Abstract
Remarkably well-preserved soft tissues in Mesozoic fossils have yielded substantial insights into the evolution of feathers1. New evidence of branched feathers in pterosaurs suggests that feathers originated in the avemetatarsalian ancestor of pterosaurs and dinosaurs in the Early Triassic2, but the homology of these pterosaur structures with feathers is controversial3,4. Reports of pterosaur feathers with homogeneous ovoid melanosome geometries2,5 suggest that they exhibited limited variation in colour, supporting hypotheses that early feathers functioned primarily in thermoregulation6. Here we report the presence of diverse melanosome geometries in the skin and simple and branched feathers of a tapejarid pterosaur from the Early Cretaceous found in Brazil. The melanosomes form distinct populations in different feather types and the skin, a feature previously known only in theropod dinosaurs, including birds. These tissue-specific melanosome geometries in pterosaurs indicate that manipulation of feather colour—and thus functions of feathers in visual communication—has deep evolutionary origins. These features show that genetic regulation of melanosome chemistry and shape7–9 was active early in feather evolution.
Subject terms: Palaeontology, Evolutionary developmental biology
Melanosomes preserved in the skin and feathers of a tapejarid pterosaur from the Early Cretaceous found in Brazil provide evidence of the early use of feathers for visual communication.
Main
Feathers are remarkable integumentary innovations that are intimately linked to the evolutionary success of birds10 and occur in diverse non-avian dinosaurs from the Middle Jurassic onwards1. The early evolutionary history of feathers, however, remains controversial as relevant fossils are rare3,11. Integumentary appendages in pterosaurs, traditionally termed pycnofibres, were recently reinterpreted as feathers on the basis of preserved branching2 but their homology with feathers is debated3,11 and their functions remain unclear4. The small size and lack of secondary branching in pterosaur feathers precludes functions in active flight, but their dense packing and distribution over the body are consistent with thermoregulation12. This in turn is consonant with functional hypotheses for small, simple feathers in theropod dinosaurs1,4. Even simple unbranched feathers in theropods, however, functioned in visual signalling, as evidenced by melanosome-based colour patterning13,14. Whether feathers in earlier-diverging taxa also functioned in patterning is unclear: feathers and filamentous integumentary structures in non-coelurosaurian dinosaurs and pterosaurs are rare and their taphonomy is difficult to interpret. As a result, the timing and phylogenetic and ecological context of the evolution of melanin-based colour patterning in feathers is unknown.
Resolution of this issue requires evidence of colour patterning, including spatial zonation of melanosomes15, but this could be a taphonomic artefact. More definitive evidence includes variation in the morphology of melanosomes, as this is linked to feather colour in extant birds16. Previous observations of feather melanosomes in pterosaurs have revealed indiscriminate ovoid geometries2. These resemble melanosome geometries in the skin of extant reptiles (where visible colour is independent of melanosome geometry6) and preserved melanosomes in the skin of fossil non-dinosaurian reptiles. These data indicate that within Avemetatarsalia, the ability to vary melanosome geometry (and control the colour of integumentary appendages) is unique to theropods. Variable melanosome geometries in extant mammals, however, suggest earlier origins for this feature in a common amniote ancestor and a secondary loss in pterosaurs.
Here we resolve this issue using a new specimen of an adult tapejarid pterosaur from the Lower Cretaceous Crato Formation17 (Araripe Basin, Brazil; Fig. 1, Extended Data Fig. 1, Supplementary Information). The specimen comprises an incomplete cranium associated with preserved skin, monofilaments and branched integumentary structures. These integumentary tissues preserve melanosomes that show tissue-specific geometries, a feature previously known only from theropod dinosaurs, including extant birds18. Collectively, these results confirm that branched integumentary structures in pterosaurs are feathers and provide evidence that tissue-specific partitioning of melanosome geometry—critical for melanin-based plumage patterning—has deep evolutionary origins in ancestral avemetatarsalians in the Early to Middle Triassic.
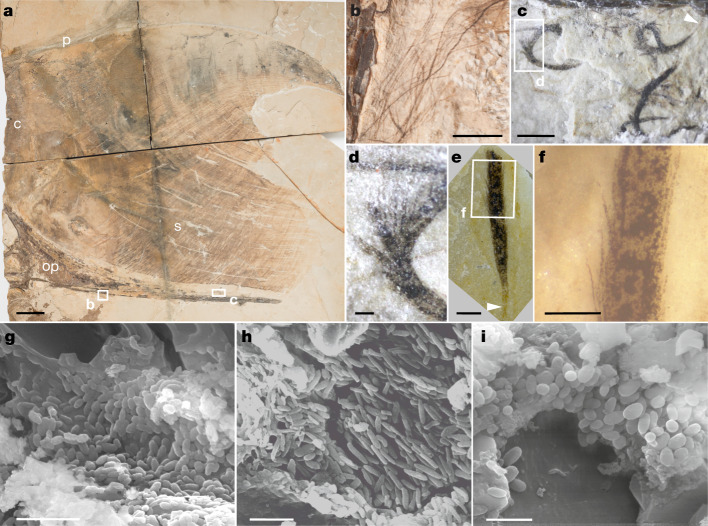
Fig. 1. Details of the cranial crest of MCT.R.1884, a new specimen of Tupandactylus cf. imperator (Pterosauria: Tapejaridae) from the Lower Cretaceous Crato Formation, Brazil.
a, Incomplete cranium showing preserved soft tissue crest. b–f, Detail of the integumentary structures associated with the posterior part of the skull. b, Monofilaments. c, Branched feathers. d, Detail of curved branched feather in c. e, f, Straight branched feather (e) with detail (f). White arrowhead in e indicates the basal calamus. g–i, SEM of melanosomes in the soft tissues of MCT.R.1884. g, Ovoid melanosomes from the elongate fibres of the soft tissue crest. h, Elongate melanosomes from a monofilament. i, Ovoid melanosomes from a branched feather. c, cristae; p, postmaxillary process; op, occipital process; s, skin. Scale bars, 50 mm (a); 5 mm (b); 2 mm (c); 250 μm (d–f); 2 μm (g–i).
Extended Data Fig. 1. Location of the samples collected from the soft tissue cranial crest, monofilaments and branched feathers and sedimentary matrix.
The soft tissue crest is characterized by elongate brown fibres. The posterodorsal part of the crest is darker than the rest of the crest and the brown fibres are faint or not evident in that area. Scale bar, 100 mm.
Preserved pterosaur feathers
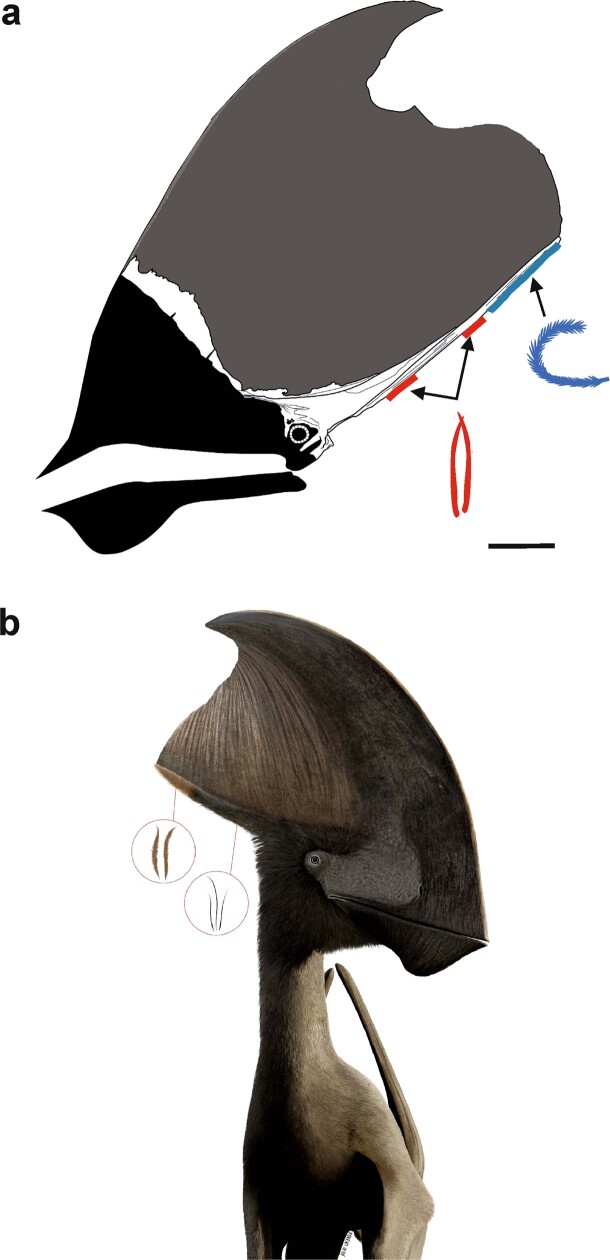
The cranium of a new specimen of Tupandactylus cf. imperator (MCT.R.1884; Pterosauria: Tapejaridae) (Supplementary Information) is preserved on five limestone slabs from the Lower Cretaceous Crato Formation in Brazil. Only the posterior portion of the cranium is present, comprising part of the left orbit, left nasoanteorbital fenestra, fibrous crista and occipital process. The preserved soft tissue cranial crest extends between the postpremaxillary and occipital processes (Fig. 1a, Supplementary Information). Two types of filamentous integumentary structure occur close to (within 15 mm of) the occipital process (Fig. 1b–f). The proximal portion of the occipital process is mostly associated with monofilaments (approximately 30 mm long and 60–90 μm wide; Fig. 1b, Extended Data Figs. 1, 2). These resemble stage I feathers19,20 and monofilaments in the anurognathid Jeholopterus ningchengensis21,22, Sordes pilosus23,24 juvenile anurognathids2, the ornithischian dinosaur Tianyulong25 and the theropod Beipiaosaurus26.
Extended Data Fig. 2. Distribution of feather types in the tapejarid pterosaur Tupandactylus cf. imperator (MCT.R.1884).
a, Schematic illustration of MCT.R.1884. Monofilaments (red) are restricted to the region immediately adjacent to the proximal part of the occipital process and the branched feathers (blue) to the region adjacent to the distal part of the occipital process. The cranial soft tissue crest is shown in dark grey and the preserved bones are shown in white. The proximal part of the skull (in black) is not present on the slab. b, Reconstruction of MCT.R.1884 showing the distribution of feathers along the occipital process (colours are not reconstructed here). Image credit, Julio Lacerda. Scale bar in (a), 100 mm.
The distal part of the occipital process is associated with short (2–5 mm long) branched integumentary structures (Fig. 1c–f, Extended Data Fig. 2). Each shows a poorly defined central shaft (approximately 60 μm wide; Extended Data Fig. 3) that thins close to the proximal tip (Fig. 1c, e). This narrow, light-toned proximal portion of the shaft resembles a basal calamus (Fig. 1e). Short (approximately 100–200 µm long), straight and closely spaced secondary fibres extend from the shaft along almost its entire length, forming a branched structure (Fig. 1d–f). These branched structures can be straight but are often curved; when curved, the branches are characteristically splayed (Fig. 1c, d). Such splaying can be generated only where a central shaft and lateral branches are stiff and where the branches diverge along the length of the shaft, rather than diverging from a single point or limited region of the shaft (Extended Data Fig. 3). This mode of branching is directly comparable to that in stage IIIa feathers19,20 of extant birds, that is, with barbs branching from a central rachis. This is strong evidence that the fossil branched structures are feathers comprising a rachis and barbs. This is consistent with and supports recent claims of branched feathers in other pterosaurs1. The monofilaments are thus most plausibly interpreted as stage I feathers.
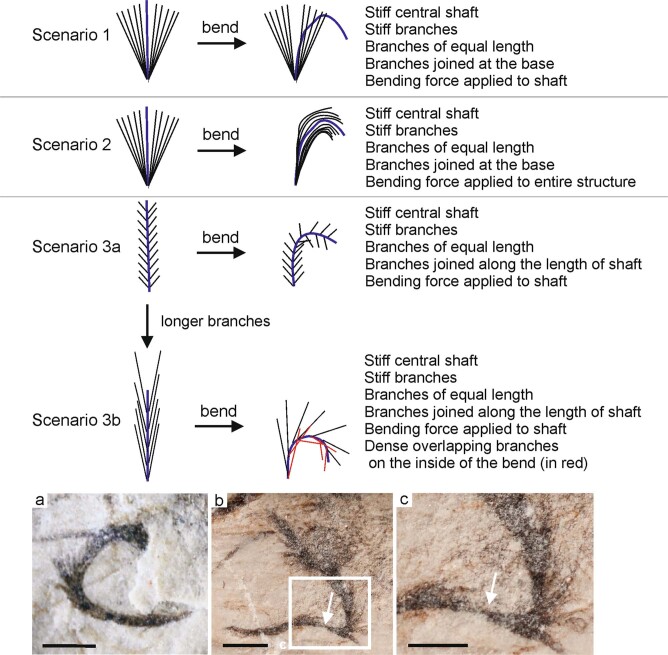
Extended Data Fig. 3. Taphonomic scenarios to explain the origin of the splayed appearance of the branched feathers, based on different styles of feather branching and stiffness.
Only scenario 3, in particular scenario 3b, with a stiff central shaft and stiff barbs of equal length, can explain the particular structures observed in Tupandactylus feathers. a–c, Branched feathers from MCT.R.1884. c, Close-up of the splayed structure in (b) showing branching and a thin shaft at the point of flexure of the barbs (arrow). Scale bars, 1 mm (a, b), 250 μm (c).
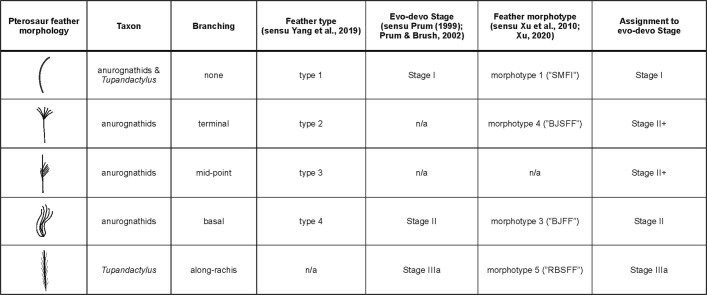
To our knowledge, stage IIIa feathers have not previously been reported in pterosaurs. The Tupandactylus branched structures resemble those in the dromaeosaurid dinosaur Sinornithosaurus millenii27, which are considered homologous to avian feathers28, and differ from the three types of branched feathers described in anurognathid pterosaurs2. Branching in the anurognathid feathers can be distal (brush-like ‘type 2’ feathers2), near the midpoint (brush-like ‘type 3’ feathers2) or proximal (tuft-like ‘type 4’ feathers2; see Extended Data Table 1 for comparison of fossil feather nomenclature systems). Unlike these three anurognathid feather types, all of which branch in a narrow zone along the feather shaft, the branched feathers in Tupandactylus branch along almost the entire length of the rachis. Further, the consistent length of the Tupandactylus secondary fibres (barbs) differs from the varying length of those in anurognathid feathers2.
Extended Data Table 1.
Classification of pterosaur feathers
Assignment of pterosaur feathers, including those reported in Tupandactylus cf. imperator (this manuscript) and two anurognathid pterosaurs2, to existing classification systems; i.e. feather type (sensu Yang et al., 2019), evo-devo stage (sensu Prum et al. 1999 and Prum & Brush, 2002) and feather morphotype (sensu Xu et al., 2010 and Xu, 2020). SMFI: slender monofilamentous integument, BJFF: basally joining filamentous feather, BJSFF: basally joining shafted filamentous feather, RBSFF: radially branched shafted filamentous feather.
The Tupandactylus feathers are not taphonomic artefacts. Both monofilaments and branched feathers occur in the specimen, which is consistent with the presence of multiple feather types in anurognathids2, feathered dinosaurs29–31 and fossil32,33 and extant birds34. Critically, Tupandactylus includes many isolated (non-superimposed) feathers where branching is obvious (Fig. 1c–f) and thus cannot be explained by superposition of monofilaments35. Nor does branching reflect degradation of monofilaments35—branched feathers show a consistent morphology, unlike the random pattern of fragmentation expected from decay. Further, the branched feathers do not represent structural fibres of the skin that have decayed, as the feathers are restricted to a portion of the skull (occipital process) that should be devoid of such fibres. Moreover, the cranial crest lacks feathers despite the preservation of long straight fibres (100–150 µm wide; up to approximately 300 mm long) that presumably represent preserved structural skin fibres (Supplementary Information and Extended Data Figs. 1, 4).
Extended Data Fig. 4. Integumentary structures of the cranial crest of MCT.R.1884.
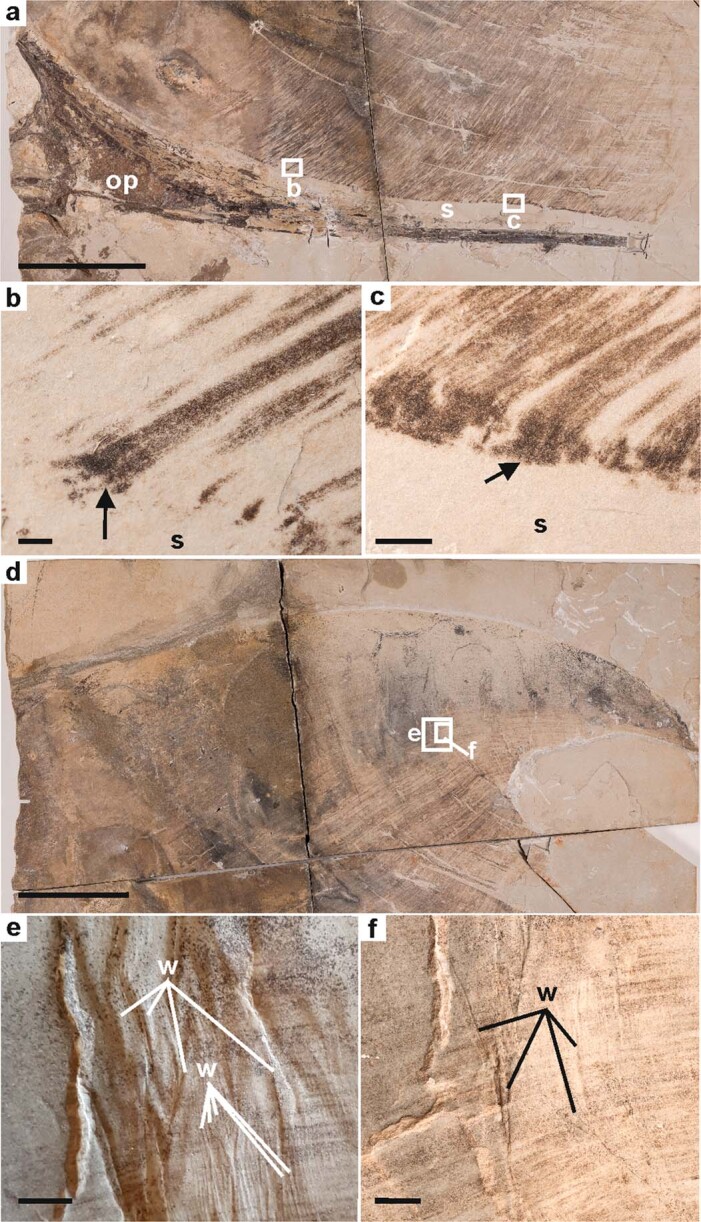
a, Ventral part of the soft tissue crest separated from the occipital process (op) by a zone lacking soft tissue and showing only sediment (s). b, c, Detail of the basal part of the cranial crest showing dark brown structures at the base of the fibres (see arrows). d, Posterodorsal part of the cranial crest. e, f, Details of regions indicated in (d). The brown fibres of the soft tissue crest are oriented perpendicular to prominent wrinkles, expressed as variation in the topography of the specimen. Scale bars, 10 mm (a, d); 2 mm (b, c); 5 mm (e, f). op, occipital process; s, sediment; w, wrinkle.
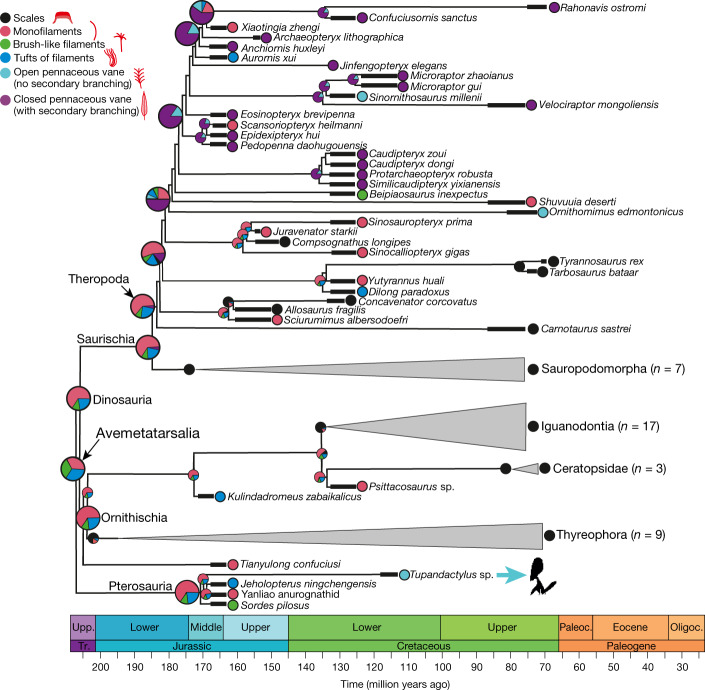
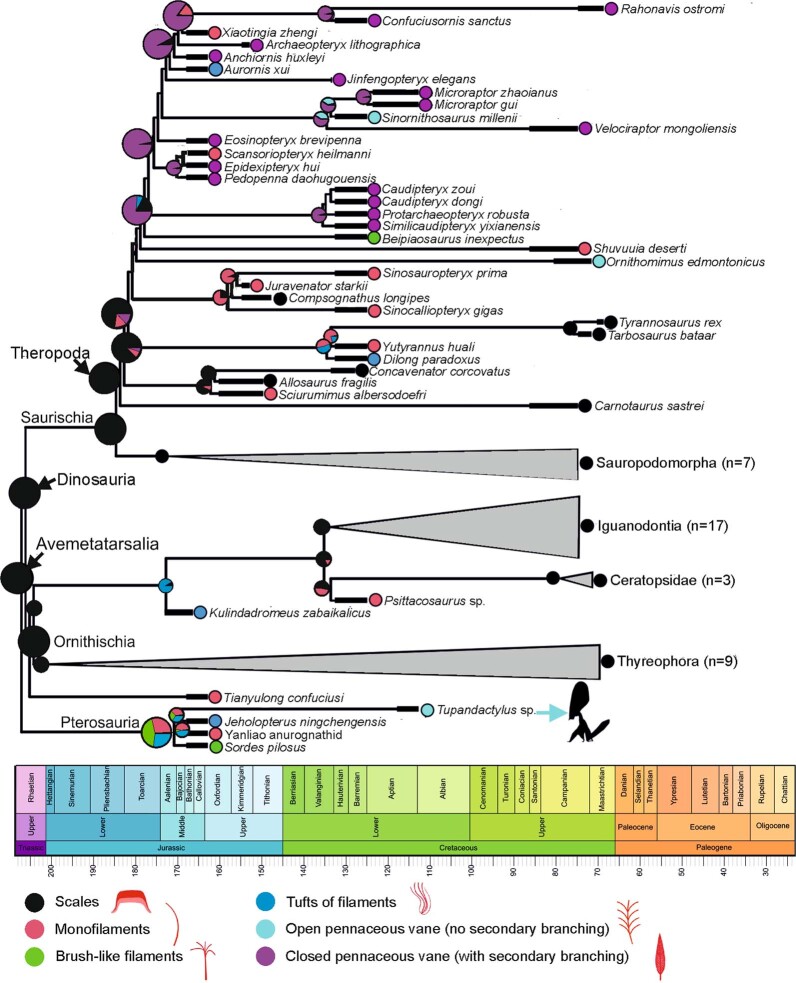
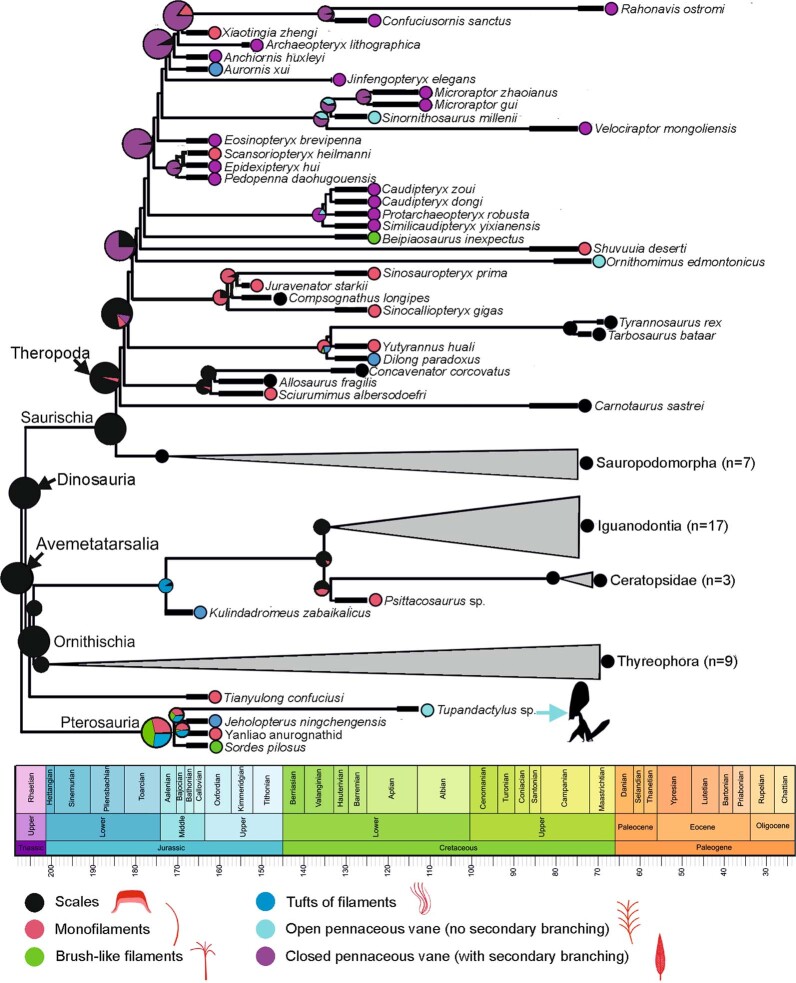
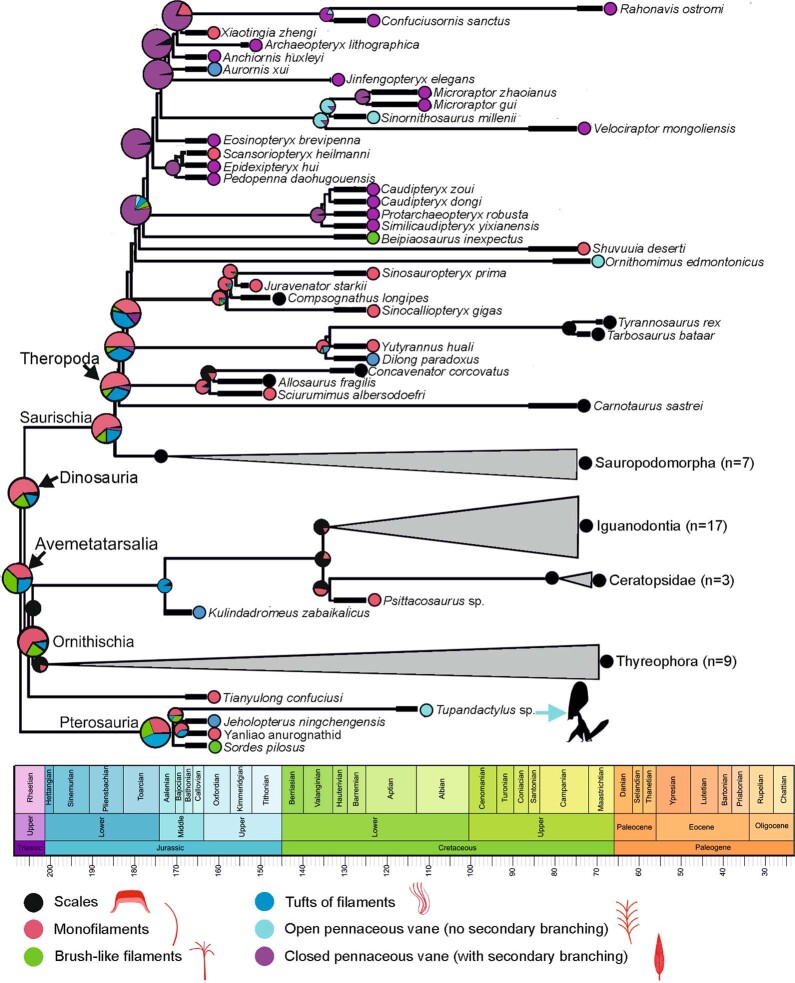
Our phylogenetic reconstruction used a recently published phylogeny for pterosaurs, birds and non-avialan dinosaurs2 that preserve integumentary structures. Given their lack of secondary branching (that is, barbules), branched feathers in Tupandactylus correspond to an open pennaceous vane. Ancestral-state estimations indicate that the statistically most likely result (corrected Akaike information criterion (AICc) weight = 84%) is that the avemetatarsalian ancestor of pterosaurs and dinosaurs possessed integumentary filaments, with approximately equal likelihood of possessing monofilaments, tufted feathers and brush-like feathers (Fig. 2, Extended Data Figs. 5–7, Extended Data Table 2). This is not inconsistent with the hypothesis that filamentous integumentary structures originated independently in both groups36. The more parsimonious interpretation, however, is that monofilaments and branched feather morphologies have a single origin in Avemetatarsalia. Our model predicts that progressively more complex integumentary structures arose within both Pterosauria and Theropoda (Fig. 2, Extended Data Figs. 5–7, Extended Data Table 2). This does not imply that identical feather types evolved in each group. Some feather morphologies are shared (that is, monofilaments, brush-like and tufted feathers and feathers with along-rachis branching), but others are not—for example, feathers with midpoint branching in pterosaurs and all feathers with barbules in theropods. Barbules are thus a unique innovation of theropod feathers. Progressive evolution of feather complexity is consistent with the younger age of Tupandactylus (with open vane branched feathers) relative to the previously studied anurognathids (with branching restricted to a narrow zone on the shaft).
Fig. 2. Time-tree phylogeny of Avemetatarsalia.
The phylogeny shows the results of ancestral-state estimations for the origin of feathers with the highest likelihood (−72.52), in addition to the lowest AICc (168.32) and the highest AICc weighting (64.56). Only the most complex integumentary structure present is shown for each taxon. Feathers are reconstructed as ancestral to the common avemetatarsalian ancestor of dinosaurs and pterosaurs. Branch lengths are estimated using the mbl branch length estimation and reconstructed according to the best model (that is, with the highest likelihood, lowest AICc and highest AICc weighing), which estimates trait transition rates following ordered evolution. The pie charts at the nodes show the scaled likelihoods of different integumentary structures. The likelihood values for model parameters are shown in Extended Data Table 2. The Tupandactylus silhouette is drawn by E. Boucher from www.phylopic.org. Silhouettes of integumentary appendages are reproduced from ref. 2, Springer Nature Limited.
Extended Data Fig. 5. Time-tree phylogeny of Avemetatarsalia, estimated using the ‘mbl’ branch-length estimation and reconstructed according to the ‘equal rates’ evolutionary model.
The likelihood values for model parameters are shown in Extended Data Table 2. The different categories of integumentary structures represent: scales, monofilaments, brush-like filaments, tufts of filaments joined basally, open pennaceous vane lacking secondary branching and closed pennaceous feathers comprising a rachis-like structure associated with lateral branches (see material and methods in the main text for more details). Tupandactylus silhouette by Evan Boucher from www.phylopic.org. Silhouettes of integumentary appendages are reproduced from ref. 2. (Fig. 3).
Extended Data Fig. 7. Time-tree phylogeny of Avemetatarsalia, estimated using the ‘mbl’ branch-length estimation and reconstructed according to the ‘all rates different’ (ARD) evolutionary model.
The likelihood values for model parameters are shown in Extended Data Table 2. The different categories of integumentary structures represent: scales, monofilaments, brush-like filaments, tufts of filaments joined basally, open pennaceous vane lacking secondary branching and closed pennaceous feathers comprising a rachis-like structure associated with lateral branches (see material and methods in the main text for more details). Tupandactylus silhouette by Evan Boucher from www.phylopic.org. Silhouettes of integumentary appendages are reproduced from ref. 2. (Fig. 3).
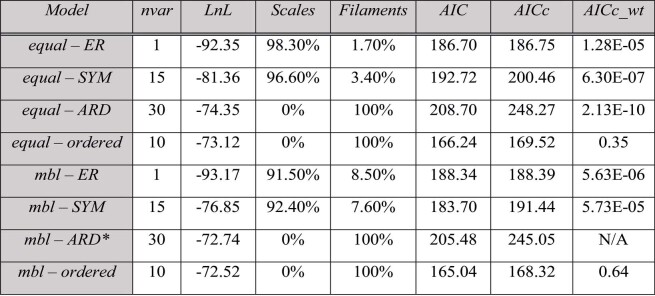
Extended Data Table 2.
Model performance of the phylogenetic reconstructions using different methods for branch length reconstruction and different transition rates
Parameters shown are the number of variables (nvar), log-likelihood (Lnl), probability of scales being ancestral (scales), probability of feather-like structures being present (filaments), Akaike Information Criterion (AIC), second order bias correction of AIC (AICc) and relative weight of the corrected AIC (AICc_wt). * mbl-ARD was calculated using a different method (make.simmap) and was not used in the weighted AICc calculations.
Tissue-specific melanosome geometries
We analysed samples of soft tissue from the fossil monofilaments, branched feathers and fibrous soft tissues from the cranial crest (Extended Data Fig. 8). Scanning electron microscopy shows that all soft tissue samples contain abundant ovoid or elongate microbodies approximately 0.5–1 μm in length (Extended Data Table 3). These microbodies are often embedded in an amorphous matrix similar to that preserved in feathers of other pterosaurs2,6 and some non-avialan dinosaurs and early-diverging birds13,36,37 and interpreted as the degraded remains of the feather keratin matrix2,37,38. Samples of sedimentary matrix adjacent to the cranial crest lack microbodies (Extended Data Fig. 1, samples 1 and 9), confirming that the latter are restricted to the soft tissues. Microbodies with relatively homogeneous ovoid geometries were previously reported in fibrous soft tissues of the crest of another Tupandactylus specimen from the Crato Formation5 and in filamentous structures from a pterosaur from the Jehol Group6. In each case, the microbodies were interpreted as preserved melanosomes5,6. This is consistent with the broad consensus (based on extensive morphological, ultrastructural, chemical and contextual evidence) that similar microbodies, preserved in dark carbonaceous soft tissue films associated with other fossil vertebrates, represent fossil melanosomes39,40.
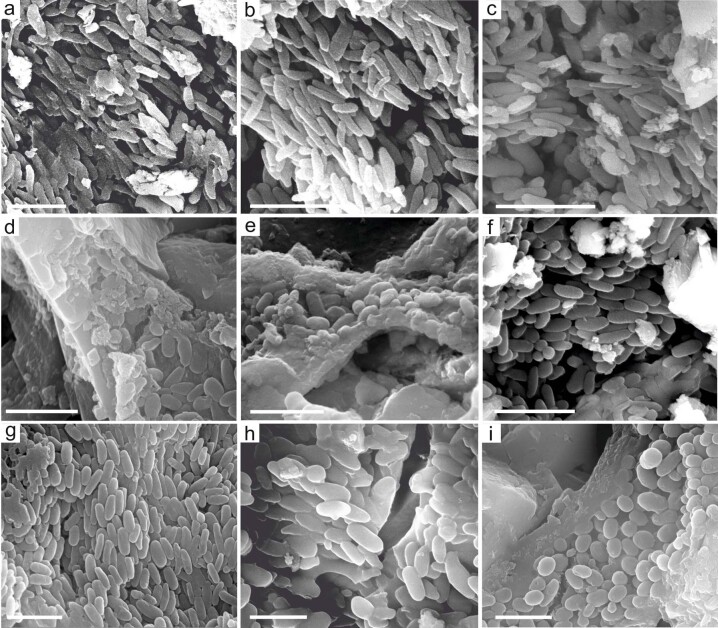
Extended Data Fig. 8. Scanning electron micrographs of melanosomes in the soft tissues of MCT.R.1884.
a–c, Elongate melanosomes from monofilaments. d–f, Ovoid melanosomes from the branched feathers. g–i, Ovoid melanosomes from the soft tissue crest (area 1, Extended Data Table 2). Scale bars, 2 μm.
Extended Data Table 3.
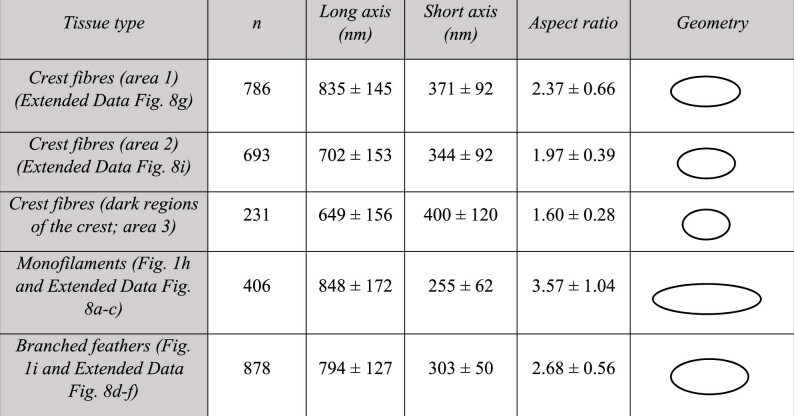
Geometry of melanosomes (mean plus standard deviation) from various soft tissues in Tupandactylus imperator (MCT.R.1884)
Schematic melanosome morphology is shown for each tissue analyzed. n, number of individual melanosomes measured for each tissue type.
In Tupandactylus, melanosomes from the skin fibres in the crest, monofilaments and branched feathers differ significantly in geometry (analysis of variance (ANOVA): F(4, 2,989) = 449.3, P < 0.0001, n = 2,994). Elongate melanosomes are restricted to the monofilaments (Fig. 1h, Extended Data Fig. 8) (848 ± 172 nm long and 255 ± 62 nm wide; n = 406). Melanosomes in the branched feathers are ovoid (794 ± 127 nm long and 303 ± 50 nm wide; n = 878; Fig. 1i, Extended Data Fig. 8). Melanosomes are ovoid in skin fibres located between the base of the cranial crest and the occipital process (Fig. 1g, Extended Data Fig. 8; area 1, Extended Data Table 3; 835 ± 145 nm long and 371 ± 92 nm wide; n = 786) and in the posterior part of the cranial crest (Extended Data Fig. 8; area 2, Extended Data Table 3; 702 ± 153 nm long and 344 ± 92 nm wide; n = 693). In the dorsal part of the crest (area 3, Extended Data Table 3), melanosomes are spheroidal (649 ± 156 nm long and 400 ± 120 nm wide; n = 231). Similar tissue-specific partitioning of melanosome geometry has been reported in diverse other fossil and extant vertebrates40–42. The absence of multiple distinct melanosome populations in the other studied specimen5 of Tupandactylus may reflect limited sampling.
The diversity of melanosome morphologies reported here expands the known range2,6 of geometries of pterosaur melanosomes (Extended Data Fig. 9c): rods and spheres had previously been reported only from mammalian hair and dinosaur (non-avialan and avialan) feathers. The geometry of the melanosomes in Tupandactylus overlaps with that of extant animals (Extended Data Fig. 9a–d). This further supports the hypothesis that the branched integumentary structures in pterosaurs are feathers. It does not, however, completely exclude the alternative (albeit unlikely) hypothesis that pterosaur filamentous integumentary structures represent a third type of vertebrate integumentary outgrowth (in addition to hair and feathers) that is capable of imparting, and varying, melanin-based coloration.
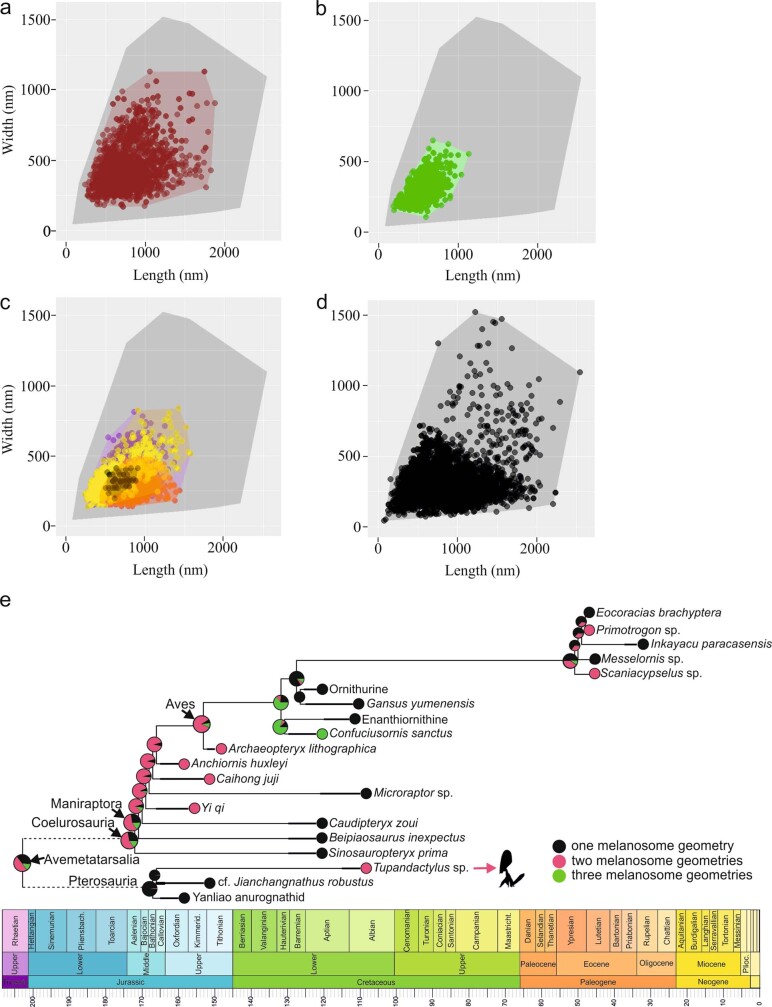
Extended Data Fig. 9. Scatterplots of melanosome geometry in amniotes and ancestral-state estimation of the diversity of melanosome geometries within Avemetatarsalia.
a–d, Melanosome geometry in amniotes; data from refs. 2,6. and this study. a, Mammal hair6 (n = 1984). b, Squamate skin6 (n = 734). c, Pterosaur skin (this study, n = 2115; melanosomes imaged from ten independent samples; purple datapoints) and pterosaur feathers (n = 2173; orange datapoints, this study (n = 1284; melanosomes imaged from four independent samples); black and yellow datapoints, previous studies2,6). d, extinct and extant bird feathers6 (n = 3643). Data from non-avialan dinosaurs are not shown here. Polygon with dark grey shading in (a–d) shows the range of melanosome geometries known for extant and extinct bird feathers. Darker shades in (a) and (d) indicate more than one data point with similar measurements. e, Simplified time-tree phylogeny estimated using the ‘mbl’ branch-length estimation and reconstructed according to the best evolutionary model, i.e.‘equal rates’ (ER) model. The different categories (or ‘states’) of melanosome geometry are: one geometry (in black), two geometries (in red) and three geometries (in green). Only taxa for which melanosome length and aspect ratio was known have been included in our dataset (n = 20). *taxa showing spheroidal melanosomes in addition to any other category. Tupandactylus silhouette (in e) by Evan Boucher from www.phylopic.org.
The different geometries of the preserved melanosomes in the monofilaments and branched feathers are suggestive of different visible colours. Irrespective of the actual colour produced, the data confirm tissue-specific melanosome populations in MCT.R.1884. In turn, this strongly suggests that the genomic and developmental mechanisms required for tuning melanosome geometry were already in place in the avemetatarsalian ancestor of pterosaurs.
Origins for visual signalling in feathers
Our study has important implications for understanding the evolution of melanin-based colouration. Melanosomes in other pterosaur fossils have ovoid to spheroidal shapes, even in integumentary filaments or feathers2,5,6. This low melanosome diversity resembles that in the skin of extant reptiles, where many colours are generated by non-melanin pigments housed in iridophores and xanthophores41–43. Preservation of ovoid and spheroidal melanosomes in pterosaur feathers and skin was therefore previously interpreted as evidence for retention of the ancestral state in pterosaurs40. Unlike those fossils, however, MCT.R.1884 shows important differences in melanosome geometry between the skin and feathers, with evidence for expanded diversity of melanosome geometry (that is, elongate melanosomes) in the feathers. This tissue-specific partitioning of melanosome geometry—and, in particular, the greater morphological diversity of melanosomes in integumentary appendages (feathers and hair) than in skin—also characterizes extant birds and mammals6. This feature may reflect preferential selection of more extreme, oblate melanosome geometries in order to expand melanin-based colour space40 into regions associated with eumelanin-dominated darker and iridescent hues. In turn, this may be a response to the loss of non-melanin-containing chromatophores during the evolution of integumentary appendages44. Alternatively, these fundamental changes in skin structure may derive from changes in metabolism6 and immunity40 during the evolution of endothermy. At a genomic and developmental level, the production of elongate, eumelanin-rich melanosomes reflects earlier activation of α-melanocyte-stimulating hormone7(α-MSH) and/or enhanced production of premelanosome proteins8,45 that form a scaffold for eumelanin deposition during melanosome development8. The discovery of elongate melanosomes in the feathers, but not skin, of the specimen of Tupandactylus described here expands the known range of feather melanosome geometries in pterosaurs and confirms that pterosaurs show similar tissue-specific trends in melanosome geometry to fossil and extant birds and other theropods46,47. This could reflect one of three evolutionary scenarios related to the timing of origin of the genomic regulatory networks governing melanogenesis (especially linked to α-MSH, agouti signaling protein, SRY-box transcription factor 10 (Sox10) and melanocortin-1-receptor)45 and their phenotypic expression. The genotypic and phenotypic characters could both be ancestral to avemetatarsalians; alternatively, both evolved independently in theropods and pterosaurs, or the genes are ancestral and the phenotypic expression occurred independently in the two groups. Our ancestral-state estimations (Extended Data Fig. 9e) reveal that the most parsimonious scenario is that feathers in the avemetatarsalian ancestor had melanosomes with different geometries. This is consistent with a single, deep evolutionary origin for this feature, whereby critical shifts in the genetic machinery facilitating plasticity in melanosome shape occurred in the common ancestor of pterosaurs and birds. Key genomic controls on melanin-based colouration that define the plumage colours of theropods and fossil and extant birds were therefore already in place in early-diverging avemetatarsalians in the Middle to Late Triassic.
Methods
Fossil material
Twenty-two soft tissue samples were collected using sterile tools from MCT.R.1884. These samples represent: (1) six distinct integumentary appendages located close to the posterior part of the occipital process (Extended Data Fig. 1, samples 3, 4, 6, 7, 23 and 24); (2) three skin fibres projecting from the crest towards the occipital process (Extended Data Fig. 1, samples 2, 5 and 8); (3) four skin fibres from the posterior part of the crest (Extended Data Fig. 1, samples 10, 11, 15 and 18); (3) nine skin fibres situated on the anterior portion of the crest (Extended Data Fig. 1, samples 12–14, 16, 17, 19–22). We also collected two samples of the sedimentary matrix (Extended Data Fig. 1, samples 1 and 9) in the region located between the cranial crest and the posterior extension of the skull.
Scanning electron microscopy
Samples of soft tissue were mounted on double-sided carbon tape and sputter-coated with gold. Scanning electron microscopy (SEM) was performed with an environmental FEI Quanta 200 SEM and a FEI Quanta 650 FEG-SEM, using a working distance of 8.6–13 mm, accelerating voltage of 10–30 kV and a probe current of 1.5–3.0.
Measurements of melanosome geometry
Long and short axis were measured for a total of 2,994 melanosomes using ImageJ48 (version 64-bit Java 1.8.0_172; http://imagej.nih.gov/ij/). Orientation was measured for selected samples. For melanosomes in each sample, values for the mean, standard deviation, skew and coefficient of variance were calculated for melanosome length, width and aspect ratio. The significance of variation in the data was tested statistically using the ANOVA test in the freeware PAST49 (version 4.09; palaeontological statistics: https://www.nhm.uio.no/english/research/infrastructure/past/).
Ancestral-state estimations
Data on melanosome geometry were analysed using quadratic discriminant analysis and multinomial logistic regression using the MASS package50 and the Nnet-package, both implemented in R using a published melanosome dataset51.
Ancestral-state estimations for integumentary appendages in Avemetatarsalia were performed using the methodology and data in ref. 2. In short, the integumentary appendages were assigned to one of six possible categories: scales, monofilaments, brush-like filaments, tufts of filaments joined basally, open pennaceous vane lacking secondary branching and closed pennaceous feathers comprising a rachis and barbs. We extended the above-mentioned database2 via the inclusion of data on feathers from MCT.R.1884 as an open-vaned structure. We used maximum-likelihood estimations implemented in the ‘ace’ function of the ape 4 package52. Tree branch lengths were estimated using two methods: ‘equal branch’ length and ‘minimum branch’ length (mbl); using the “DatePhylo’ function in the strap R package53. For more details, see ref. 2.
We ran our analyses using four evolutionary models with different state transition rates: an equal-rates model, a symmetrical rates model, an all-rates-different and an ordered-rates model. In the last example, transition can occur only to and from successive states; that is, feathers with a closed vane can evolve only if open-vaned feathers have already evolved. We compared models by calculating log-likelihood, Akaike information criterion (AIC) and AICc; the latter model corrects for sample size and is summarized as weighed AICc values (Extended Data Table 2). Because of the large parameter space, ‘ace’ was not able to estimate ancestral states for the mbl-ARD model. As such, we used the ‘make.simmap’ function of the phytools package54.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-04622-3.
Supplementary information
This file contains supporting text and supplementary references.
Acknowledgements
This work was funded by a Fonds National pour la Recherche Scientifique (F.R.S.–FNRS) FRIA grant (F3/5/5-MCF/ROI/BC-2319784), an Irish Research Council Government of Ireland Postdoctoral Fellowship (GOIPD/2018/768) awarded to A.C. and an ERC Starting Grant H2020-2014-StG-637691-ANICOLEVO and an ERC Consolidator Grant H2020-2020-CoG-101003293-PALAEOCHEM awarded to M.N. We thank M. Benton for providing the original data and code used in the phylogenetic reconstruction2, Z. Yang for providing raw melanosome measurements used to compare melanosome geometry in pterosaurs and J. Cillis for assistance with SEM. MCT.R.1884 was photographed by T. Hubin (RBINS).
Extended data figures and tables
Extended Data Fig. 6. Time-tree phylogeny of Avemetatarsalia, estimated using the ‘mbl’ branch-length estimation and reconstructed according to the ‘SYM’ evolutionary model.
The likelihood values for model parameters are shown in Extended Data Table 2. The different categories of integumentary structures represent: scales, monofilaments, brush-like filaments, tufts of filaments joined basally, open pennaceous vane lacking secondary branching and closed pennaceous feathers comprising a rachis-like structure associated with lateral branches (see material and methods in the main text for more details). Tupandactylus silhouette by Evan Boucher from www.phylopic.org. Silhouettes of integumentary appendages are reproduced from ref. 2. (Fig. 3).
Author contributions
A.C. conceived the study, designed and performed analyses (SEM, melanosome measurements, taphonomy and ANOVA), interpreted data, prepared figures and tables and co-wrote the paper with M.M. M.N. performed analyses (ancestral-state estimations), described the specimen, interpreted data, prepared figures and tables and revised drafts of the paper. H.B.N.C. described the specimen; M.M. performed analyses (SEM), interpreted data, prepared figures and co-wrote the paper with A.C. L.D. supervised the study, prepared figures and provided data. M.D.S. supervised the study and provided data. E.-E.K. provided data. J.Y. supervised the study and revised drafts of the paper. R.C. performed analyses and interpreted data. F.E. provided data. P.G. conceived, designed and supervised the study, described the specimen and revised drafts of the paper. All authors discussed the manuscript and approved the submitted version.
Peer review
Peer review information
Nature thanks Michael Benton, Stephen Brusatte and Xing Xu for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Additional data on melanosome geometry and the character matrix used in the phylogenetic analyses are available in the Zenodo.org data repository at 10.5281/zenodo.6122213. SEM images and samples are available from the corresponding authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aude Cincotta, Email: acincotta@naturalsciences.be.
Maria McNamara, Email: maria.mcnamara@ucc.ie.
Extended data
is available for this paper at 10.1038/s41586-022-04622-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-04622-3.
References
- 1.Xu, X. In The Evolution of Feathers (eds Foth, C. & Rauhut, O. W. M.) 67–78 (Springer, 2020).
- 2.Yang Z, et al. Pterosaur integumentary structures with complex feather-like branching. Nat. Ecol. Evol. 2019;3:24–30. doi: 10.1038/s41559-018-0728-7. [DOI] [PubMed] [Google Scholar]
- 3.Unwin DM, Martill DM. No protofeathers on pterosaurs. Nat. Ecol. Evol. 2020;4:1590–1591. doi: 10.1038/s41559-020-01308-9. [DOI] [PubMed] [Google Scholar]
- 4.Benton MJ, Dhouailly D, Jiang B, McNamara M. The early origin of feathers. Trends Ecol. Evol. 2019;34:856–869. doi: 10.1016/j.tree.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Pinheiro FL, et al. Chemical characterization of pterosaur melanin challenges color inferences in extinct animals. Sci. Rep. 2019;9:15947. doi: 10.1038/s41598-019-52318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, et al. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature. 2014;507:350–353. doi: 10.1038/nature12973. [DOI] [PubMed] [Google Scholar]
- 7.Rees JL. Genetics of skin and hair colour. Annu. Rev. Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 8.Eliason CM, Shawkey MD, Clarke JA. Evolutionary shifts in the melanin‐based color system of birds. Evolution. 2016;70:445–455. doi: 10.1111/evo.12855. [DOI] [PubMed] [Google Scholar]
- 9.Raposo G, Marks MS. Melanosomes—dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brusatte SL, O’Connor JK, Jarvis ED. The origin and diversification of birds. Curr. Biol. 2015;25:R888–R898. doi: 10.1016/j.cub.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, et al. Reply to: No protofeathers on pterosaurs. Nat. Ecol. Evol. 2020;4:1592–1593. doi: 10.1038/s41559-020-01309-8. [DOI] [PubMed] [Google Scholar]
- 12.Marsh, R. L. & Dawson, W. R. In Animal Adaptation to Cold (ed Wang, L. C. H.) 205–253 (Springer, 1989).
- 13.Li Q, et al. Plumage color patterns of an extinct dinosaur. Science. 2010;327:1369–1372. doi: 10.1126/science.1186290. [DOI] [PubMed] [Google Scholar]
- 14.Smithwick FM, Nicholls R, Cuthill IC, Vinther J. Countershading and stripes in the theropod dinosaur Sinosauropteryx reveal heterogeneous habitats in the Early Cretaceous Jehol Biota. Curr. Biol. 2017;27:3337–3343. doi: 10.1016/j.cub.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Vinther J, Briggs DE, Prum RO, Saranathan V. The colour of fossil feathers. Biol. Lett. 2008;4:522–525. doi: 10.1098/rsbl.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGraw, K. J. In Bird Coloration: Mechanisms and Measurements (eds Hill, G. E. & McGraw, K. J.) 243–294 (Harvard Univ. Press, 2006).
- 17.Martill, D. M., Bechly, G. & Loveridge, R. F. The Crato Fossil Beds of Brazil: Window into an Ancient World (Cambridge Univ. Press, 2007).
- 18.Rossi V, McNamara ME, Webb SM, Ito S, Wakamatsu K. Tissue-specific geometry and chemistry of modern and fossilized melanosomes reveal internal anatomy of extinct vertebrates. Proc. Natl Acad. Sci. USA. 2019;116:17880–17889. doi: 10.1073/pnas.1820285116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prum RO. Development and evolutionary origin of feathers. J. Exp. Zool. 1999;285:291–306. doi: 10.1002/(SICI)1097-010X(19991215)285:4<291::AID-JEZ1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 21.Kellner AW, et al. The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane. Proc. R. Soc. Lond. B. 2010;277:321–329. doi: 10.1098/rspb.2009.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zhou Z, Zhang F, Xu X. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and “hairs” from Inner Mongolia, northeast China. Chin. Sci. Bull. 2002;47:226–230. doi: 10.1360/02tb9054. [DOI] [Google Scholar]
- 23.Sharov, A. G. Phylogeny of the Orthopteroidea (No. 595.72 SHA) (NTIS, 1971).
- 24.Unwin DM, Bakhurina NN. Sordes pilosus and the nature of the pterosaur flight apparatus. Nature. 1994;371:62–64. doi: 10.1038/371062a0. [DOI] [Google Scholar]
- 25.Zheng XT, You HL, Xu X, Dong ZM. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature. 2009;458:333–336. doi: 10.1038/nature07856. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Tang ZL, Wang XL. A therizinosauroid dinosaur with integumentary structures from China. Nature. 1999;399:350–354. doi: 10.1038/20670. [DOI] [Google Scholar]
- 27.Xu X, Wang XL, Wu XC. A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature. 1999;401:262–266. doi: 10.1038/45769. [DOI] [Google Scholar]
- 28.Xu X, Zhou ZH, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, et al. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 30.Hu D, Hou L, Zhang L, Xu X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461:640–643. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- 31.Godefroit P, et al. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science. 2014;345:451–455. doi: 10.1126/science.1253351. [DOI] [PubMed] [Google Scholar]
- 32.Chiappe, L. M., Ji, S. A., Ji, Q. & Norell, M. A. Anatomy and systematics of the Confuciusornithidae (Theropoda, Aves) from the late Mesozoic of northeastern China. Bull. Am. Mus. Nat. Hist. 242 (1999).
- 33.Zhang F, Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 2000;290:1955–1959. doi: 10.1126/science.290.5498.1955. [DOI] [PubMed] [Google Scholar]
- 34.Stettenheim PR. The integumentary morphology of modern birds—an overview. Am. Zool. 2000;40:461–477. [Google Scholar]
- 35.Unwin DM, Martill DM. No protofeathers on pterosaurs. Nat. Ecol. Evol. 2020;4:1590–1591. doi: 10.1038/s41559-020-01308-9. [DOI] [PubMed] [Google Scholar]
- 36.Campione, N. E., Barrett, P. M. & Evans, D. C. In The Evolution of Feathers: From their Origin to the Present (eds Foth, C. & Rauhut, O. M. W.) 213–243 (Springer, 2020).
- 37.Zhang F, et al. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature. 2010;463:1075–1078. doi: 10.1038/nature08740. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, et al. Reconstruction of Microraptor and the evolution of iridescent plumage. Science. 2012;335:1215–1219. doi: 10.1126/science.1213780. [DOI] [PubMed] [Google Scholar]
- 39.D’Alba L, Shawkey MD. Melanosomes: biogenesis, properties, and evolution of an ancient organelle. Physiol. Rev. 2019;99:1–19. doi: 10.1152/physrev.00059.2017. [DOI] [PubMed] [Google Scholar]
- 40.McNamara ME, et al. Decoding the evolution of melanin in vertebrates. Trends Ecol. Evol. 2021;36:430–443. doi: 10.1016/j.tree.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Rogers CS, et al. Synchrotron X-ray absorption spectroscopy of melanosomes in vertebrates and cephalopods: implications for the affinity of Tullimonstrum. Proc. R. Soc. B. 2019;286:20191649. doi: 10.1098/rspb.2019.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi V, Webb S, McNamara ME. Hierarchical biota-level and taxonomic controls on the chemistry of fossil melanosomes revealed using synchrotron X-ray fluorescence. Sci. Rep. 2020;10:8970. doi: 10.1038/s41598-020-65868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landmann, L. In Biology of the Integument (eds Bereiter-Hahn, L. et al.) 150–187 (Springer, 1986).
- 44.Alexander NJ, Fahrenbach WH. The dermal chromatophores of Anolis carolinensis (Reptilia, lguanidae) Am. J. Anat. 1969;126:41–55. doi: 10.1002/aja.1001260105. [DOI] [PubMed] [Google Scholar]
- 45.Cooper, W. & Greenberg, N. In Biology of the Reptilia (eds Gans, E. C. & Crews, D.), 298–422 (Univ. Chicago Press, 1992).
- 46.Vinther J. A guide to the field of palaeo colour: melanin and other pigments can fossilise: reconstructing colour patterns from ancient organisms can give new insights to ecology and behaviour. Bioessays. 2015;37:643–656. doi: 10.1002/bies.201500018. [DOI] [PubMed] [Google Scholar]
- 47.Baxter LL, Pavan WJ. Pmel17 expression is Mitf-dependent and reveals cranial melanoblast migration during murine development. Gene Expr. Patterns. 2003;3:703–707. doi: 10.1016/j.modgep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 49.Hammer Ø, Harper DA, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electr. 2001;4:9. [Google Scholar]
- 50.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S (Springer, 2002).
- 51.Babarović F, et al. Characterization of melanosomes involved in the production of non-iridescent structural feather colours and their detection in the fossil record. J. R. Soc. Interface. 2019;16:20180921. doi: 10.1098/rsif.2018.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paradis, E. Analysis of Phylogenetics and Evolution with R (Springer, 2011).
- 53.Bell MA, Lloyd GT. Strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology. 2015;58:379–389. doi: 10.1111/pala.12142. [DOI] [Google Scholar]
- 54.Revell LJ. Phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains supporting text and supplementary references.
Data Availability Statement
Additional data on melanosome geometry and the character matrix used in the phylogenetic analyses are available in the Zenodo.org data repository at 10.5281/zenodo.6122213. SEM images and samples are available from the corresponding authors on request.