sample size at a given time).
Fig. 1.
Recovery curve of self-reported dyspnea (in orange) and fatigue (in green) symptoms. Opacity indicates the sample size at that follow-up time. Asterisks represent then mean values taken at T0, T1 and T2 follow-up periods.
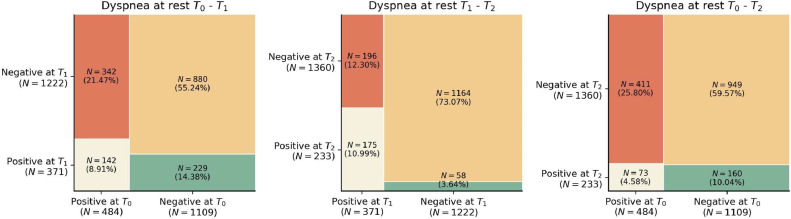
As it can be observed, the prevalence of dyspnea decreased from 30.8% (n=484) at hospital admission (T0), to 23.3% (n=371) at T1, to 14.6% (n=233) at T2. Fig. 2 shows mosaic plots of dyspnea prevalence comparing T0 to T1, T0 to T2, and T1 to T2. Looking at Fig. 2, 70% of patients (n=342/484) experiencing dyspnea at hospital admission (T0) had recovered eight months after (T1), whereas 229/371 (61.7%) patients with dyspnea at T1, had developed it “de novo” (did not suffered from dyspnea at T0). Similarly, almost 25% (n=58/233) of patients reporting dyspnea at T2 did not experience this symptom at T1 (Fig. 2).
Fig. 2.
Mosaic plots dyspnea at (from left to right): T0 (hospital admission) vs T1 (8.4 months after hospital discharge), T1 vs T2 (13.2 months after hospital discharge), and T0 vs T2.
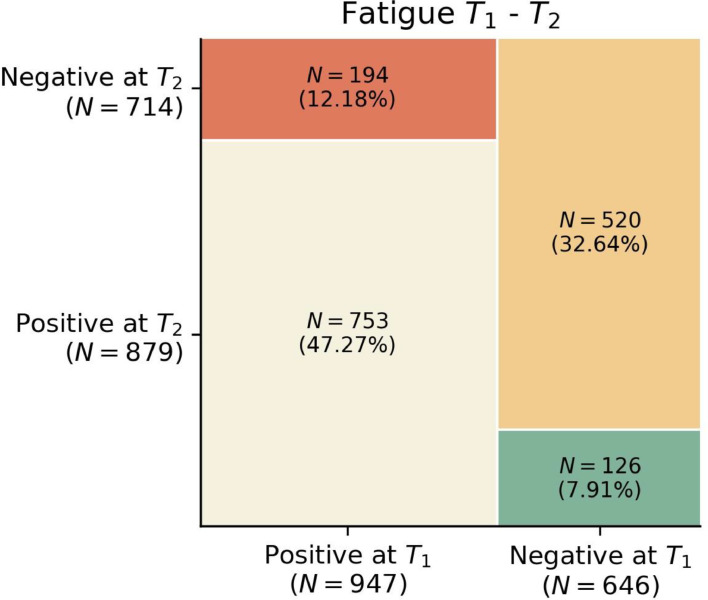
The prevalence of fatigue slightly decreased from 59.4% (n=947) at T1 to 55.2% (n=879) at T2 (Fig. 3 ). Only 20.5% of patients (n=194/947) experiencing fatigue at T1 had recovered at T2, while 14% (126/879) individuals with fatigue at T2 had developed it “de novo”.
Fig. 3.
Mosaic plots of fatigue at T1 (8.4 months after hospital discharge) vs T2 (13.2 months after hospital discharge).
To the best of our knowledge, this is the first time analyzing the longitudinal curve of recovery of post-COVID dyspnea and fatigue in previously hospitalized COVID-19 survivors. The mosaic plots showed that a large number of patients developed “de novo” dyspnea, whereas fatigue was considered as a “de novo” post-COVID symptom. Despite this, more individuals recovered than those that developed dyspnea “de novo”, explaining the downwards prevalence trend observed. This decrease was, however, less pronounced in the case of post-COVID fatigue, which seems to be a long-lasting symptom.
Our prevalence rates of dyspnea and fatigue at the different follow-up periods are similar to those reported in current literature [3,4]; however, we recognized that collecting fatigue as an onset symptom at hospital admission during the first wave of the pandemic was difficult because several subjects confused it with generalized viral-induced myalgias (general tiredness painful sensation). In fact, dyspnea and fever are the most bothersome COVID-19 symptoms self-reported by the patients at hospital admission.
Since lungs exhibit high levels of the angiotensin-converting enzyme II (ACE2) and transmembrane serine protease 2 (TMPRSS2) receptors, they are invaded by SARS-CoV-2 virus resulting in pulmonary affectation, and, hence, the development of post-COVID dyspnea and fatigue. The exponential recovery curve identified in our study suggests that post-COVID dyspnea could be present up to four years after the infection. This may be explained to the fact that almost 62% of COVID-19 survivors develop “de novo” dyspnea the following months after hospitalization. Similarly, the huge prevalence of post-COVID fatigue developed after hospitalization would explain the slow recovery curve presented by this symptom. Since recovery of post-COVID fatigue will be longer than expected, this could suggest an enhanced exposure and significant viral load for a prolonged time period. Nevertheless, evidence suggests complex mechanisms underlying post-COVID dyspnea and fatigue. For instance, the systemic immune response against SARS-CoV-2 virus, reflected by significant lower levels of antibodies, has been found to be strongly correlated with more severe post-COVID fatigue [9]. Therefore, identifying the risk factors associated with the development of post-COVID dyspnea and fatigue may help to better understand the management of these symptoms [10].
An important finding revealed by the mosaic plots is that the development of post-COVID symptoms, in this case dyspnea or fatigue, can appear at any time, e.g., almost 25% individuals without dyspnea at T1 self-reported this symptom at T2, suggesting that longitudinally evolution of post-COVID symptoms should be analyzed patient by patient and not just as the total sample [8].
We acknowledge potential weaknesses of the study. First, only hospitalized patients aged around 60-years old were included. Second, we collected self-reported dyspnea and fatigue. Although some tools are used for the assessment of fatigue, all consists of self-reported questionnaires. Third, we focused on these post-COVID symptoms. Interaction between different post-COVID symptoms is common. Future studies investigating the interactions between different post-COVID symptoms during the following years are now needed
In conclusion, our tendency analysis revealed that post-COVID dyspnea tend to slowly recover during the three years after SARS-CoV-2 infection, whereas the recovery of post-COVID fatigue is much slower in previously hospitalized COVID-19 survivors and could last several years after the infection.
Author contributions
All authors contributed to the study concept and design. CFdlP, JMG and OPV conducted literature review and did the statistical analysis. All authors recruited participants and collected data. OPV supervised the study. All authors contributed to interpretation of data. All authors contributed to drafting the paper. All authors revised the text for intellectual content and have read and approved the final version of the manuscript.
Declaration of Competing Interest
No conflict of interest is declared by any of the authors
Acknowledgments
Consent to participate
Provided informed consent before collecting data.
Consent for publication
No personal info of any patient is provided in the text.
Ethics approval
The Ethics Committees of all involved hospitals approved the study (HCSC20/495E, HSO25112020, HUFA 20/126, HUIL/092-20, HUF/EC1517).
Role of the funding source
The LONG-COVID-EXP-CM is supported by a grant associated to the Fondo Europeo De Desarrollo Regional - Recursos REACT-UE del Programa Operativo de Madrid 2014-2020, en la línea de actuación de proyectos de I+D+i en materia de respuesta a COVID 19. The sponsor had no role in the design, collection, management, analysis, or interpretation of the data, draft, review, or approval of the manuscript or its content. The authors were responsible for the decision to submit the manuscript for publication, and the sponsor did not participate in this decision.
References
- 1.Borges do Nascimento IJ, O'Mathúna DP, von Groote TC, et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis. 2021;21:52. doi: 10.1186/s12879-021-06214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Int Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao S, Benzouak T, Gunpat S, et al. Fatigue symptoms associated with covid-19 in convalescent or recovered COVID-19 patients: a systematic review and meta-analysis. Ann Behav Med. 2021 doi: 10.1093/abm/kaab081. Oct kaab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL): a systematic review and meta-analysis. J Med Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staudt A, Jörres RA, Hinterberger T, Lehnen N, Loew T, Budweiser S. Associations of post-acute COVID syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur J Intern Med. 2022;95:50–60. doi: 10.1016/j.ejim.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;39:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab611. Jul ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-de-las-Peñas C. Are patients exhibiting post-COVID symptoms at 12-months the same than at 5- or 9-months the fluctuating nature of Post-COVID. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molnar T, Varnai R, Schranz D, et al. Severe fatigue and memory impairment are associated with lower serum level of Anti-SARS-CoV-2 antibodies in patients with post-COVID symptoms. J Clin Med. 2021;10:4337. doi: 10.3390/jcm10194337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal F, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]