Abstract
Previous studies in our laboratory have demonstrated that microinjection of N-methyl-D-aspartate NMDA agonist into the nucleus. magnocellularis (NMC) of the medial medulla increases muscle tone and/ or produces locomotion, while injection of corticotropin-releasing factor (CRF) and non-NMDA agonists into the same or nearby sites suppresses muscle tone. In the first paper of this series, we report. that myoclonic twitches or coordinated rhythmic leg movement locomotion can be induced by either NMDA or hemorrhagic bilateral. lesion of the ventral mesopontine junction (vMPJ). In this paper, we report that microinjection of CRF (10 nM) or non-NMDA agonists, kainic acid (0.1–0.2 mM) and quisqualic acid (1–10 mM), into the NMC block locomotion and myoclonic twitches. The latency and duration of CRF and non-NMDA agonist-induced blockade of motor activity were short, at 34 s and 3.6 min, respectively. However, microinjection of the NMDA antagonists DL-2-amino-5-phosphonovaleric acid (APV; 50 mM) or. DL-2-amino-5-phosphonopentanoic acid (AP5, 20 mM) block myoclonus at a latency of 0.6–3 min with the block lasting for a mean of 7 h. Thus, activation of non-NMDA. receptors or inactivation of NMDA receptors in NMC can block myoclonus. An imbalance between the inputs to these receptor systems may contribute to the generation of abnormal motor activation in waking and sleep.
Keywords: N-Methyl-D-aspartate (NMDA), Non-NMDA, Corticotropin-releasing factor, DL-2-Amino-5-phosphonovaleric acid (APV), REM behavior disorder, Periodic leg movement, Nucleus magnocellularis
1. Introduction
The nucleus magnocellularis NMC of the medulla has been shown to play an important role in the control of muscle activity. Electrical stimulation of the NMC in decerebrate animals produces inhibition of spinal reflexes [1,7,22], suppression of muscle tone [9,20] and locomotion [3,12]. Intracellular recording has shown that stimulation of the NMC produces inhibitory (IPSPs). and excitatory postsynaptic potentials (EPSPs). in both forelimb and hindlimb, back, and neck motoneurons [11,21,26,27].
We have demonstrated that corticotropin-releasing factor (CRF).[18] and non-NMDA agonists [17] microinjected into the NMC produce muscle atonia, while N-methyl-Dasparate NMDA agonists [12,17] produce increased muscle tone andror locomotion. In the first paper of this series, we reported that lesion of the ventral part of the mesopontine junction (vMPJ). and retrorubral nucleus (RRN) produced spontaneous or sensory induced locomo-. tion or muscle twitches [19] We have also demonstrated that the areas of RRN and vMPJ have very dense projections to the NMC [15] The present study was undertaken to investigate the pharmacology of NMC control of locomotion and myoclonus.
2. Materials and methods
Fifty-one cats weighing 2.5–4.5 kg, 18 males and 33 females, were used in this experiment. The protocol for surgical preparation, stimulation and lesion was described in the first paper of this series [19]. Among these 51 cats, 6 of 7 NMDA lesioned cats and 11 of 40 cats without NMDA injection developed spontaneous or sensory induced locomotion or myoclonus. The remaining 4 cats received Ringer’s saline injection and did not generate muscle hyperactivity over the period of the experiment.
The NMC of the ventromedial medulla, which is located at P8–P11, 1 mm from the midline, and dorsoventral at −8 to −10 [5], was physiologically identified [20] by electrical stimulation which produced muscle atonia. Stimulation consisted of 500 ms trains with 100 Hz, 0.2 ms, and 20–70 μA rectangular cathodal pulses applied through a stainless steel monopolar microelectrode (A-M Systems, Model 5710) After spontaneous movement or myoclonus. developed, microinjections into the NMC consisted of 0.5 μl of one of the following: CRF, NMDA and non-NMDA agonists, and NMDA antagonists. Microinjections were delivered through a 1 μl Hamilton microsyringe with a 25-gauge needle (7001 N). over a period of 1 min. The injection needle was retained in position for another 5 min after the injection. At least 1 h elapsed between injections.
2.1. Chemicals
The concentration of the chemicals used in this experiment was based on our previous studies [17,18], which showed the optimal doses for inducing muscle tone effects in the NMC. The chemicals and concentration used for microinjection into the NMC were as follows: kainic acid (KA; 0.1–0.2 mM), quisqualic acid. (QA; 1–10 mM),. NMDA (7 mM),. DL-2-amino-5-phosphonovaleric acid (APV; 50 mM),. DL-2-amino-5-phosphonopentanoic acid (AP5; 20 mM), and CRF. (10 nM). All chemicals were. dissolved in Ringer’s saline and adjusted to a pH of 7.4.
2.2. Histology
Iron was deposited into the injection sites of the medullary reticular formation through a stainless steel monopolar microelectrode A-M Systems, 50 μA anodal DC current for 19 s at the end of the experiment. The cats. were anesthetized (sodium pentobarbital, 35 mg/rkg, i.p.). and perfused intra-cardially with saline followed by buffered formalin solution. The brainstem was removed and stored in 30% sucrose buffered formalin. Then, serial coronal sections were cut at 60 μm. Staining with neutral red and counterstaining with ferrocyanide were done to identify the Prussian blue reaction at the injection site.
3. Results
Fifty-seven microinjections (CRF: 6, KA: 12, QA: 15, NMDA: 8, APV: 11, AP5: 5) in 29 sites within the NMC,. 4 injections APV: 2, AP5: 2) at the border of NMC and. nucleus paramedianus (NPM), and 3 injections. (KA: 2, QA: 1) at the border of NMC and nucleus gigantocellularis. (NGC) were performed.
One half microliter of CRF, non-NMDA agonists, or NMDA antagonists injected into the NMC had a global effect on blocking (42 out of 57 injections) or reducing (15 Injections) the frequency and magnitude of locomotor ac-. tivity, tonic contractions and myoclonic events Fig. 1 and Table 1) Such microinjections were effective both in. animals with NMDA lesions and in those with hemorrhagic lesions in the RRN and vMPJ as reported in the first paper of this series 19. As we reported previously [17,18], the concentration of NMC muscle tone suppressants (CRF: 10 nM, KA: 0.2 mM, QA: 10 mM) used in this study had a. short latency (20–47 s) and short duration (2.1–7.8 min) suppressive effect (Table 1) on muscle tone. This suppressive effect coincided with their blockade of myoclonus, locomotor and phasic muscle contractions. Three non-NMDA agonist injections which were located at the border of NMC and NGC produced a longer latency (0.8–1.7 min) of blocking effect on motor activities. The duration of. this blocking effect was not different from those of NMC injections, with the blockade lasting 1.8–7.5 min.
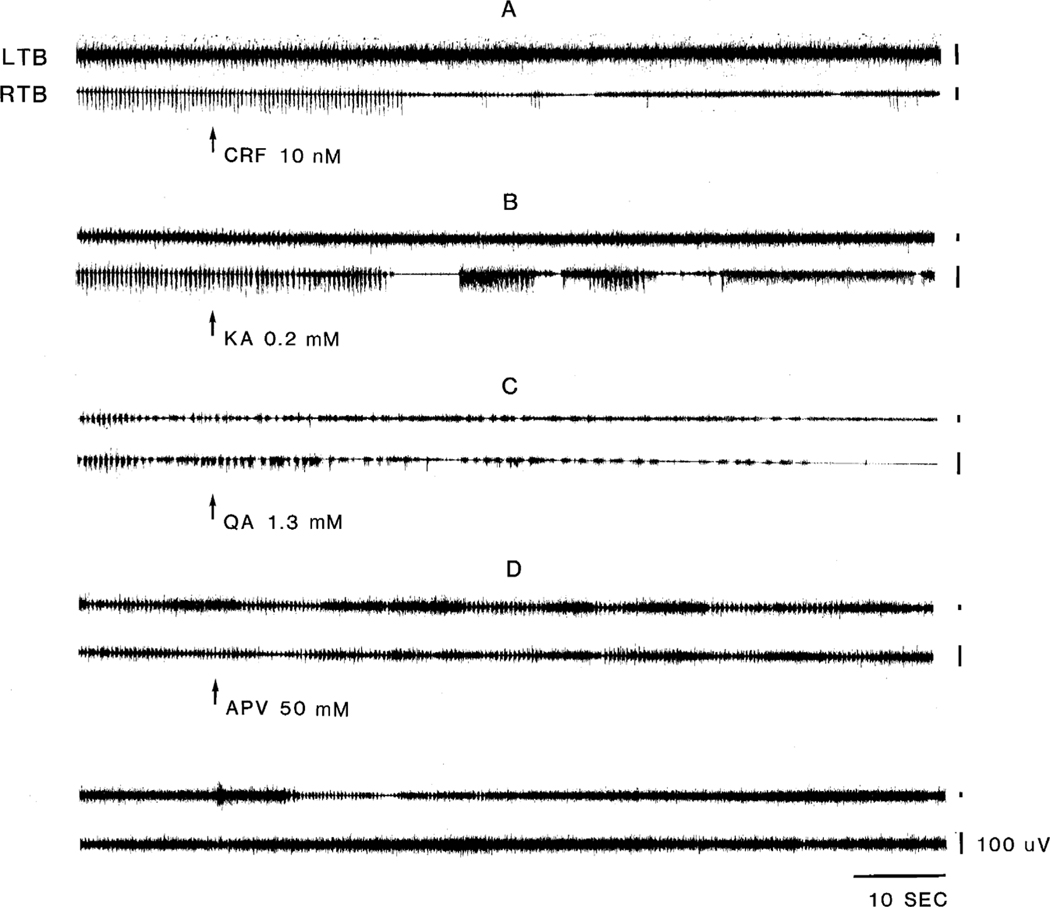
Fig. 1.
Effect of microinjections on muscle activity. Microinjection of corticotropin-releasing factor (CRF; A), kainic acid (KA; B), quisqualic acid (QA;C) and 2-amino-5-phosphonovaleric acid (APV; D) into the nucleus magnocellularis NMC of the medulla suppressed motor hyperactivity. All injection were made into the same site. CRF and KA were injected on day 2, QA was injected on day 3, and APV was injected on day 4 post-decerebration. LTB, left triceps brachii; RTB, right triceps brachii.
Table 1.
Latency and duration of suppression of motor activity by CRF, non-NMDA agonists, NMDA agonists, and NMDA antagonists microinjected into the NMC
| Chemicals | n | Latency (s) | Duration (min) |
|---|---|---|---|
|
| |||
| CRF (10 nM) | 6 | 29±12.1 | 3.8±1.42 |
| Kainate (0.2 mM) | 12 | 38±20.7 a | 4.7±1.06 |
| Quisqualate (10 mM) | 15 | 36±21.5 a | 4.3±0.87 |
| NMDA (6.8 mM) | 8 | 31±10.4 | 3.4±0.46 |
| APV (50 mM) | 11 | 46±22.6 b | 624±48.6 |
| AP5 (20 mM) | 5 | 36±25.8 b | 586±53.8 |
Results from injections at the border of NMC and NGC are not included.
Results from injections at the border of NMC and NPM are not included.
DL-2-Amino-5-phosphonovaleric acid (50 mM) and AP. 5 (20 mM), which are specific NMDA antagonists, produced. long lasting (8–11 h) effects on locomotion or myoclonus (Fig. 1 and Fig. 2) The efficacy of movement blockade for. both APV or AP5 was not significantly different. The latency of onset of the movement blocking effect for APV and AP5 microinjected into NMC was 46±22.6 (n=11). and 36±25.8 (n=5).s, respectively. DL-2-Amino-5-phos-phanopentanoic acid and APV microinjected into the border of NMC and NPM were also found to block muscle contractions and twitches (Fig. 2). The duration and magnitude of the effect of injections at the border of NMC and NGC on muscle activity did not differ from those of NMC injection. However, the latency was longer at 135±48 s (72–235 s).
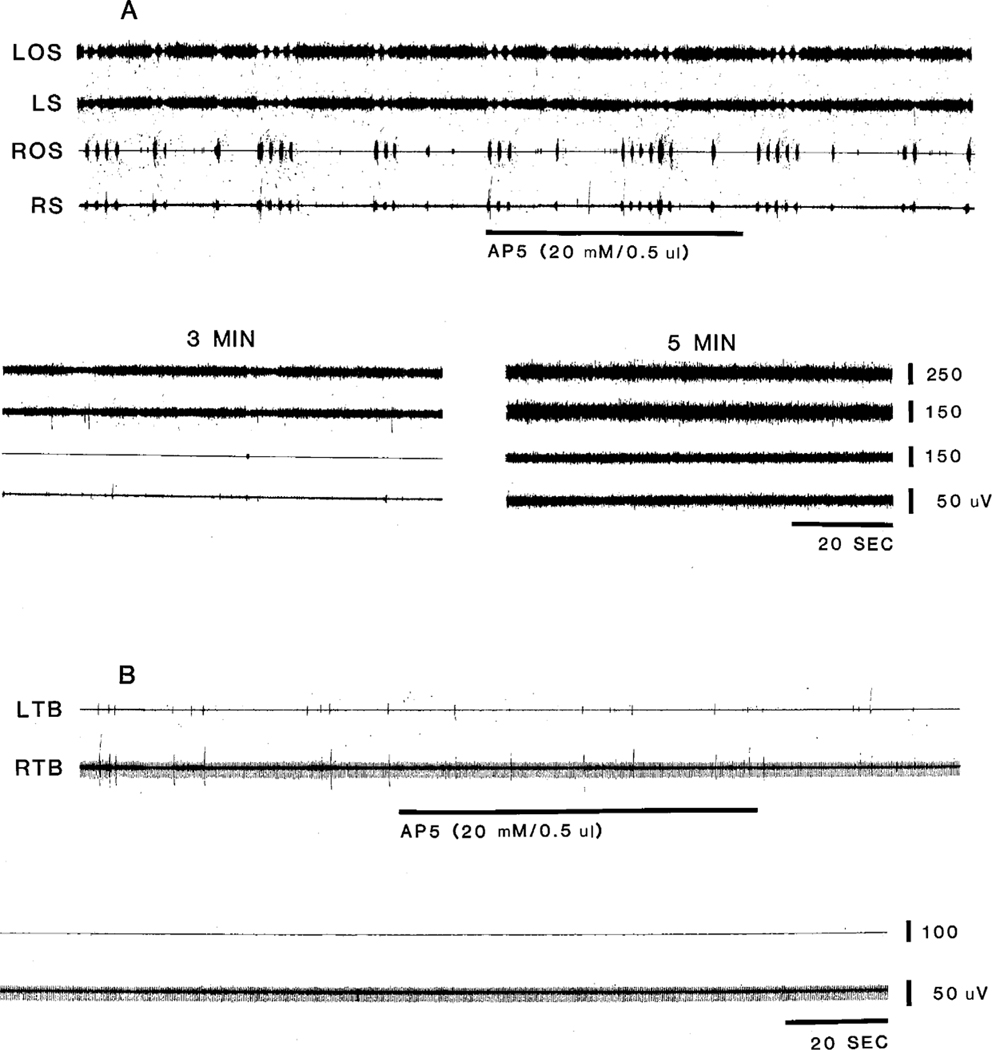
Fig. 2.
Effect of AP5 on longer duration muscle contractions (A) and twitches (B). Microinjection of AP5 into the caudal portion of the NMC blocked. movement at latency longer than that of CRF, KA and QA. Effect was also longer lasting. Injections were made in different animals. LOS and ROS, left and right occipitoscapularis; LS and RS, left and right splenius.
NMDA agonists, which produced increased muscle tone or locomotion in the normal decerebrate cat, produced muscle hyperactivity but blocked rhythmic locomotor movement when injected during locomotor episodes (Fig. 3). As in our prior study 17, the latency and duration of the NMDA effect was short at 31 s and 3.4 min, respectively.
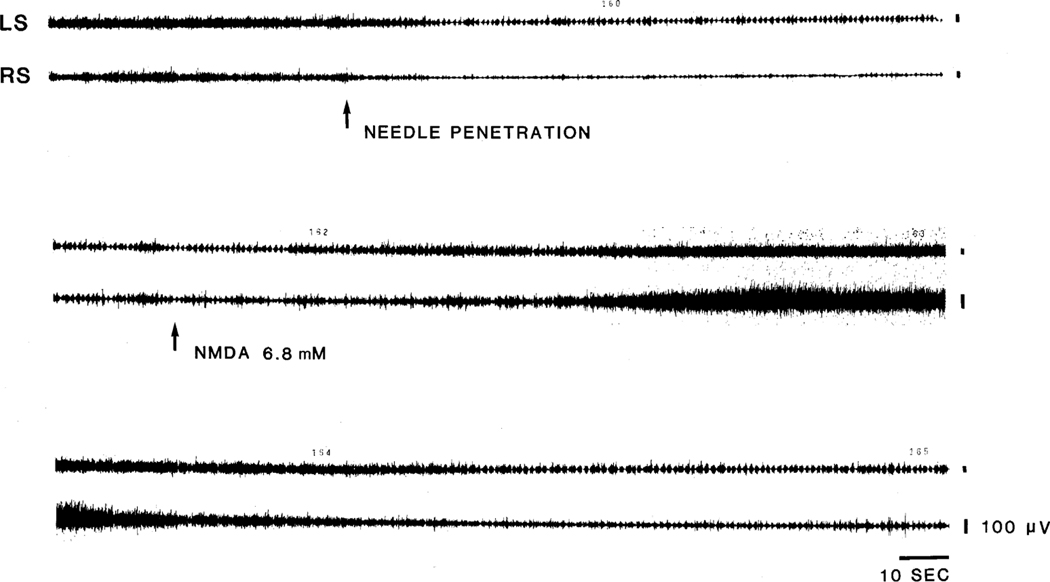
Fig. 3.
Effect of NMDA on mechanically induced locomotion. Needle penetration into the NMC produced muscle tone suppression intermixed with rhythmic muscle activity on day 2 post-decerebration in this cat which had hemorrhagic lesion of the ventral part of the mesopontine junction. NMDA injection into the NMC produced an increase of basal muscle tone and blocked locomotion. Locomotion recovered when muscle tone returned to the baseline level (bottom panel). LS, left splenius; RS, right splenius.
Serial coronal sections of the brainstem were obtained. All of the sites of chemical microinjections which blocked hyperactivity were either in the NMC, on the border between NMC and NGC, or on the border between NMC and NPM (Fig. 4).
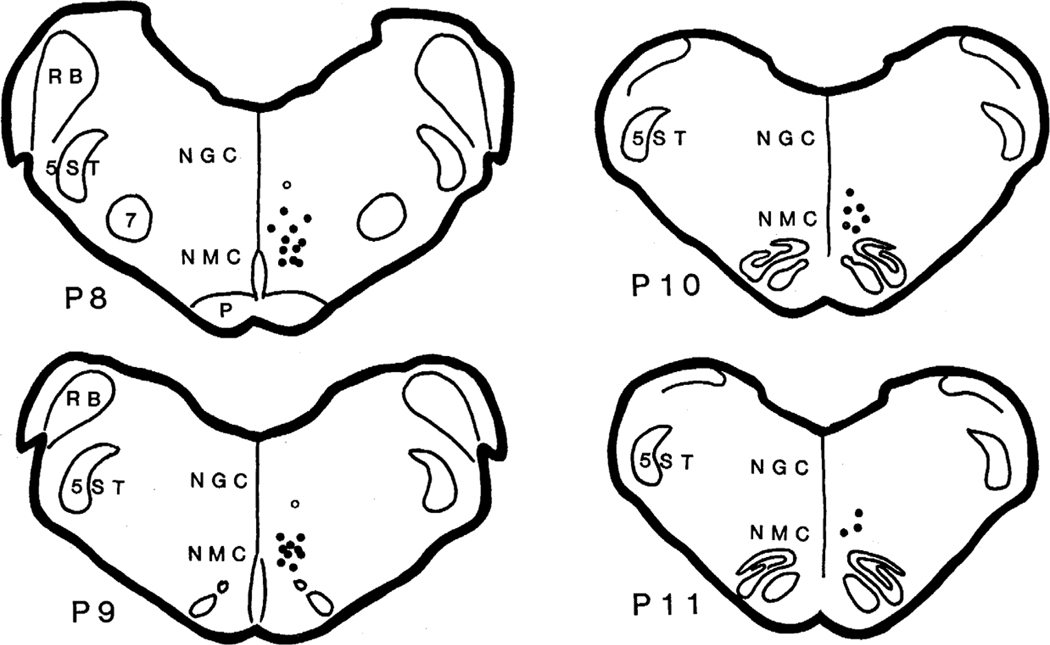
Fig. 4.
Reconstruction of the location of microinjections into the medullary reticular formation. All sites are plotted on the right side of the brain, although sites were located on both sides. Corticotropin-releasing factor, non-NMDA agonists and NMDA antagonists microinjected into the NMC (dot) blocked. locomotion or myoclonus with a short latency. Open circles shown in the ventral part of the nucleus gigantocellularis represent the sites which when injected with non-NMDA agonists produced a longer latency (longer than 3 min) blockade of locomotion and muscle twitches. All sites received multiple injections. NGC, nucleus gigantocellularis; NMC, nucleus magnocellularis; P, pyramidal tract; RB, restiform body; 7, facial nucleus; 5ST, spinal trigeminal tract.
4. Discussion
In the previous paper, we found that after lesion of the RRN and vMPJ, spontaneous or sensory induced locomotion and myoclonus occurred in the decerebrate cat. The present paper demonstrates that NMC microinjection of CRF and non-NMDA agonists produces a short duration blockade of myoclonus and locomotion. APV and AP5 produced the same effects for a longer duration. NMDA injection in locomoting animals produced increased muscle tone and also blocked locomotion and muscle twitches.
All microinjections of CRF, NMDA agonists and antagonists, and non-NMDA agonists were found to block or reduce muscle hyperactivity in this study. However, variations of the latency and duration of blocking effect were found across the injections. The site of injection and the areas of chemical diffusion can explain these variations. The latency was longer when the injection was made at the border of either the NMC and NGC or the NMC and NPM. These latency data suggest that the NMC is mediating these effects. It is likely that the neuronal damage caused by multiple needle insertions contributed to some variation in latency across the injections [17]
The ventral horn of the spinal cord receives a major projection from the NMC [2,6,10,13,14,29,31] The nucleus magnocellularis receives a major glutamatergic projection from the RRN and vMPJ [15]. The vMPJ has been found to produce muscle tone suppression during electrical stimulation and locomotion during inter-stimulus intervals upon repetitive stimulation [16] Electrophysiological studies have demonstrated that neurons of the NMC monosynaptically excite and inhibit neck motoneurons [8,25,26] Electrical stimulation of the NMC produces either locomotion [3,4,24] or inhibitory effects on muscle activity [20,22] in the decerebrate animal as a function of stimulus parameters. A similar effect of electrical stimulation in the nucleus gigantocellularis alpha and ventralis on muscle tone has been also found in the decerebrate rat [9]. Furthermore, microinjection studies have demonstrated that non-NMDA and CRF agonist injection in the NMC produces muscle atonia [17,18], while NMDA injection produces locomotion and muscle tone facilitation [12,17] in the decerebrate cat. We suggest that glutamate release, possibly from RRN and vMPJ projections, activates NMDA and non-NMDA receptors in the same or different neuronal populations in the NMC and can produce a phasic muscle activity super-imposed on muscle atonia.
NMDA receptors may play an important role in involuntary movements in animals. MK-801, an NMDA antagonist, has been reported to block myoclonic jerks in the neonatal rat when injected systemically [28]. Systemic injection of the specific NMDA antagonists, AP5 and AP7, have also been shown to prevent tremors and myoclonus in the high-pressure neurological syndrome in the rat [30]. In the present studies, we found that microinjection of NMDA antagonists into the NMC had a potent anti-myoclonic effect.
The level of muscle tone has been reported to modulate locomotor response to brain stimulation. Both very low and very high levels of muscle tone are incompatible with locomotor movements [17,23] Thus the anti-myoclonic effect induced by CRF and non-NMDA agonist injections may be due to the decrease of muscle tone that CRF and non-NMDA agonists induce. In contrast, blockade of involuntary movements by NMDA injection may result from the increase of muscle tone elicited by NMDA agonists. We hypothesize that the interaction between CRF/non-NMDA and NMDA activity in the NMC maintains muscle tone at optimal levels and thus controls posture. We hypothesize that an imbalance of facilitatory and inhibitory mechanism in the NMC may contribute to involuntary movements in waking and sleep.
Acknowledgements
This study was supported by the Medical Research Service of the VA and HL41370.
References
- [1].Alderson AM and Downman CBB, Supraspinal inhibition of thoracic reflexes of somatic and visceral origin, Arch. Ital. Biol, 104 (1966) 309–327. [Google Scholar]
- [2].Alstermark B, Kummel H. and Tantisira B, Monosynaptic raphe-spinal and reticulospinal projection to forelimb motoneurones in cats, Neurosci. Lett, 74 1987. 286–290. [DOI] [PubMed] [Google Scholar]
- [3].Atsuta Y, Garcia-Rill E. and Skinner RD, Characteristics of electrically induced locomotion in rat in vitro brain stem-spinal cord preparation, J. Neurophysiol, 64 1990. 727–735. [DOI] [PubMed] [Google Scholar]
- [4].Atsuta Y, Garcia-Rill E. and Skinner RD, Electrically induced locomotion in the in vitro brainstem-spinal cord preparation, Dev. Brain Res, 42 1988. 309–312. [DOI] [PubMed] [Google Scholar]
- [5].Berman AL, The Brain Stem of the Cat, University of Wisconsin Press, Madison, WI, 1968. [Google Scholar]
- [6].Carlton SM, Chung JM, Leonard RB and Willis WD, Funicular trajectories of brainstem neurons projecting to the lumbar spinal cord in the monkey (Macaca fascicularis): a retrograde labeling study, J. Comp. Neurol, 241 1985. 382–404. [DOI] [PubMed] [Google Scholar]
- [7].Engberg E, Lundberg A. and Ryall RW, Reticulospinal inhibition of transmission in reflex pathways, J. Physiol, 194 (1968). 201–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Garcia-Rill E. and Skinner RD, The mesencephalic locomotor region. II. Projections to reticulospinal neurons, Brain Res., 411 (1987) 13–20 [DOI] [PubMed] [Google Scholar]
- [9].Hajnik T, Lai YY and Siegel JM, Atonia related regions in the rodent pons and medulla, Sleep Res., 24A (1995) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Holstege G. and Kuypers HGJM, The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. In Kuypers HGJM and Martin GM (Eds.),. Descending Pathways to the Spinal Cord. Progress in Brain Research, Vol. 57, Elsevier, Amsterdam, 1982, pp. 145–175. [DOI] [PubMed] [Google Scholar]
- [11].Jankowska E, Lundberg A, Roberts WJ and Stuart D, A long propriospinal system with direct effect on motoneurones and interneurones in the cat lumbosacral cord, Exp. Brain Res, 21 (1974). 169–194. [DOI] [PubMed] [Google Scholar]
- [12].Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD and Garcia-Rill E, Medioventral medulla-induced locomotion, Brain Res. Bull, 24 1990. 509–516. [DOI] [PubMed] [Google Scholar]
- [13].Kuypers HGJM and Huisman AM, The new anatomy of the descending brain pathways. In Sjolund B. and Björklund A. (Eds)’ Brain Stem Control of Spinal Mechanisms, Elsevier, Amsterdam, 1982, pp. 29–54. [Google Scholar]
- [14].Kuypers HGJM and Maisky VA, Funicular trajectories of descending brain stem pathways in cat, Brain Res., 136 (1977). 159–165. [DOI] [PubMed] [Google Scholar]
- [15].Lai YY, Clements JR and Siegel JM, Afferents to the nucleus magnocellularis of the medulla, Soc. Neurosci. Abstr, 19 (1993). 1437. [Google Scholar]
- [16].Lai YY and Siegel JM, Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation, J. Neurosci, 10 (1990) 2727–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lai YY and Siegel JM, Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression, J. Neurosci, 11 (1991) 2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai YY and Siegel JM, Corticotropin-releasing factor mediated muscle atonia in pons and medulla, Brain Res., 575 (1992) 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lai YY and Siegel JM, Brainstem-mediated locomotion and myoclonic jerks. I. Neural substrates, Brain Res., 745 1997. 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai YY, Siegel JM and Wilson WJ, Effect of blood pressure on medial medulla-induced muscle atonia, Am. J. Physiol, 252 (1987) H1249–H1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Llinas T. and Terzuolo CA, Mechanisms of supraspinal actionś upon spinal cord activities. Reticular inhibitory mechanisms on α-extensor motoneurons, J. Neurophysiol, 27 (1964) 579–591. [DOI] [PubMed] [Google Scholar]
- [22].Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- [23].Mori S, Ohta Y, Sakamoto T. and Nonaka S, Excitability level-setting mechanisms in the pons: their behavioral support in decerebrate, reflex standing and freely moving, intact cats, Brain Dev., 8 (1986) 408–415. [DOI] [PubMed] [Google Scholar]
- [24].Noga BR, Kettler J. and Jordan LM, Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip, J. Neurosci, 8 (1988) 2074–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peterson BW, Reticulospinal projections to spinal motor nuclei, Annu. Rev. Physiol, 41 (1979) 127–140. [DOI] [PubMed] [Google Scholar]
- [26].Peterson BW, Pitts NG, Fukushima K. and Mackel R, Reticulospinal excitation and inhibition of neck motoneurons, Exp. Brain Res, 32 (1978) 471–489. [DOI] [PubMed] [Google Scholar]
- [27].Peterson BW, Pitts NG and Fukushima K, Reticulospinal connections with limb and axial motoneurons, Exp. Brain Res, 36 (1979) 1–20. [DOI] [PubMed] [Google Scholar]
- [28].Pranzatelli MR, Antimyoclonic effect of MK-801: a possible role for NMDA receptors in developmental myoclonus of the neonatal rat, Clin. Neuropharmacol, 13 (1990) 329–338. [PubMed] [Google Scholar]
- [29].Tohyama M, Sakai K, Salvert D, Touret M. and Jouvet M, Spinal projections from the lower brain stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectories, Brain Res., 173 (1979) 383–403. [DOI] [PubMed] [Google Scholar]
- [30].Wardley-Smith B, Meldrum BS and Halsey MJ, The high pressure neurological syndrome and 2APH: differences between fed and fasted rats, Neurosci. Lett, 48 (1984) 155–160. [DOI] [PubMed] [Google Scholar]
- [31].Zagon A. and Bacon SJ, Evidence of a monosynaptic pathway between cells of the ventromedial medulla and the motoneuron pool of the thoracic spinal cord in rat: electron microscopic analysis of synaptic contacts, Eur. J. Neurosci, 3 (1991) 55–65. [DOI] [PubMed] [Google Scholar]