Abstract
Objetives
The present study aimed to evaluate the effect of a 13-week COVID-19 lockdown on health-related parameters in women aged 60–70 years.
Study design
Thirty women participated in this longitudinal study. All the assessments were performed before and 13 weeks after the initial phase of the COVID-19 pandemic in Spain (March to June 2020).
Main outcome measures
A sociodemographic questionnaire was provided, and tests were performed to assess muscle strength, anthropometric measurements, densitometry, blood pressure, blood parameters, Mediterranean diet adherence, and physical activity levels.
Results
After the lockdown, both healthy and unhealthy lifestyle groups showed a significant worsening of HDL, cholesterol/HDL lipids, and isometric knee extension strength parameters. However, individuals with an unhealthy lifestyle also showed a significant worsening in LDL lipids, Non-HDL lipids, bone mineral density of the femoral neck, fat mass/height (%), truncal fat mass (absolute and percentage), total fat mass (absolute and percentage) and handgrip strength.
Conclusions
The COVID-19 pandemic had a negative impact on the overall health status of Spanish older women, potentially increasing their susceptibility to comorbidities, such as lipid cholesterol, body fat, and decreased muscle strength.
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; BP, Blood parameters; Chol, total cholesterol; COVID-19, Coronavirus disease; DBP, Diastolic blood pressure; DXA, Dual energy X-ray absorptiometry; FM, Fat mass; GPAQ, Global Physical Activity Questionnaire; HDL, High-Density Lipoprotein cholesterol; HL, healthy lifestyle; HG, Handgrip strength; IKE, maximal isometric knee extension strength; IPAQ, International Physical Activity Questionnaire; ISAK, International Society for the Advancement on Kinanthropometry; LDL, Low-Density Lipoprotein cholesterol; MDA, Mediterranean diet adherence; MVPA, moderate to vigorous physical activity; PR, Pulse rate; PREDIMED, prevention with Mediterranean diet; SBP, Systolic blood pressure; Trig, triglycerides; UL, unhealthy lifestyle; WHO, World Health Organization
Keywords: Aging, Densitometry, Life style, Sarcopenia, SARS-CoV-2
1. Introduction
The Coronavirus disease (COVID-19) has become the world's leading health news headline. Health authorities in most countries have imposed certain restrictions to prevent the spread of the virus. Unfortunately, these restrictions inevitably disrupted the daily activities of tens of millions of people, with lockdown becoming a trigger for the deterioration of physical capacity, specially in older population who may have suffered the most from confinement and post-confinement [1].
COVID-19 lockdown could have affected some health parameters, such as level of physical activity and diet, which are risk factors for several cardiovascular and metabolic diseases, such as hypertension, dyslipidaemia and obesity 2, 3, 4. About the reduction of the physical activity, long hours at home may lead to increased sedentary behavior and inactivity, long periods of sitting and lying down; all of which increases the risk of developing or exacerbating chronic diseases 5, 6. Additionally, this isolation resulted in changes in dietary intake of adults and older people that may have increase body fat, accelerated sarcopenia and led to a decline in muscle mass and strength [7].
Most of the longitudinal studies on the effects of lockdown due to COVID-19 have been conducted with young or adult populations, but there are hardly any studies with older people [8]. Therefore, the aim of the present study was to analyze the effects of 13-week of lockdown due to COVID-19, on different health parameters: physical activity level, Mediterranean diet adherence, anthropometric measurements, blood parameters, densitometry parameters, and muscle strength levels of older women.
2. Methods
2.1. Design
This study is a part of the ongoing project (ClinicalTrials.gov Identifier: NCT04958499). Pre-test measurements were taken to begin the intervention specified in this clinical trial. However, this intervention could not be carried out due to COVID-19 pandemic. Instead, the present research was conducted, for which external funding was obtained, with additional permission provided by the institutional ethics committee for it to be carried out. This longitudinal study design followed the Strobe Statement [9].
2.2. Participants
The participants were volunteer woman form the nearby areas of the institution. The sample was collected through announcements in women's centers, senior centers, social centers and health centers in nearby areas. Thirty Spanish women between 60 and 70 years of age participated in the study. The inclusion criteria were: being between 60 and 70 years of age and being female. Participants with smoking, self-reported alcohol dependence, chronic kidney disease, infectious diseases and/or coronary heart disease were excluded from the study. This research could present response bias. Women who came voluntarily to participate in the research could be the most concerned about their health; leaving out of it those women who have a worse lifestyle.
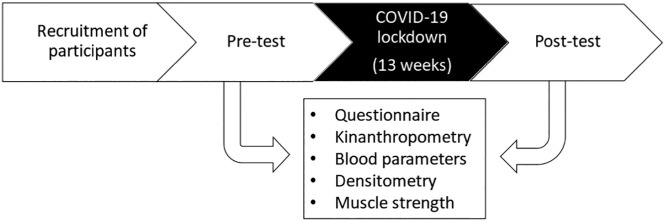
Before the start of the research protocol, all the participants were informed about the experimental protocol (objectives, methods, benefits and risks). All the women underwent assessments before (pre) and after (post) the 13-week period of COVID-19 pandemic isolation (Fig. 1 ). The pre-tests were performed in February 2020, and the post-tests were performed in June 2020.
Fig. 1.
Experimental design.
Rstudio 3.15.0 software was used to establish the sample size. The significance level was set at α = 0.05. According to the standard deviation established for the Femoral neck bone mineral content (BMC) in a previous study [10] and a estimated error of 0.04 g, a valid sample size of 30 was needed for a confidence interval of 95%.
2.3. Ethics approval and consent to participate
The study obtained approval CE052002 from the Catholic University of Murcia ethics committee on research, in accordance with the Declaration of Helsinki. All patients signed written informed consent forms prior to participating in the study.
2.4. Variables and instruments
The same researchers performed all the measurements in a single session between 8:00 and 14:00 h. The participants were examined barefoot with the temperature of laboratory standardized at 24 °C. Before the test measurement, the participants did not perform warm-up or stretching exercises. There was a 5-minute rest between measurements.
2.4.1. Mediterranean diet adherence
The prevention with mediterranean diet (PREDIMED) questionnaire was used, an instrument for dietary evaluation that provides information on the Mediterranean diet adherence (MDA) of older adults, which was previously validated in the Spanish population [12].
2.4.2. Physical activity level
The Global Physical Activity Questionnaire (GPAQ) is a modified version of the International Physical Activity Questionnaire (IPAQ), and was developed by the World Health Organization (WHO) [13]. This questionnaire classifies participants according to activity level as the main objective of physical activity measurement, and allows for the study of trends and associations with other types of behavior or health outcomes.
2.4.3. Kinanthropometric measurements
Kinanthropometric parameters were measured based on the standards of the International Society for the Advancement on Kinanthropometry (ISAK) [14]. Weight (kg) was evaluated in light clothing without footwear by using an electronic scale (Seca 877, Birmingham, UK), and height (cm) was measured using a stadiometer (Seca 763, Birmingham, UK).
2.4.4. Blood parameters
Blood parameters (BP) were measured following the recommendations for older patients [15]. Systolic blood pressure (SBP), diastolic BP (DBP), and pulse rate (PR) were measured using a calibrated automatic arm device (OMRON, model HEM-7113). The final value for BP, SBP and DBP was the mean of two recordings with three minutes apart.
Finger stick blood samples were collected before 9-h. The Afinion™ Analyzer (Alere, Ltd., Stockport, UK) device was used for the lipid panel test. This test measured total cholesterol (Chol), High-Density Lipoprotein cholesterol (HDL), Low-Density Lipoprotein cholesterol (LDL), Triglycerides (Trig), Non-HDL Lipids (LipidNonHDL) and the Chol/HDL ratio in whole blood, serum and plasma to be used in the diagnosis and treatment of lipid disorders.
2.4.5. Densitometry measurements
Bone mineral content (BMC), bone mineral density (BMD) and body composition were measured with Dual energy X-ray absorptiometry (DXA) (QDR 4500A, fan-beam densitometer, Hologic, Waltham, MA, USA). This method is used for body composition measurements for the evaluation of soft tissue composition, fat mass (FM), lean mass and total mass [16]. The variables measured included: Spine BMC and BMD; Femoral neck BMC and BMD; FM/Height; Lean mass/Height; Total and percentage FM; and Total and percentage trunk FM.
2.4.6. Muscle strength measurement
To measure muscle strength, the participants performed two tests:
Handgrip strength test (HG). HG was measured in a standing position with the arms at the sides. The participants performed one repetition in each hand to familiarize themselves with the device and the test. Each participant was asked to squeeze the grip with maximal strength for 3 s with the right hand. The highest peak strength (kg) recorded between the three attempts was considered for analysis. A digital grip strength dynamometer was used for this (TKK 5401; Takei Scientific Instruments Co., Ltd., Tokyo, Japan).
To determine the maximal isometric knee extension (IKE) strength, the participants were assessed while seated with a knee and hip angle of 90°. Participants seated in a chair were instructed to push as strong as possible for three seconds while provided with verbal encouragement. The extension test was assessed with a force transducer (Musclelab, Ergotest, Norway) sampling at 1000 Hz. The subjects performed three IKE tests with 2 min of rest between tests. The maximum peak force in Newton (Nm) was collected.
2.5. Statistical analysis
The Kolmogorov–Smirnov test and Mauchly's W-test were used to evaluate the normality and the sphericity of the data. The mean and standard deviation were calculated from the quantitative variables, and frequency and percent were used for the qualitative variables. The participants were categorized as having a healthy lifestyle (HL) or unhealthy lifestyle (UL). HL was considered to be those who complied with at least 300 min of moderate to vigorous physical activity (MVPA) (WHO extended recommendation to achieve greater benefits than the recommended 150 min), and who maintained a MDA (above 9 points, calculated according to the mean and median of the sample) vs UL, those who did not comply with either or both parameters. Analysis of covariance (ANCOVA) using to comparing the change from baseline between groups. This analysis was performed unadjusted and adjusted by age as in previous research studies [7]. A two-way ANOVA with repeated measures in 1 factor (time) was used to analyze inter- and intra-group differences. An error of p ≤ 0.05 was established. The statistical analysis was performed using the statistical package SPSS 21.0 for Windows.
3. Results
A total of 40 women volunteered for the study. Two women were eliminated for not meeting the inclusion and exclusion criteria. Thirty-eight women were included in the study and were measured at pre-test. All of them were contacted to attend the post-test. Three women were infected with Covid at the time of the post-test and 5 women were reluctant to come forward to those detected for fear of contracting Covid. Thirty women attended the post-test and are analyzed in this study.
Table 1 show the characteristics of the sample. The sample consisted of Spanish women between 60 and 70 years old. More than 50% of them were married, retired, and with an educational level corresponding to elementary school. Approximately three-quarters of them lived with a partner, and two out of three had an UL.
Table 1.
Characteristic of the sample.
| Variable | %(n) or X ± SD |
|---|---|
| Age (year-old) | 64.43 ± 4.58 |
| Height (cm) | 154.15 ± 7.49 |
| Body mass (kg) | 71.27 ± 12.60 |
| Marital status | |
| Single | 6.67 (2) |
| Married | 56.67 (17) |
| Separated | 6.67 (2) |
| Widow | 30 (9) |
| Occupation | |
| Full-Time Worker | 13.33 (4) |
| Part-Time Worker | 6.67 (2) |
| Unemployed | 20 (6) |
| Retired | 60 (18) |
| Education level | |
| No education | 13.33 (4) |
| Elementary school | 56.67 (17) |
| High school | 16.67 (5) |
| Bachelor's Degrees or higher | 13.33 (4) |
| Living status | |
| Living with someone | 73.33 (22) |
| Living alone | 26.67 (8) |
| Lifestyle (Active and Mediterranean diet adherence vs non-active or no adherence) | |
| Healthy | 30 (9) |
| Unhealthy | 70 (21) |
Table 2 shows that LDL increased (+10.30 ± 3.19; p = 0.003), HDL decreased (−7.01 ± 1.78; p = 0.001), Non-HDL lipids increased (+8.73 ± 3.02; p = 0.007), Chol/HDL lipids increased (+0.42 ± 0.08; p < 0.001), femoral neck BMD decreased (−0.02 ± 0.01; p = 0.017), percent FM/height increased (+0.37 ± 0.10; p = 0.002), total FM (kg) increased (+0.81 ± 0.24; p = 0.002), percent FM (%) increased (+0.67 ± 0.21; p = 0.003), total trunk FM (kg) increased (+0.53 ± 0.20; p = 0.013), percentage trunk FM (%) increased (+1.06 ± 0.33; p = 0.003), HG decreased (−0.87 ± 0.36; p = 0.023) and IKE decreased (−302.80 ± 24.14; p < 0.001) after the lockdown, significantly. When these changes were adjusted by age values are maintained.
Table 2.
Effect of lockdown due to Covid pandemic (Unadjusted and adjusted by age).
| Unadjusted |
Adjusted by age |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre-test (M ± SD) |
Post-test (M ± SD) |
Difference post-pre (M ± SD) | p | 95% CI (Mpost-Mpre) |
Difference post-pre (M ± SD) | p | 95% CI (Mpost-Mpre) |
| BMI (kg/m2) | 29.97 ± 4.54 | 30.25 ± 4.67 | 0.28 ± 0.16 | 0.097 | −0.05;0.61 | 0.26 ± 0.15 | 0.097 | −0.05;0.56 |
| BP (mmHg) | 97.43 ± 11.16 | 95.5 ± 11.29 | −1.89 ± 2.22 | 0.403 | −6.43;2.67 | −2.00 ± 2.24 | 0.38 | −6.59;2.6 |
| SBP (mmHg) | 132.37 ± 21.61 | 130.03 ± 19.89 | −2.46 ± 4.84 | 0.616 | −12.40;7.48 | −2.70 ± 4.89 | 0.585 | −12.74;7.34 |
| DBP (mmHg) | 79.97 ± 10.13 | 78.23 ± 10.79 | −1.60 ± 1.62 | 0.332 | −4.2;1.72 | −1.64 ± 1.65 | 0.327 | −5.03;1.74 |
| PR (bpm) | 68.87 ± 11.63 | 66.03 ± 11.24 | −2.52 ± 1.52 | 0.11 | −5.64;0.61 | −2.48 ± 1.55 | 0.121 | −5.67;0.7 |
| Chol (mg/dL) | 190.1 ± 29.44 | 195.7 ± 24.53 | 1.73 ± 3.99 | 0.669 | −6.47;9.92 | 1.50 ± 4.01 | 0.712 | −6.75;9.75 |
| LDL (mg/dL) | 101.79 ± 25.82 | 114.13 ± 20.42 | 10.30 ± 3.19 | 0.003 | 3.76;16.84 | 10.07 ± 3.17 | 0.004 | 3.55;16.58 |
| HDL (mg/dL) | 66.17 ± 12.61 | 60.27 ± 12.96 | −7.01 ± 1.78 | 0.001 | −10.67;-3.35 | −6.88 ± 1.78 | 0.001 | −10.54;-3.23 |
| Trig (mg/dL) | 110.72 ± 58.83 | 106.3 ± 41.82 | −8.30 ± 10.95 | 0.455 | −30.75;14.16 | −8.93 ± 10.99 | 0.424 | −31.53;13.66 |
| LipidNonHDL (mg/dL) | 123.93 ± 24.01 | 135.43 ± 20.09 | 8.73 ± 3.02 | 0.007 | 2.55;14.92 | 8.38 ± 2.87 | 0.007 | 2.48;14.28 |
| Chol/HDL (mg/dL) | 2.92 ± 0.49 | 3.35 ± 0.59 | 0.42 ± 0.08 | <0.001 | 0.26;0.57 | 0.40 ± 0.06 | <0.001 | 0.27;0.53 |
| Spine BMC (kg) | 1.82 ± 0.27 | 1.83 ± 0.26 | 2.95 ± 9.71 | 0.763 | −16.97;22.88 | 2.55 ± 9.83 | 0.798 | −17.66;22.76 |
| Spine BMD (g/cm2) | 1.03 ± 0.08 | 1.03 ± 0.09 | −0.00 ± 0.00 | 0.756 | −0.01;0.01 | −0.00 ± 0.00 | 0.796 | −0.01;0.01 |
| Femoral neck BMC (g) | 3.14 ± 0.78 | 3.15 ± 0.79 | 0.02 ± 0.03 | 0.634 | −0.05;0.08 | 0.02 ± 0.03 | 0.612 | −0.05;0.08 |
| Femoral neck BMD (g/cm2) | 0.7 ± 0.1 | 0.69 ± 0.11 | −0.02 ± 0.01 | 0.017 | −0.03;-0.00 | −0.02 ± 0.01 | 0.02 | −0.03;0.00 |
| FM/Height (%) | 13.71 ± 3.34 | 14.1 ± 3.4 | 0.37 ± 0.10 | 0.002 | 0.15;0.58 | 0.36 ± 0.11 | 0.002 | 0.15;0.58 |
| Lean mass/Height (kg/m2) | 14.84 ± 1.42 | 14.81 ± 1.5 | −0.02 ± 0.10 | 0.817 | −0.24;0.19 | −0.05 ± 0.08 | 0.58 | −0.21;0.12 |
| Trunk FM (kg) | 16.09 ± 4.15 | 16.59 ± 4.15 | 0.53 ± 0.20 | 0.013 | 0.12;0.95 | 0.54 ± 0.21 | 0.015 | 0.11;0.96 |
| Trunk FM (%) | 46.2 ± 4.95 | 47.23 ± 4.52 | 1.06 ± 0.33 | 0.003 | 0.38;1.73 | 1.10 ± 0.30 | 0.001 | 0.49;1.72 |
| Total FM (kg) | 32.66 ± 8.70 | 33.54 ± 8.78 | 0.81 ± 0.24 | 0.002 | 0.32;1.31 | 0.81 ± 0.25 | 0.003 | 0.30;1.31 |
| Total FM (%) | 46.24 ± 4.43 | 46.98 ± 4.25 | 0.67 ± 0.21 | 0.003 | 0.25;1.09 | 0.69 ± 0.20 | 0.002 | 0.29;1.1 |
| HG (kg) | 23.56 ± 5.68 | 22.78 ± 5.13 | −0.87 ± 0.36 | 0.023 | −1.60;-0.13 | −0.85 ± 0.36 | 0.027 | −1.6;-0.11 |
| IKE (Nm) | 548.02 ± 167.46 | 248 ± 68.91 | −302.80 ± 24.14 | <0.001 | −352.34;-253.26 | −303.67 ± 24.50 | <0.001 | −354.03;-253.32 |
Abbreviations: BMI = body mass index; BMC = bone mineral content; BMD = bone mineral density; BP = blood pressure; bpm = beats per minute; DBP = diastolic blood pressure; FM = fat mass; HDL = high density lipoprotein; HG = Handgrip; IKE = Isometric knee extension strength; LDL = low density lipoprotein; MBP = mean blood pressure; mmHg = millimeters of mercury; Nm = newton meter; PR = pulse rate; SBP = systolic blood pressure; Chol = total cholesterol; Trig = triglycerides.
Table 3 shows the changes before and after lockdown according to HL and UL. The HL group showed a decrease in HDL (−6.85 ± 3.04; p = 0.033), increase in Chol/HDL lipids (0.29 ± 0.11; p = 0.012), increase trunk FM (1.16 ± 0.51; p = 0.032) and decrease in IKE (−303.06 ± 41.90; p < 0.001), significantly. The UL group showed a increased BMI (0.41 ± 0.16; p = 0.015), and increased LDL (+13.76 ± 3.33; p < 0.001), decreased HDL (−6.91 ± 1.87; p = 0.001), increased Non-HDL lipids (+14.15 ± 3.02; p < 0.001), increased Chol/HDL lipids (+0.51 ± 0.07; p p < 0.001), decreased femoral neck BMD (−0.02 ± 0.01; p = 0.009), increased FM/height (%) (+0.46 ± 0.11; p < 0.001), increased trunk FM (kg) (+0.52 ± 0.22; p = 0.024), increased trunk FM (%) (+1.04 ± 0.32; p = 0.003), increased total FM (kg) (+1.05 ± 0.26; p < 0.001), increased total FM (%) (+0.87 ± 0.21; p < 0.001), decreased HG (−0.80 ± 0.38; p = 0.046) and decreased IKE (−304.29 ± 25.75; p < 0.001), significantly. No differences were observed between groups in the pre-post lockdown change.
Table 3.
Effects of lockdown according to healthy or unhealthy lifestyle + Time*Group Interaction (all age-adjusted).
| Variable | Pre-test (M ± SD) | Post-test (M ± SD) | Adjusted by age |
Group * time interaction |
||||
|---|---|---|---|---|---|---|---|---|
| Difference post-pre (M ± SD) | p | 95% CI (Mpost-Mpre) | F | Sig | ||||
| BMI (kg/m2) | HL | 30.65 ± 4.64 | 30.73 ± 4.96 | 0.11 ± 0.26 | 0.682 | −0.42;0.63 | 1.008 | 0.328 |
| UL | 29.67 ± 4.57 | 30.05 ± 4.65 | 0.41 ± 0.16 | 0.015 | 0.09;0.73 | |||
| BP (mmHg) | HL | 96.22 ± 13.8 | 96.63 ± 12.82 | −1.19 ± 3.83 | 0.758 | −9.06;6.67 | 0.127 | 0.724 |
| UL | 97.95 ± 10.17 | 95.02 ± 10.88 | −2.8 ± 2.35 | 0.244 | −7.63;2.03 | |||
| SBP (mmHg) | HL | 129.78 ± 20.3 | 130.56 ± 16.71 | −2.03 ± 8.36 | 0.81 | −19.21;15.15 | 0.018 | 0.893 |
| UL | 133.48 ± 22.55 | 129.81 ± 21.49 | −3.37 ± 5.14 | 0.518 | −13.93;7.19 | |||
| DBP (mmHg) | HL | 79.44 ± 15.38 | 79.67 ± 16.02 | −0.77 ± 2.81 | 0.786 | −6.56;5.01 | 0.276 | 0.604 |
| UL | 80.19 ± 7.35 | 77.62 ± 8.05 | −2.52 ± 1.73 | 0.158 | −6.07;1.04 | |||
| PR (bpm) | HL | 68.56 ± 10.42 | 65.89 ± 8.96 | −2.03 ± 2.65 | 0.451 | −7.47;3.42 | 0.086 | 0.772 |
| UL | 69 ± 12.35 | 66.1 ± 12.28 | −2.94 ± 1.63 | 0.082 | −6.29;0.41 | |||
| Chol (mg/dL) | HL | 198.88 ± 33.69 | 200.33 ± 26.39 | −4.24 ± 6.87 | 0.542 | −18.35;9.87 | 2.013 | 0.168 |
| UL | 186.76 ± 27.81 | 193.71 ± 24.09 | 7.23 ± 4.22 | 0.098 | −1.44;15.91 | |||
| LDL (mg/dL) | HL | 105.12 ± 26.43 | 114.44 ± 16.44 | 6.37 ± 5.42 | 0.251 | −4.78;17.52 | 1.34 | 0.258 |
| UL | 100.52 ± 26.13 | 114 ± 22.28 | 13.76 ± 3.33 | <0.001 | 6.91;20.62 | |||
| HDL (mg/dL) | HL | 72.13 ± 15.39 | 67.56 ± 17.71 | −6.85 ± 3.04 | 0.033 | −13.11;-0.60 | 0.000 | 0.987 |
| UL | 63.9 ± 10.95 | 57.14 ± 9.16 | −6.91 ± 1.87 | 0.001 | −10.76;-3.07 | |||
| Trig (mg/dL) | HL | 108.25 ± 54.67 | 90.78 ± 29 | −19.94 ± 18.8 | 0.299 | −58.59;18.70 | 0.988 | 0.329 |
| UL | 111.67 ± 61.61 | 112.95 ± 45.22 | 2.07 ± 11.55 | 0.859 | −21.68;25.82 | |||
| LipidNonHDL (mg/dL) | HL | 126.75 ± 25.68 | 132.78 ± 18.55 | 2.61 ± 4.91 | 0.599 | −7.48;12.71 | 3.976 | 0.057 |
| UL | 122.86 ± 23.91 | 136.57 ± 21.04 | 14.15 ± 3.02 | <0.001 | 7.95;20.35 | |||
| Chol /HDL (mg/dL) | HL | 2.81 ± 0.51 | 3.1 ± 0.67 | 0.29 ± 0.11 | 0.012 | 0.07;0.52 | 2.913 | 0.1 |
| UL | 2.96 ± 0.49 | 3.46 ± 0.53 | 0.51 ± 0.07 | <0.001 | 0.38;0.65 | |||
| Spine BMC (kg) | HL | 1.82 ± 0.25 | 1.82 ± 0.24 | −4.43 ± 16.82 | 0.794 | −39.00;30.14 | 0.496 | 0.488 |
| UL | 1.82 ± 0.28 | 1.83 ± 0.28 | 9.53 ± 10.34 | 0.365 | −11.72;30.77 | |||
| Spine BMD (g/cm2) | HL | 1.03 ± 0.09 | 1.02 ± 0.09 | −0.00 ± 0.01 | 0.934 | −0.02;0.01 | 0.013 | 0.909 |
| UL | 1.03 ± 0.08 | 1.03 ± 0.09 | −0.00 ± 0.00 | 0.722 | −0.01;0.01 | |||
| Femoral neck BMC (g) | HL | 3.21 ± 0.56 | 3.19 ± 0.57 | 0.01 ± 0.05 | 0.851 | −0.10;0.12 | 0.036 | 0.852 |
| UL | 3.11 ± 0.87 | 3.13 ± 0.88 | 0.02 ± 0.03 | 0.509 | −0.05;0.09 | |||
| Femoral neck BMD (g/cm2) | HL | 0.7 ± 0.08 | 0.69 ± 0.1 | −0.01 ± 0.01 | 0.252 | −0.03;0.01 | 0.219 | 0.644 |
| UL | 0.7 ± 0.11 | 0.68 ± 0.11 | −0.02 ± 0.01 | 0.009 | −0.03;-0.01 | |||
| FM/Height (%) | HL | 14.24 ± 3.21 | 14.48 ± 3.57 | 0.27 ± 0.18 | 0.152 | −0.10;0.64 | 0.816 | 0.375 |
| UL | 13.49 ± 3.45 | 13.94 ± 3.4 | 0.46 ± 0.11 | <0.001 | 0.23;0.69 | |||
| Lean mass/Height (kg/m2) | HL | 14.94 ± 1.63 | 14.9 ± 1.72 | −0.09 ± 0.14 | 0.506 | −0.38;0.19 | 0.346 | 0.561 |
| UL | 14.8 ± 1.36 | 14.78 ± 1.44 | 0.00 ± 0.09 | 0.976 | −0.17;0.18 | |||
| Trunk FM (kg) | HL | 16.59 ± 4.66 | 17.05 ± 5.44 | 0.55 ± 0.35 | 0.127 | −0.17;1.27 | 0.007 | 0.934 |
| UL | 15.87 ± 4.02 | 16.39 ± 3.62 | 0.52 ± 0.22 | 0.024 | 0.08;0.96 | |||
| Trunk FM (%) | HL | 47.07 ± 3.47 | 47.93 ± 4.41 | 1.16 ± 0.51 | 0.032 | 0.11;2.22 | 0.039 | 0.844 |
| UL | 45.82 ± 5.5 | 46.92 ± 4.64 | 1.04 ± 0.32 | 0.003 | 0.39;1.69 | |||
| Total FM (kg) | HL | 33.91 ± 9.99 | 34.39 ± 10.58 | 0.56 ± 0.42 | 0.196 | −0.31;1.42 | 0.998 | 0.327 |
| UL | 32.13 ± 8.31 | 33.17 ± 8.15 | 1.05 ± 0.26 | <0.001 | 0.52;1.59 | |||
| Total FM (%) | HL | 47.14 ± 3.81 | 47.52 ± 4.34 | 0.51 ± 0.34 | 0.139 | −0.18;1.21 | 0.806 | 0.378 |
| UL | 45.85 ± 4.71 | 46.75 ± 4.3 | 0.87 ± 0.21 | <0.001 | 0.45;1.30 | |||
| HG (kg) | HL | 23.12 ± 5.36 | 22.35 ± 5.73 | −0.91 ± 0.62 | 0.157 | −2.18;0.37 | 0.021 | 0.886 |
| UL | 23.75 ± 5.92 | 22.97 ± 4.99 | −0.80 ± 0.38 | 0.046 | −1.59;-0.02 | |||
| IKE (Nm) | HL | 534.05 ± 148.7 | 246.51 ± 64.51 | −303.06 ± 41.90 | <0.001 | −389.19;-216.93 | 0.001 | 0.98 |
| UL | 554.01 ± 178.02 | 248.64 ± 72.25 | −304.29 ± 25.75 | <0.001 | −357.23;-251.36 | |||
Abbreviations: BMC = bone mineral content; BMD = bone mineral density; BMI = body mass index; BP = blood pressure; bpm = beats per minute; DBP = diastolic blood pressure; FM = fat mass; HDL = high density lipoprotein; HG = Handgrip; HL = healthy lifestyle; IKE = Isometric knee extension strength; LDL = low density lipoprotein; MBP = mean blood pressure; mmHg = millimeters of mercury; Nm = newton meter; PR = pulse rate; SBP = systolic blood pressure; Chol = total cholesterol; Trig = triglycerides; UL = unhealthy lifestyle.
4. Discussion
The main objective of this study was to analyze the effects of 13-week period of lockdown due to COVID-19 on different health parameters: physical activity level, Mediterranean diet adherence, anthropometric measurements, blood parameters, densitometry parameters and muscle strength levels of women aged 60–70 years. The results showed that blood HDL, LDL and Non-HDL lipid values worsened after lockdown, with a significant increase in LDL and a decrease in HDL, as in previous studies [17]. In addition, in the present study, a significant worsening of HDL and cholesterol/HDL lipid values was observed in individuals who had a HL and UL due to the lockdown, but those who had an UL also presented a worsening LDL, and Non-HDL lipid values. Therefore, maintaining a healthy lifestyle during the lockdown period, including physical activity and a healthy diet, could decrease the negative effects of habit changes on blood parameters during lockdown 17, 18. These changes in daily life may increase the risk of developing heart disease in the long term [18], with this being especially relevant in the population of the present investigation as they are adult and older women; thus, age should be taken into consideration as it increases the predisposition to cardiovascular disease [19].
Regarding blood pressure, SBP significantly increased with age. This may pose a risk, as a high SBP associated with age has been associated to the possible occurrence of major cardiovascular disease events, coronary heart disease, stroke, and heart failure in other studies [24]. In contrast, no significant changes were found in SBP, DBP or BP pre and post lockdown. Furthermore, differences were not found between HL and UL. The lack of changes may be due to the average time period elapsed [25].
The present investigation also found a significant increase in trunk FM (absolute and percentage), total FM (absolute and percentage), and FM/height percentage after lockdown, in line with previous studies [26]. Considering that in the adult and older population, height does not usually change in the short term [27], the increase in FM/height percentage could be due to the changes found in FM. Previous studies have shown that lockdown led to a decrease in the level of physical activity or a worsening of eating habits, including a higher frequency of snacking, with these factors affecting the female gender to a greater extent [28]. Not surprisingly, in the present study, it was found that those with an UL were women who showed the greatest significant increase in the variables related to total and trunk fat accumulation and increased significantly in BMI as well. Another possible explanation could be that there was an increase in total FM and trunk FM with age [29]. The area of fat mass distribution is a factor to consider, as total FM has been correlated with different levels of cardiometabolic risk, as well as trunk FM, which is associated with a high risk of cardiovascular disease [29]. Therefore, in light of the results of the present study, older women who have an UL would have a higher probability of suffering from this type of pathologies.
Regarding bone mass, in general no differences were found when comparing the pre-post lockdown variables. However, when dividing the women according to their lifestyle, a significant decrease in femoral neck BMD was found in those with an UL. These changes in normal (non-lockdown) situations may be due to multiple reasons, including genetics, some types of medication, other diseases, nutrition and lifestyle [30]. With respect to the latter, it is worth noting that lifestyle was one of the most affected factors during the lockdown [23]. The first cause may be physical inactivity and a sedentary lifestyle [31]. Disuse or prolonged periods of inactivity such as lockdown or the absence of stimulus on the skeleton, favor the reduction of bone mass, making it more fragile, while subjecting it to a mechanical load through exercise increases bone mass [31]. On the other hand, home lockdown reduced exposure to the sun over a long period of time [31], thus affecting vitamin D synthesis, and therefore negatively affecting calcium absorption in the intestine, the maintenance of adequate levels of calcium and phosphate for optimal bone formation, and the promotion of proper functioning of the parathyroid hormone, which maintains serum calcium levels [31]. For these reasons, the sum of these factors may have been the main cause for the reduced BMD observed in the population with an UL.
Another objective of this study was to test the effects of lockdown on muscle strength production capacity. We found a significant worsening of the results obtained in the IKE test by both groups after the lockdown. These results are in agreement with those expected, due to the effect of age on the decrease in the capacity to produce strength [21], in addition to a situation of restricted mobility and, therefore, a decrease in physical activity [32]. On the other hand, only the UL older women showed a significantly worsening HG test results. This may be due to the maintenance of the active lifestyle by the HL older women, whereas the UL older women were able to greatly reduce their daily physical activity during the lockdown than post lockdown due to the restrictive mobility policies. This may be because the muscle response to inactivity is stronger than to physical activity [32]. The loss of muscle strength, in addition to the absence of significant differences in the Lean mass/Height variable, reinforces the idea found in previous research, where it was observed that the magnitude of strength loss observed due to aging and inactivity was greater than the degree of muscle mass loss [32]. Therefore, it could be said that age, inactivity, and an UL during lockdown, promoted the loss of muscle strength in the population studied.
The main strength of the present research was the combined study of physical, lifestyle and physiological parameters during this period, with a longitudinal study which included a field test to analyze the effects of lockdown on the health of older women. Therefore, the data collected in the present study could be very useful in similar, future situations to predict the changes in health variables in this population group, as well as to improve the health management system by political entities. On the other hand, more research will be necessary, especially in the most affected or at-risk groups, in this case on their health in relation to physical activity and lifestyle. Regarding the limitations encountered, the post-lockdown measurements could not be performed until the mobility restrictions imposed allowed the sample to access the center where tests were performed, as well as the absence of a control group that was not in lockdown.
As conclusion, it was observed that lockdown had a great negative impact on Spanish older women in most of the measured parameters: cholesterol, femoral neck BMD, FM and strength values. In addition, it was found that a HL could be a protective factor against the age-associated increase in LDL, decrease in HDL, increase in FM, and decrease strength values. These results should be taken into account due to the potentially large negative impact on public health that a situation of lockdown and social isolation such as the one experienced could have, so that non-pharmacological strategies such as physical exercise and a healthy diet are deemed necessary to ensure the health of individuals during possible future lockdown situations. Furthermore, it is crucial to highlight the need for future studies investigating not only the impact of COVID-19 lockdown restrictions on health and muscle strength parameters, but also the short- and long-term effects of specific interventions that aim to improve overall health and include a home-tailored physical exercise program.
Funding
This work was supported by Research Support and Promotion Measures Plan of the Vice-Rectorate for Research of the Catholic University of Murcia 2020/2021 grant number PMAFI-COVID19/17; and by Spanish Ministry of Science, Innovation and Universities, Colaboration-Retos 2017 Project grant number RTC-2017-6145-1, 2017.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the Vice-Rectorate for Research of the Catholic University of Murcia and the study participants.
Section Editor: Christiaan Leeuwenburgh
Data availability
Data will be made available on request.
References
- 1.Palmer K., Monaco A., Kivipelto M., Onder G., Maggi S., Michel J.-P., Prieto R., Sykara G., Donde S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: consequences for healthy ageing. Aging Clin. Exp. Res. 2020;32:1189–1194. doi: 10.1007/S40520-020-01601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P., Mao L., Nassis G.P., Harmer P., Ainsworth B.E., Li F. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020;9:103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellettiere J., LaMonte M.J., Evenson K.R., Rillamas-Sun E., Kerr J., Lee I.-M., Di C., Rosenberg D.E., Stefanick M.L., Buchner D.M., Hovell M.F., LaCroix A.Z., Rossouw J., Ludlam S., Burwen D., McGowan J., Ford L., Geller N., Anderson G., Prentice R., Kooperberg C., Manson J.E., Jackson R., Thomson C.A., Wactawski-Wende J., Limacher M., Wallace R., Kuller L., Shumaker S., Shumaker S., Howard B.v. Sedentary behavior and cardiovascular disease in older women. Circulation. 2019;139:1036–1046. doi: 10.1161/CIRCULATIONAHA.118.035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colpani V., Baena C.P., Jaspers L., van Dijk G.M., Farajzadegan Z., Dhana K., Tielemans M.J., Voortman T., FreakPoli R., Veloso G.G.V., Chowdhury R., Kavousi M., Muka T., Franco O.H. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur. J. Epidemiol. 2018;33:831–845. doi: 10.1007/s10654-018-0374-z. [DOI] [PubMed] [Google Scholar]
- 5.Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin. Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharr J.R., Coughenour C.A., Bungum T.J. An assessment of the relationship of physical activity, obesity, and chronic diseases/conditions between active/obese and sedentary/normal weight American women in a national sample. Public Health. 2018;156:117–123. doi: 10.1016/j.puhe.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Marcos-Pardo P.J., González-Gálvez N., López-Vivancos A., Espeso-García A., Martínez-Aranda L.M., Gea-García G.M., Orquín-Castrillón F.J., Carbonell-Baeza A., Jiménez-García J.D., Velázquez-Díaz D., Cadenas-Sanchez C., Isidori E., Fossati C., Pigozzi F., Rum L., Norton C., Tierney A., Äbelkalns I., Klempere-Sipjagina A., Porozovs J., Hannola H., Niemisalo N., Hokka L., Jiménez-Pavón D., Vaquero-Cristóbal R. Sarcopenia, diet, physical activity and obesity in European middle-aged and older adults: the LifeAge study. Nutrients. 2021;13 doi: 10.3390/nu13010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro de Lima J.G., Abud G.F., de Freitas E.C., Bueno Júnior C.R. Effects of the COVID-19 pandemic on the global health of women aged 50 to 70 years. Exp. Gerontol. 2021;150 doi: 10.1016/j.exger.2021.111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Bristow S.M., Gamble G.D., Horne A.M., Reid I.R. Longitudinal changes in bone mineral density, bone mineral content and bone area at the lumbar spine and hip in postmenopausal women, and the influence of abdominal aortic calcification. Bone Rep. 2019;10 doi: 10.1016/j.bonr.2018.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schröder H., Fitó M., Estruch R., Martínez-González M., Corella D., Salas-Salvadó J., Lamuela-Raventós R., Ros E., Salaverría I., Fiol M., Lapetra J., Vinyoles E., Gómez-Gracia E., Lahoz C., Serra-Majem L., Pintó X., Ruiz-Gutierrez V., Covas M. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011;141:1140–1145. doi: 10.3945/JN.110.135566. [DOI] [PubMed] [Google Scholar]
- 13.de la Cámara M., Higueras-Fresnillo S., Cabanas-Sánchez V., Sadarangani K., Martinez-Gomez D., Veiga O. Criterion validity of the sedentary behavior question from the global physical activity questionnaire in older adults. J. Phys. Act. Health. 2020;17:2–12. doi: 10.1123/JPAH.2019-0145. [DOI] [PubMed] [Google Scholar]
- 14.Esparza-Ros F., Vaquero-Cristóbal R., Marfell-Jones M.J. International Society for Advancement in Kinanthropometry; Murcia: 2019. International Standards for Anthropometric Assessment (2019) -Full profile- [Google Scholar]
- 15.Benetos A., Petrovic M., Strandberg T. Hypertension management in older and frail older patients. Circ. Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236. [DOI] [PubMed] [Google Scholar]
- 16.Bi X., Loo Y.T., Henry C.J. Body fat measurements in Singaporean adults using four methods. Nutrients. 2018;10:303. doi: 10.3390/nu10030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrone M.A., Feola A., Pieri M., Donatucci B., Salimei C., Lombardo M., Perrone A., Parisi A. The effects of reduced physical activity on the lipid profile in patients with high cardiovascular risk during COVID-19 lockdown. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18168858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogataj Jontez N., Novak K., Kenig S., Petelin A., Jenko Pražnikar Z., Mohorko N. The impact of COVID-19-related lockdown on diet and serum markers in healthy adults. Nutrients. 2021;13 doi: 10.3390/nu13041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfie J., Aparicio L.S., Cuffaro P.E. The influence of resting heart rate on central pulse pressure is age-dependent. High Blood Press. Cardiovasc. Prev. 2021;28:27–34. doi: 10.1007/s40292-020-00432-8. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarmozzino F., Visioli F. Covid-19 and the subsequent lockdown modified dietary habits of almost half the population in an Italian sample. Foods. 2020;9 doi: 10.3390/foods9050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., Chalmers J., Rodgers A., Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 25.Veloudi P., Sharman J.E. Methodological factors affecting quantification of blood pressure variability: a scoping review. J. Hypertens. 2018;36:711–719. doi: 10.1097/HJH.0000000000001606. [DOI] [PubMed] [Google Scholar]
- 26.Karatas S., Yesim T., Beysel S. Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim. Care Diabetes. 2021;15:424–427. doi: 10.1016/j.pcd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahoney P., Miszkiewicz J.J., Chapple S., Le Luyer M., Schlecht S.H., Stewart T.J., Griffiths R.A., Deter C., Guatelli-Steinberg D. The biorhythm of human skeletal growth. J. Anat. 2018;232:26–38. doi: 10.1111/joa.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh A., Arora B., Gupta R., Anoop S., Misra A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab Syndr. 2020;14:917–920. doi: 10.1016/j.dsx.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coin A., Ruggiero E., Giannini S., Pedrazzoni M., Minisola S., Rossini M., Del Puente A., Inelmen E.M., Manzato E., Sergi G. Trunk and lower limb fat mass evaluated by dual-energy X-ray absorptiometry in a 20- to 80-year-old healthy Italian population. Ann. Nutr. Metab. 2012;61:151–159. doi: 10.1159/000342086. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari S.L., Rizzoli R. Gene variants for osteoporosis and their pleiotropic effects in aging. Mol. Asp. Med. 2005;26:145–167. doi: 10.1016/j.mam.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Umeda-Raffa S., Pergolizzi J.V., Jr., Raffa R.B. Bone fractures during the time of coronavirus. J. Clin. Pharm. Ther. 2021;46:543–546. doi: 10.1111/jcpt.13297. [DOI] [PubMed] [Google Scholar]
- 32.Shur N.F., Creedon L., Skirrow S., Atherton P.J., MacDonald I.A., Lund J., Greenhaff P.L. Age-related changes in muscle architecture and metabolism in humans: the likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 2021;68 doi: 10.1016/j.arr.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.