Summary
Background
Cognitive decline is a growing public health concern. However, presently, only a few large-scale studies are available on the prevalence of cognitive decline worldwide, and the relationship between nutrition and cognitive decline remains unclear and requires further investigation, especially among Chinese centenarians and oldest-old adults. This study aimed to assess the prevalence of cognitive decline among Chinese centenarians and oldest-old adults, its associated factors, and explore a possible connection with nutrition, to provide new directions for the prevention of cognitive decline in Chinese centenarians and oldest-old adults.
Methods
Based on the China Hainan Centenarian Cohort Study (CHCCS), a household survey was conducted among all the centenarians and oldest-old adults residing in 16 cities and counties of Hainan province from June 2014 to June 2016. This study included 946 centenarians and oldest-old adults (412 and 534, respectively). Cognitive function was measured using the mini-mental state examination (MMSE).
Findings
The total prevalence of cognitive decline was 76·6% (725 participants). Centenarians had a significantly higher prevalence of cognitive decline compared to oldest-old adults [359 centenarians (87·1%) vs. 366 oldest-old adults (68·5%)]. Centenarians and oldest-old adults with cognitive decline had significantly lower prognostic nutritional index (PNI) and mini nutrition assessment-short form (MNA-SF) than those without cognitive decline (P < 0·05). Multivariate logistic regression analyses showed that participants with higher PNI and MNA-SF were less likely to have cognitive decline. Multivariate linear regression analyses showed that PNI and MNA-SF were positively associated with MMSE (P < 0·05).
Interpretation
Malnutrition was positively associated with cognitive decline among Chinese centenarians and oldest-old adults. It is therefore important for clinicians and community health workers to pay attention to malnutrition in these populations and provide supplemental nutrients to prevent cognitive decline.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81900357, 81903392, 81941021, 81901252, 82001476, 81802804, 81801251), the Military Medical Science and Technology Youth Incubation Program (20QNPY110, 19QNP060), the Excellent Youth Incubation Program of Chinese People's Liberation Army General Hospital (2020-YQPY-007), the Military Medicine Youth Program of Chinese People's Liberation Army General Hospital (QNF19069, QNF19068), the National Key R&D Program of China (2018YFC2000400), the National S&D Resource Sharing Service Platform Project of China (YCZYPT[2018]07), the Innovation Platform for Academinicians of Hainan Province, the Hainan Major Scientific and Technological Cooperation Project (2016KJHZ0039), the China Postdoctoral Science Foundation funded project (2019M650359, 2020M682816, 2021T140298), the Medical Big Data R&D Project of Chinese People's Liberation Army General Hospital (MBD2018030), the National Geriatric Disease Clinical Medicine Research centre Project (NCRCG-PLAGH-2017-014), the Central Health Care Scientific Research Project (W2017BJ12), the Hainan Medical and Health Research Project (16A200057), the Sanya Medical and Health Science and Technology Innovation Project (2016YW21, 2017YW22, 2018YW11), and the Clinical Scientific Research Supporting Fund of Chinese People's Liberation Army General Hospital (2017FC-CXYY-3009).
Keywords: Cognitive decline, Centenarians, Malnutrition, Oldest-old adults
Research in context.
Evidence before this study
We searched MEDLINE, Embase, and the Cochrane Database of Systematic Reviews from 2001 to 2020. Search terms included Aged, 80 and over, Centenarians, Cognitive Dysfunction, and Malnutritionm [MeSH Terms]. We observed that the prevalence of malnutrition has been reported to rise among the elderly and found to be directly related to cognitive decline. However, presently, few studies has focused on the relationship between nutrition and cognitive decline among Chinese centenarians and oldest-old adults.
Added value of this study
We found malnutrition positively associated with cognitive decline among Chinese centenarians and oldest-old adults, and supported avoiding the development of malnutrition as a strategy for preventing cognitive decline.
Implications of all the available evidence
It is important for clinicians and community health workers to pay attention to malnutrition in the elderly, and provide supplemental nutrients to prevent cognitive decline.
Alt-text: Unlabelled box
Introduction
With the population of older adults steadily increasing, the prevalence of cognitive decline, a gradual process of transition in cognitive capacity with increasing age, is also increasing.1 Age, gender, education level and genetic susceptibility are well-known risk factors for cognitive decline,2 and its prevalence increases exponentially from the age of 65.3 In 2015, 8·5% of the global population (617 million) were aged over 65 years, and this rate is expected to rise to 12% (1 billion) by 2030, and 16·7% (1·6 billion) by 2050.4 Furthermore, there are nearly 10 million new cases of cognitive decline every year, and this number is expected to triple by 2050. Approximately, this disease affects 50 million people worldwide and incurs 2 trillion in healthcare costs per year. Cognitive decline is the main cause of disability and dependence among older adults, and the high social and economic burden caused by this disease makes it a public health concern.5 Additionally, cognitive decline raises the risk of many age-related diseases, as described previously in the literature.6
While ageing is the strongest factor associated with cognitive decline, other factors like malnutrition might be involved.7 Malnutrition refers to the lack of energy and other nutrients usually due to inadequate diet, poor absorption and excessive loss of nutrients, and it has adverse effects on health. A previous study showed that 8·4% of older adults were malnourished, and 42·7% were at risk of malnutrition.8 The Singapore Longitudinal Ageing Studies also indicated that the prevalence of malnutrition was 42%, and its prevalence among the cognitive decline was 63%.9 The prevalence of malnutrition has been found to be high among the elderly, and directly related to cognitive decline.10, 11, 12, 13, 14 Nutritional status might play an important role in the management and prevention of cognitive decline.15 However, presently, there is a lack of large-scale research on the prevalence of cognitive decline worldwide, including Europe and USA, and the relationship between nutrition and cognitive decline remains unclear and requires further investigation, especially among Chinese centenarians and oldest-old adults. Therefore, this study aimed to assess the prevalence of cognitive decline and its associated factors. Furthermore, we planned to determine whether there is a relationship between cognitive decline and nutrition, to provide new directions for preventing cognitive decline in Chinese centenarians and oldest-old adults.
Methods
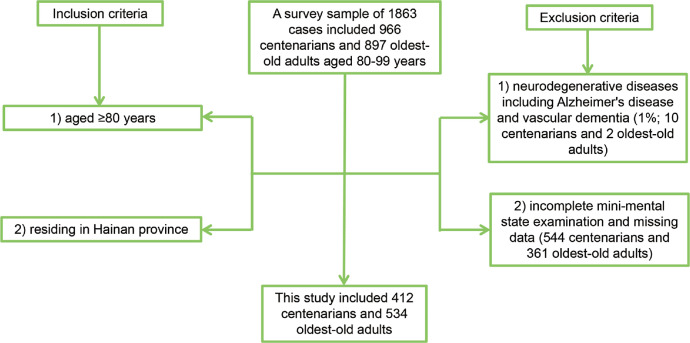
Based on the China Hainan Centenarian Cohort Study (CHCCS), a household survey was conducted among all the centenarians and oldest-old adults residing in 16 cities and counties of Hainan province from June 2014 to June 2016 based on a demographics list provided by the Department of Civil Affairs in Hainan province, China.16 A survey sample of 1863 cases included 966 centenarians and 897 oldest-old adults aged 80–99 years. Inclusion criteria: (1) aged ≥80 years; (2) residing in Hainan province. Exclusion criteria: (1) presence of neurodegenerative diseases including Alzheimer's disease and vascular dementia (1%; 10 centenarians and 2 oldest-old adults); (2) incomplete mini-mental state examination (MMSE) and missing data (nonresponse rate: 49%; 544 centenarians and 361 oldest-old adults). Alzheimer's disease was diagnosed by chief physicians based on medical history, symptoms of memory loss, language impairment, personality change and cognitive decline, and cerebral imaging.17 Vascular dementia referred to dementia caused by cerebrovascular disease.18 Finally, this study included 412 centenarians and 534 oldest-old adults (Figure 1). This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Chinese PLA General Hospital (301hn11-206-01). All participants or their legal guardians provided written informed consent before participation.
Figure 1.
The numbers of cases included and excluded in this study.
The household survey method was used to collect basic information with interview questionnaires. Physical examinations and blood tests were conducted by trained doctors and nurses who could communicate in the local language. Variables assessed in this study included age, gender, body mass index (BMI), education level (i.e., illiteracy, elementary school level and junior high school level), living situation (alone or not), work type (mental or manual labour), smoking, drinking, hypertension, diabetes, coronary artery disease (CAD), anaemia, white blood cells, neutrophils, lymphocytes, albumin, C-reactive protein, red blood cell distribution width (RDW), mean corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC), blood glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), prognostic nutritional index (PNI) and mini nutrition assessment-short form (MNA-SF). Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of anti-hypertensive drug.19 Diabetes was defined as fasting blood glucose ≥7·0 mmol/L, or the use of antidiabetic drug/insulin.20 CAD was defined by chief physicians based on medical history, symptoms of typical angina, cardiac markers and tests, such as electrocardiogram, echocardiogram, computed tomography, and coronary arteriography, according to the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines.21, 22, 23 Anaemia was defined if haemoglobin level was lower than 120 g/L in males or 110 g/L in females.24 PNI is a nutritional screening tool, calculated as follows: serum albumin (g/L) + 0·005 × total lymphocyte count (109/L).25
The core observation index was MMSE, as its association with cognitive function has been established;26 the Georgia Centenarian Study confirmed that age and education could significantly affect MMSE. The Chinese version of the MMSE has been validated in several previous studies.27 Cognitive decline was defined when older adults had reduced MMSE after excluding neurodegenerative diseases such as Alzheimer's disease and vascular dementia. The cut-off points for cognitive decline differed with respect to the level of education: illiteracy at 17 points, elementary school level at 20 points, and junior high school level at 24 points.28
Statistical analyses
Quantitative data with normal distribution are described descriptively with mean ± standard deviation, and their differences were compared using the independent sample t-test. Quantitative data with skewed distribution are described with median (interquartile range), and their differences were compared using the Mann–Whitney U test. Distribution was determined using Kolmogorov–Smirnov and Shapiro–Wilk tests. Categorical data are described as number (percentage), and their differences were compared using Chi-square test. Receiver operator characteristic (ROC) curve and area under the curve (AUC) were used to analyse the efficacy of PNI or MNA-SF in identifying all participants without cognitive decline. Multivariate logistic regression analysis was performed with cognitive decline as the dependent variable, and with PNI (MNA-SF), age, being female, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT, and AST as the independent variables. Multivariate linear regression analysis was performed with MMSE as the dependent variable, and with PNI (MNA-SF), age, being female, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT, and AST as independent variables. Another multivariate logistic regression analysis was performed with oldest-old adults/centenarians as the dependent variable, and with being female, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT, AST, PNI (MNA-SF), and MMSE as independent variables. A p level of 0·05 was considered significant. Statistical analyses were performed using SPSS version 19·0 (IBM Corp; Armonk, NY).
Role of the funding source
The sponsors had no role in the design, conduct, interpretation, review, approval, or control of this article. All authors had full access to all the data in the study and had final responsibility for the decision to submit
Results
Univariate analyses
among 946 cases over 80 years old, the total prevalence of cognitive decline was 76·6% (359 centenarians and 366 oldest-old adults). Characteristics of all participants are shown in Table 1; centenarians (412 cases) and oldest-old adults (534 cases) with and without cognitive decline are shown in supplementary Table 1 and supplementary Table 2, respectively. PNI and MNA-SF were significantly lower in the cases with cognitive decline than those without cognitive decline for all participants (P < 0·05). PNI was negatively associated with cognitive decline in univariate logistic regression analysis (P < 0·001; Odds ratio: 0.86; 95CI: 0.83–0.90) and positively associated with MMSE in univariate linear regression analysis (P < 0·001; B: 0.60; 95CI: 0.50–0.71) in centenarians and oldest-old adults. MNA-SF was negatively associated with cognitive decline in univariate logistic regression analysis (P < 0·001; Odds ratio: 0.75; 95CI: 0.68–0.82) and positively associated with MMSE in univariate linear regression analysis (P < 0·001; B: 1.11; 95CI: 0.90–1.33) in centenarians and oldest-old adults.
Table 1.
Characteristics of centenarians and oldest-old adults with and without cognitive decline.
| Characteristics | With cognitive decline (n = 725) |
Without cognitive decline (n = 221) |
P |
|---|---|---|---|
| Age (year) | 99(85,102) | 85(81,96) | <0·001 |
| Gender, n (%) | <0·001 | ||
| Males | 184(25·4) | 110(49·8) | |
| Females | 541(74·6) | 111(50·2) | |
| BMI (kg/m2) | 19(17,22) | 20(18,23) | <0·001 |
| BMI <18.5 kg/m2 | 300(41·4) | 60(27·1) | <0·001 |
| BMI 18.5 kg/m2 to 24 kg/m2 | 337(46·5) | 111(50·2) | |
| BMI ≥24 kg/m2 | 88(12·1) | 50(22·6) | |
| Education degree, n (%) | <0·001 | ||
| Illiteracy | 624(86·1) | 149(67·4) | |
| Elementary school level | 75(10·3) | 47(21·3) | |
| Junior high school level | 26(3·6) | 25(11·3) | |
| Living alone, n (%) | 114(15·7) | 46(20·8) | 0·077 |
| Mental labour, n (%) | 710(97·9) | 206(93·2) | <0·001 |
| Smoking, n (%) | 68(9·4) | 39(17·6) | 0·001 |
| Drinking, n (%) | 92(12·7) | 40(18·1) | 0·042 |
| Hypertension, n (%) | 523(72·1) | 160(72·4) | 0·940 |
| Diabetes, n (%) | 73(10·1) | 22(10·0) | 0·961 |
| CAD, n (%) | 37(5·1) | 19(8·6) | 0·054 |
| Anaemia, n (%) | 502(73·4) | 223(81·5) | <0·001 |
| White blood cells (109/L) | 5·96(5·02,7·10) | 6·22(5·26,7·53) | 0·027 |
| Neutrophils (109/L) | 0·55(0·49,0·62) | 0·57(0·48,0·64) | 0·281 |
| Lymphocytes (109/L) | 0·31(0·25,0·38) | 0·31(0·24,0·37) | 0·278 |
| Albumin (g/L) | 40·7(37·8,42·9) | 42·6(40·0,44·7) | <0·001 |
| C-reactive protein (mg/dL) | 0·15(0·06,0·37) | 0·14(0·07,0·33) | 0·590 |
| RDW (%) | 14·1(13·3,15·2) | 13·8(13·1,14·7) | <0·001 |
| MCV (fl) | 92·8(87·4,96·5) | 93·8(89·4,97·0) | 0·016 |
| MCHC (g/L) | 314·0(305·0,320·5) | 317·0(309·0,324·0) | <0·001 |
| Blood glucose (mmol/L) | 4·68(4·05,5·50) | 4·38(3·87,5·15) | 0·005 |
| ALT (U/L) | 10·8(8·4,14·5) | 12·2(9·9,15·8) | <0·001 |
| AST (U/L) | 21·5(18·6,25·7) | 21·7(19·0,26·1) | 0·421 |
| MMSE | 10(6,13) | 23(20,26) | <0·001 |
| PNI | 40·7(37·8,42·9) | 42·6(40·0,44·7) | <0·001 |
| MNA-SF | 9(8,10) | 10(9,11) | <0·001 |
Abbreviations: BMI: body mass index; CAD: cardiovascular diseases; RDW: red blood cell distribution width; MCV: mean corpuscular volume; MCHC: mean corpuscular haemoglobin concentration; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MMSE: mini-mental state examination; PNI: prognostic nutritional index; MNA-SF: mini nutrition assessment-short form.
Multivariate analyses
Multivariate logistic regression analyses showed that participants with higher PNI and MNA-SF were less likely to have cognitive decline (Table 2, Table 3, Table 4 and Supplementary Table 3). Multivariate linear regression analyses showed that PNI and MNA-SF were positively associated with MMSE (P < 0·05 Table 2, Table 3, Table 4; and Supplementary Table 3). PNI was negatively associated with cognitive decline in multivariate logistic regression analysis (P = 0·017; Odds ratio: 0.94; 95CI: 0.89–0.99) and positively associated with MMSE in multivariate linear regression analysis (P < 0·001; B: 0.33; 95CI: 0.20–0.45) in centenarians and oldest-old adults. MNA-SF was negatively associated with cognitive decline in multivariate logistic regression analysis (P = 0·042; Odds ratio: 0.86; 95CI: 0.75–1.00) and positively associated with MMSE in multivariate linear regression analysis (P = 0·001; B: 0.51; 95CI: 0.22–0.81) in centenarians and oldest-old adults.
Table 2.
Multivariate analysis of PNI with cognitive decline or MMSE in centenarians and oldest-old adults.
| Characteristics | Cognitive declinea |
MMSEb |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | B | 95% CI | P | |
| PNI | 0·94 | 0·89–0·99 | 0·017 | 0·33 | 0·20–0·45 | <0·001 |
| Age | 1·05 | 1·02–1·07 | <0·001 | -0·18 | -0·23–0·13 | <0·001 |
| Females | 2·08 | 1·40–3·10 | <0·001 | -3·31 | -4·32–2·30 | <0·001 |
| BMI <18.5 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 1·15 | 0·67–1·98 | 0·613 | -0·08 | -0·98–0·83 | 0·870 |
| BMI ≥24 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 1·17 | 0·73–1·88 | 0·508 | 0·02 | -1·19–1·24 | 0·971 |
| Living alone | 0·77 | 0·50–1·16 | 0·212 | 1·01 | -0·04–2·05 | 0·059 |
| Mental labour | 1·31 | 0·57–3·05 | 0·527 | -1·93 | -4·23–0·38 | 0·101 |
| Smoking | 0·83 | 0·50–1·39 | 0·476 | -0·50 | -1·85–0·86 | 0·472 |
| Drinking | 0·89 | 0·55–1·41 | 0·609 | -0·36 | -1·53–0·80 | 0·542 |
| Hypertension | 1·05 | 0·71–1·55 | 0·819 | -0·68 | -1·59–0·24 | 0·147 |
| Diabetes | 0·62 | 0·31–1·23 | 0·620 | 1·36 | -0·28–2·99 | 0·103 |
| CAD | 0·80 | 0·42–1·51 | 0·483 | 0·68 | -0·99–2·35 | 0·426 |
| Anaemia | 0·99 | 0·62–1·60 | 0·972 | 0·15 | -0·88–1·19 | 0·772 |
| White blood cells | 0·91 | 0·82–1·01 | 0·070 | 0·14 | -0·11–0·38 | 0·274 |
| Neutrophils | 0·97 | 0·36–2·59 | 0·950 | -0·05 | -2·02–1·93 | 0·963 |
| C-reactive protein | 1·02 | 0·85–1·22 | 0·846 | -0·09 | -0·50–0·33 | 0·687 |
| RDW | 1·19 | 1·02–1·40 | 0·029 | -0·26 | -0·55–0·04 | 0·086 |
| MCV | 1·01 | 0·99–1·04 | 0·312 | -0·02 | -0·08–0·03 | 0·406 |
| MCHC | 0·99 | 0·97–1·01 | 0·154 | 0·03 | 0·00–0·07 | 0·042 |
| Blood glucose | 1·20 | 1·05–1·37 | 0·007 | -0·34 | -0·59–0·08 | 0·009 |
| ALT | 0·99 | 0·96–1·02 | 0·325 | 0·03 | -0·05–0·10 | 0·508 |
| AST | 1·01 | 0·98–1·04 | 0·557 | 0·00 | -0·07–0·07 | 0·911 |
Abbreviations: PNI: prognostic nutritional index; MMSE: mini-mental state examination; CI: confidence interval; BMI: body mass index; CAD: cardiovascular diseases; RDW: red blood cell distribution width; MCV: mean corpuscular volume; MCHC: mean corpuscular haemoglobin concentration; ALT: Alanine aminotransferase; AST: aspartate aminotransferase.
Notes:
Multivariate Logistic regression analysis was used to performed with cognitive decline as the dependent variables, and with PNI, age, being female, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
Multivariate Linear regression analysis was used to performed with MMSE as the dependent variables, and with PNI, age, females, BMI, living alone, mental labour, smokers, drinkers, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
Table 3.
Multivariate analysis of PNI with cognitive decline or MMSE in centenarians.
| Characteristics | Cognitive declinea |
MMSEb |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | B | 95% CI | P | |
| PNI | 0·93 | 0·84–1·03 | 0·186 | 0·32 | 0·15–0·50 | <0·001 |
| Age | 1·03 | 0·92–1·16 | 0·584 | -0·12 | -0·31–0·07 | 0·222 |
| Females | 5·26 | 2·34–11·86 | <0·001 | -4·22 | -5·91–2·53 | <0·001 |
| BMI <18.5 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 0·67 | 0·16–2·84 | 0·583 | -0·03 | -1·25–1·20 | 0·967 |
| BMI ≥24 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 0·56 | 0·13–2·35 | 0·426 | -1·54 | -4·10–1·02 | 0·237 |
| Living alone | 0·59 | 0·27–1·31 | 0·197 | 1·41 | -0·22–3·04 | 0·089 |
| Mental labour | 2·97 | 0·36–24·33 | 0·311 | -5·75 | -10·99–0·51 | 0·032 |
| Smoking | 3·22 | 0·93–11·18 | 0·065 | -3·03 | -5·29–0·76 | 0·009 |
| Drinking | 1·38 | 0·52–3·69 | 0·519 | -1·15 | -2·92–0·62 | 0·201 |
| Hypertension | 1·56 | 0·73–3·33 | 0·256 | -0·78 | -2·15–0·59 | 0·264 |
| Diabetes | 0·30 | 0·07–1·36 | 0·304 | 2·12 | -0·62–4·86 | 0·129 |
| CAD | 0·65 | 0·16–2·68 | 0·554 | 0·52 | -2·53–3·57 | 0·737 |
| Anaemia | 2·24 | 1·03–4·88 | 0·043 | -0·34 | -1·63–0·94 | 0·600 |
| White blood cells | 1·02 | 0·84–1·24 | 0·832 | 0·05 | -0·29–0·39 | 0·774 |
| Neutrophils | 0·09 | 0·21–4·66 | 0·987 | -0·50 | -2·52–1·53 | 0·631 |
| C–reactive protein | 0·80 | 0·54–1·19 | 0·275 | 0·41 | -0·38–1·20 | 0·307 |
| RDW | 1·17 | 0·84–1·50 | 0·420 | 0·02 | -0·44–0·48 | 0·938 |
| MCV | 1·02 | 0·97–1·07 | 0·420 | -0·03 | -0·10–0·05 | 0·518 |
| MCHC | 1·00 | 0·98–1·03 | 0·889 | 0·04 | -0·01–0·08 | 0·099 |
| Blood glucose | 1·32 | 0·95–1·85 | 0·103 | -0·27 | -0·85–0·31 | 0·354 |
| ALT | 0·99 | 0·94–1·03 | 0·530 | -0·01 | -0·12–0·10 | 0·798 |
| AST | 0·99 | 0·95–1·03 | 0·661 | 0·04 | -0·05–0·13 | 0·408 |
Abbreviations: PNI: prognostic nutritional index; MMSE: mini-mental state examination; CI: confidence interval; BMI: body mass index; CAD: cardiovascular diseases; RDW: red blood cell distribution width;MCV: mean corpuscular volume; MCHC: mean corpuscular haemoglobin concentration; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Notes:
Multivariate Logistic regression analysis was used to performed with cognitive decline as the dependent variables, and with PNI, age, females, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
Multivariate Linear regression analysis was used to performed with MMSE as the dependent variables, and with PNI, age, females, BMI, living alone, mental labour, smokers, drinkers, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
Table 4.
Multivariate analysis of PNI with cognitive decline or MMSE in oldest-old adults.
| Characteristics | Cognitive declinea |
MMSEb |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | B | 95% CI | P | |
| PNI | 0·93 | 0·87–0·99 | 0·028 | 0·37 | 0·19–0·55 | <0·001 |
| Age | 1·11 | 1·05–1·17 | <0·001 | -0·26 | -0·38–0·14 | <0·001 |
| Females | 1·59 | 0·98–2·56 | 0·059 | -2·95 | -4·26–1·64 | <0·001 |
| BMI <18.5 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 1·20 | 0·62–2·33 | 0·588 | 0·21 | -1·57–1·15 | 0·759 |
| BMI ≥24 kg/m2/ 18.5 kg/m2 to 24 kg/m2 | 1·30 | 0·77–2·21 | 1·302 | 0·39 | -1·09–1·86 | 0·607 |
| Living alone | 0·89 | 0·54–1·49 | 0·668 | 0·71 | -0·69–2·11 | 0·318 |
| Mental labour | 1·09 | 0·42–2·80 | 0·865 | -0·55 | -3·25–2·14 | 0·686 |
| Smoking | 0·53 | 0·29–0·98 | 0·044 | 0·91 | -0·83–2·65 | 0·304 |
| Drinking | 0·78 | 0·44–1·37 | 0·384 | 0·07 | -1·63–1·50 | 0·934 |
| Hypertension | 1·00 | 0·62–1·61 | 0·992 | -0·84 | -2·11–0·43 | 0·195 |
| Diabetes | 0·68 | 0·29–1·59 | 0·370 | 0·74 | -1·44–2·93 | 0·504 |
| CAD | 0·93 | 0·44–1·99 | 0·858 | 0·46 | -1·60–2·51 | 0·664 |
| Anaemia | 0·63 | 0·32–1·22 | 0·172 | 0·81 | -0·90–2·52 | 0·354 |
| White blood cells | 0·88 | 0·77–1·00 | 0·056 | 0·12 | -0·23–0·48 | 0·498 |
| Neutrophils | 0·84 | 0·09–7·37 | 0·878 | 3·68 | -2·12–9·48 | 0·213 |
| C–reactive protein | 1·12 | 0·88–1·43 | 0·355 | -0·26 | -0·77–0·25 | 0·318 |
| RDW | 1·18 | 0·98–1·43 | 0·085 | -0·39 | -0·79–0·01 | 0·054 |
| MCV | 1·01 | 0·98–1·04 | 0·552 | -0·02 | -0·10–0·05 | 0·539 |
| MCHC | 0·98 | 0·96–1·00 | 0·080 | 0·04 | -0·01–0·10 | 0·119 |
| Blood glucose | 1·24 | 1·06–1·46 | 0·008 | -0·37 | -0·66–0·08 | 0·013 |
| ALT | 0·98 | 0·94–1·03 | 0·464 | 0·05 | -0·07–0·17 | 0·376 |
| AST | 1·02 | 0·97–1·06 | 0·482 | -0·02 | -0·13–0·10 | 0·794 |
Abbreviations: PNI: prognostic nutritional index; MMSE: mini-mental state examination; CI: confidence interval; BMI: body mass index; CAD: cardiovascular diseases; RDW: red blood cell distribution width;MCV: mean corpuscular volume; MCHC: mean corpuscular haemoglobin concentration; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Notes:
Multivariate Logistic regression analysis was used to performed with cognitive decline as the dependent variables, and with PNI, age, females, BMI, living alone, mental labour, smoking, drinking, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
Multivariate Linear regression analysis was used to performed with MMSE as the dependent variables, and with PNI, age, females, BMI, living alone, mental labour, smokers, drinkers, hypertension, diabetes, CAD, anaemia, white blood cell, neutrophil, C-reactive protein, RDW, MCV, MCHC, blood glucose, ALT and AST as the independent variables.
ROC curves
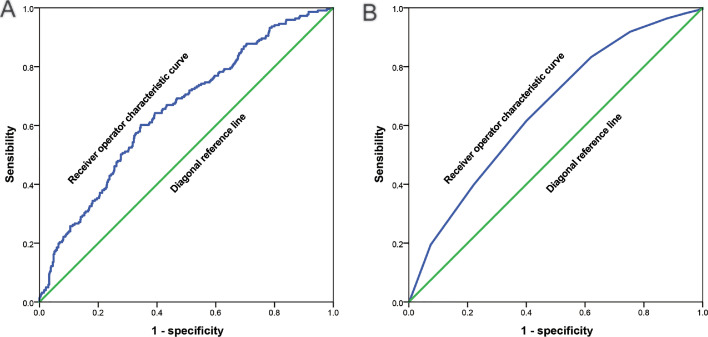
PNI and MNA-SF could identify all participants without cognitive decline. As shown in the ROC curve in Figure 2a, the AUC for PNI to identify all participants without cognitive decline was 0·652 (0·611–0·692; P < 0·001), and the cut-off point was 41·9, with a sensitivity of 0·602 and specificity of 0·655. The AUC of MNA-SF to identify all participants without cognitive decline was 0·654 (0·614–0·694; P < 0·001) (Figure 2b), and the cut-off point was 9·5 with sensitivity of 0·615 and specificity of 0·600.
Figure 2.
(a) The receiver operator characteristic curve of prognostic nutritional index to identify all participants without cognitive decline; and (b) The receiver operator characteristic curve of mini nutrition assessment-short form to identify all participants without cognitive decline.
Centenarians/oldest-old adults
Centenarians had a significantly higher prevalence of cognitive decline than oldest-old adults (87·1% vs. 68·5%, P < 0·001). Characteristics of 412 centenarians and 534 oldest-old adults among 946 cases over 80 years old were shown in Table 5. MMSE, PNI and MNA-SF were significantly lower among the centenarians than oldest-old adults (P < 0·05). Multivariate logistic analyses showed that centenarians were more likely to have lower MMSE, PNI and MNA-SF (P < 0·05; Supplementary Table 4).
Table 5.
Characteristics of centenarian and oldest-old adults.
| Characteristics | Centenarians (n = 412) |
Oldest-old adults (n = 534) |
P |
|---|---|---|---|
| Age (year) | 102(101,104) | 84(82,88) | <0·001 |
| Gender, n (%) | <0·001 | ||
| Males | 80(19·4) | 214(40·1) | |
| Females | 332(80·6) | 320(59·9) | |
| BMI (kg/m2) | 18(16,20) | 20(18,23) | <0·001 |
| BMI <18.5 kg/m2 | 222(53·9) | 138(25·8) | <0·001 |
| BMI 18.5 kg/m2 to 24 kg/m2 | 167(40·5) | 281(52·6) | |
| BMI ≥24 kg/m2 | 23(5·6) | 115(21·5) | |
| Education degree, n (%) | <0·001 | ||
| Illiteracy | 374(90·8) | 399(74·7) | |
| Elementary school level | 30(7·3) | 92(17·2) | |
| Junior high school level | 8(1·9) | 43(8·1) | |
| Living alone, n (%) | 59(14·3) | 101(18·9) | 0·062 |
| Mental labour, n (%) | 5(1·2) | 25(4·7) | 0·003 |
| Smokers, n (%) | 36(8·7) | 71(13·3) | 0·028 |
| Drinkers, n (%) | 49(11·9) | 83(15·5) | 0·108 |
| Hypertension, n (%) | 309(75·0) | 374(70·0) | 0·091 |
| Diabetes, n (%) | 38(9·2) | 57(10·7) | 0·462 |
| CAD, n (%) | 15(3·6) | 41(7·7) | 0·009 |
| Anaemia | 174(66·4) | 88(33·6) | <0·001 |
| White blood cell (109/L) | 5·93(5·05,7·14) | 6·15(5·10,7·24) | 0·321 |
| Neutrophil (109/L) | 0·55(0·48,0·63) | 0·56(0·49,0·62) | 0·614 |
| Lymphocyte (109/L) | 0·32(0·25,0·38) | 0·31(0·25,0·37) | 0·616 |
| Albumin (g/L) | 39·2(36·4,41·6) | 42·5(40·2,44·2) | <0·001 |
| C-reactive protein (mg/dL) | 0·16(0·07,0·39) | 0·13(0·06,0·33) | 0·057 |
| RDW (%) | 14·2(13·4,15·2) | 13·9(13·2,14·9) | 0·002 |
| MCV | 92·5(87·1,96·0) | 93·7(88·7,97·2) | 0·004 |
| MCHC | 313·0(306·0,320·0) | 316·0(306·0,323·0) | 0·019 |
| Blood glucose (mmol/L) | 4·83(4·23,5·64) | 4·36(3·82,5·31) | <0·001 |
| ALT | 9·55(7·70,12·48) | 12·50(10·00,15·90) | <0·001 |
| AST | 20·90(18·20,25·10) | 22·10(19·20,26·43) | 0·001 |
| MMSE | 9(5,13) | 14(10,20) | <0·001 |
| PNI | 39·2(36·4,41·6) | 42·5(40·2,44·2) | <0·001 |
| MNA-SF | 8(7,9) | 10(9,11) | <0·001 |
Abbreviations: BMI: body mass index; CAD: cardiovascular diseases; RDW: red blood cell distribution width; MCV: mean corpuscular volume; MCHC: mean corpuscular haemoglobin concentration; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MMSE: mini-mental state examination; PNI: prognostic nutritional index; MNA-SF: mini nutrition assessment-short form.
Discussion
The most significant independent risk factor for cognitive decline is age, but other contributing factors include demographic, genetic, and nutritional parameters.29 Ongoing population ageing will lead to a doubling of the number of people aged over 65 years in the coming five decades. There is evidence that malnutrition is a widespread problem among the ageing population;30 in fact, hypoalbuminemia is related to mortality in the elderly, whether they live in a community, in a hospital, or are institutionalised.31 Albumin is a good marker of nutritional status in clinically stable people, and PNI is a nutritional screening tool calculated using albumin level and lymphocyte count.32 This study found that PNI and MNA-SF were significantly lower in the centenarians and in those with cognitive decline than others, and had significantly positive association with MMSE.
Older adults are at risk of malnutrition, a major public health problem in tropical and subtropical regions of the world, and it usually occurs during a period of energy deficiency due to poor socio-economic and environmental conditions.33 Nutritional status impacts health and cognitive function, and malnutrition could lead to cognitive decline in the elderly.34 This study identified malnutrition as a significant factor for cognitive decline, and other studies have found that energy deficiency could lead to nerve cell damage, central nervous system (CNS) deregulation, and negative cognitive outcomes.35 Malnutrition is marked by insufficient protein and nutrient intake and results in energy deficiency in neurons, promoting the development of neurological disorders.36 In the ageing population, such physiological changes further accelerate the ageing process and increase the vulnerability of neurons to damage. Therefore, in older adults, malnutrition could be a significant risk factor for cognitive decline during the ageing process.
Cognitive decline might be influenced by nutritional status. Nutritional problems have been found to be related to adverse consequences, such as a decline in cognitive ability.37 Sugita et al. have realized that nutritional index, especially PNI, is significantly correlated with cognitive function.38 Kimura et al. have suggested that patients with cognitive decline had lower MNA-SF and higher prevalence of malnutrition than normal people.39 Malnutrition has been identified to be associated with cognitive function in the elderly. In the Georgia Centenarian Study designed to test the correlates of healthy longevity, the role of nutrition was focused on regarding the change in cognitive function.40
Change in the pathophysiology of cognitive decline in the oldest-old adults has many complex and heterogeneous causes. Malnutrition is a potential mechanism of cognitive decline, which might be related to the decrease of energy intake caused by the increase of metabolic disorders and energy consumption. Nutritional status might affect cognition and mood through the following pathways. As in the overeating and obesity, malnutrition could generate inflammatory responses in peripheral and central immune cells, and affect blood-brain interface and circulating factors regulating cognitive function. Neuroprotective foods might provide a means to protect the aging brain from such damage by reducing brain inflammation and oxidative stress, thereby preventing cognitive decline in the elderly.41 Oxidative stress and inflammatory responses are etiological factors for the development of insulin resistance, cardiometabolic diseases, and cognitive decline.42 As a key reaction operating interdependently during the ageing process, insulin resistance significantly diminishes the responsiveness of peripheral tissues and affects nutritional metabolism and status.43
Previous studies have found that Mediterranean diet is associated with higher cognitive ability and greater brain volume.44 Panza et al. have suggested that the consumption of Mediterranean diet might act synergistically with other protective factors to prevent and treat cognitive decline.45 Such diets can be provided as ω- 3 fatty acids, folic acid, carotenoids and vitamin E, which are related to maintaining healthy brain structure and function.46 A recent double-blind, placebo-controlled, randomized, human intervention study has demonstrated a beneficial effect of flavonoid and polyphenol on cognitive function.47 Besides, increasing intake of blueberries and strawberries has also been found to slow the rate of cognitive decline through its supplemental intake of anthocyanin and flavonoid.48
Furthermore, with the increase of age, tooth loss and chewing inability might also be one of the factors leading to the decline of cognitive ability in the elderly.49 Chewing might be a protective factor for cognitive decline because it is associated with increased blood flow in specific brain regions.50 Although the mechanisms involved in the influence of diet on cognitive function are not clear, nutritional status are likely to be involved in the change of neuronal plasticity and cognitive function.51 To achieve a normal cognitive function, it is essential to reduce malnutrition.
Tyas et al. have demonstrated that preventing cognitive decline is critical for the maintenance of healthy ageing.52 Age and educational level are determinants of cognitive ageing and decline.53 With males as the reference group, this study found that females were more likely to have cognitive decline than males because females had lower educational levels and performed less mental labour. As recommended by the World Health Organization, the best ways to prevent cognitive decline include participation in mental work and social activities, as well as the consumption of healthy diet to achieve balanced nutrition.54
This study had several strengths. First, we focused on specific populations: centenarians and oldest-old adults. Second, we analysed the interesting and valuable relationship between nutritional status and cognitive decline. Third, we presented findings of a large-scale epidemiological study. However, there were several limitations. First, being a survey conducted among specific populations in Hainan Province, China, the findings of this study might not be generalisable to all populations. Second, although surveying the oldest-old adults required huge efforts, the sample size in this study was not enough to make significant inferences among Chinese oldest-old adults. Third, nonresponse rates and self-reporting bias might affect the inferences, as well as poor transportation and communication. We tried to avoid this by using full census, household survey, and objective data; and by ensuring full communication with local language. Finally, other variables and tools for assessing nutritional status were not included due to the limitation of the variables in this study.
The findings of this study indicated that malnutrition had positive associations with cognitive decline among Chinese centenarians and oldest-old adults. It is therefore important for clinicians and community health workers to pay attention to malnutrition in these populations and provide supplemental nutrients to prevent cognitive decline.
Data sharing statement
Data underlying this study are available within the manuscript.
Contributors
SF, YZ, YY and WY contributed to the study design; SF, YZ and YY conducted the data collection; SF, LF, ZC, XQ and YZ did the statistical analyses; SF and LF wrote the first draft of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81900357, 81903392, 81941021, 81901252, 82001476, 81802804, 81801251), the Military Medical Science and Technology Youth Incubation Program (20QNPY110, 19QNP060), the Excellent Youth Incubation Program of Chinese People's Liberation Army General Hospital (2020-YQPY-007), the Military Medicine Youth Program of Chinese People's Liberation Army General Hospital (QNF19069, QNF19068), the National Key R&D Program of China (2018YFC2000400), the National S&D Resource Sharing Service Platform Project of China (YCZYPT[2018]07), the Innovation Platform for Academinicians of Hainan Province, the Hainan Major Scientific and Technological Cooperation Project (2016KJHZ0039), the China Postdoctoral Science Foundation funded project (2019M650359, 2020M682816, 2021T140298), the Medical Big Data R&D Project of Chinese People's Liberation Army General Hospital (MBD2018030), the National Geriatric Disease Clinical Medicine Research centre Project (NCRCG-PLAGH-2017-014), the Central Health Care Scientific Research Project (W2017BJ12), the Hainan Medical and Health Research Project (16A200057), the Sanya Medical and Health Science and Technology Innovation Project (2016YW21, 2017YW22, 2018YW11), and the Clinical Scientific Research Supporting Fund of Chinese People's Liberation Army General Hospital (2017FC-CXYY-3009).
Declaration of interests
The authors declare no conflict of interest.
Acknowledgments
We acknowledge the contributions of all colleagues and institutions aiding the efforts of authors. We thank the support from the Healthy Aging Consortium of the China Cohort Consortium.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101336.
Contributor Information
Weixiu Yuan, Email: Yuanweixiu301@163.com.
Yao Yao, Email: yaoyao@nsd.pku.edu.cn.
Yali Zhao, Email: zhaoyl301@163.com.
Shihui Fu, Email: fushihui@301hospital.com.cn.
Appendix. Supplementary materials
References
- 1.Blazer D.G., Wallace R.B. Cognitive aging: what every geriatric psychiatrist should know. Am J Geriatr Psychiatry. 2016;24(9):776–781. doi: 10.1016/j.jagp.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth H.W., LaFerla F.M. Mechanisms of disease: Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Stephan B.C., Bravne C. Risk factors and screening methods for detecting dementia: a narrative review. J Alzheimer's Dis. 2014;42(S4):S329–S338. doi: 10.3233/JAD-141413. [DOI] [PubMed] [Google Scholar]
- 4.Shlisky J., Bloom D.E., Beaudreault A.R., et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8(1):17–26. doi: 10.3945/an.116.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes L.L., Bennett D.A. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff. 2014;33(4):580–586. doi: 10.1377/hlthaff.2013.1353. (Millwood) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz M.J., Lipton R.B., Hall C.B., et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer's Dis Assoc Disord. 2012;26(4):335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez L.J., Barbagallo M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018;89(2):276–290. doi: 10.23750/abm.v89i2.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verlaan S., Ligthart-Melis G.C., Wijers S.L.J., Cederholm T., Maier A.B., de van der Schueren M.A.E. High prevalence of physical frailty among community-dwelling malnourished older adults-a systematic review and meta-analysis. J Am Med Dir Assoc. 2017;18(5):374–382. doi: 10.1016/j.jamda.2016.12.074. [DOI] [PubMed] [Google Scholar]
- 9.Chye L., Wei K., Nyunt M.S.Z., Gao Q., Wee S.L., Ng T.P. Strong relationship between malnutrition and cognitive frailty in the Singapore longitudinal ageing studies. J Prev Alzheimer's Dis. 2018;5(2):142–148. doi: 10.14283/jpad.2017.46. [DOI] [PubMed] [Google Scholar]
- 10.Mantzorou M., Vadikolias K., Pavlidou E., et al. Nutritional status is associated with the degree of cognitive impairment and depressive symptoms in a Greek elderly population. Nutr Neurosci. 2020;23(3):201–209. doi: 10.1080/1028415X.2018.1486940. [DOI] [PubMed] [Google Scholar]
- 11.Chen L.Y., Liu L.K., Hwang A.C., et al. Impact of malnutrition on physical, cognitive function and mortality among older men living in veteran homes by minimum data set: a prospective cohort study in Taiwan. J Nutr Health Aging. 2016;20(1):41–47. doi: 10.1007/s12603-016-0674-5. [DOI] [PubMed] [Google Scholar]
- 12.Sanders C., Behrens S., Schwartz S., et al. Nutritional status is associated with faster cognitive decline and worse functional impairment in the progression of dementia: the cache county dementia progression study1. J Alzheimer's Dis. 2016;52(1):33–42. doi: 10.3233/JAD-150528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Gómez M.E., Zapico S.C. Frailty, cognitive decline, neurodegenerative diseases and nutrition interventions. Int J Mol Sci. 2019;20(11):2842. doi: 10.3390/ijms20112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H.P., Liang J., Kuo L.M., Chen C.Y., Shyu Y.I. Trajectories of nutritional status and cognitive impairment among older Taiwanese with hip fracture. J Nutr Health Aging. 2017;21(1):38–45. doi: 10.1007/s12603-016-0756-4. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S. Nutritional management of elderly people with cognitive decline and dementia. Gerontol Int. 2014;14(suppl 2):17–22. doi: 10.1111/ggi.12252. [DOI] [PubMed] [Google Scholar]
- 16.Fu S., Hu J., Chen X., Li B., et al. Mutant single nucleotide polymorphism rs189037 in ataxia-telangiectasia mutated gene is significantly associated with ventricular wall thickness and human lifespan. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.658908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hort J., O'Brien J.T., Gainotti G., et al. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010;17(10):1236–1248. doi: 10.1111/j.1468-1331.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 18.Raz L., Knoefel J., Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz P.E., Gruhl U., Bornstein S.R., Landgraf R., Hall M., Tuomilehto J. The European perspective on diabetes prevention: development and implementation of a European Guideline and training standards for diabetes prevention (IMAGE) Diab Vasc Dis Res. 2007;4(4):353–357. doi: 10.3132/dvdr.2007.064. [DOI] [PubMed] [Google Scholar]
- 21.Fox K., Garcia M.A., Ardissino D., et al. Guidelines on the management of stable angina pectoris: executive summary: the task force on the management of stable angina pectoris of the European society of cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J.L., Adams C.D., Antman E.M., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K., Alpert J.S., White H.D., et al. Joint ESC/ACC/AHA/WHF task force for the redefinition of myocardial infarction universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 24.Drabinski T., Zacharowski K., Meybohm P., Rüger A.M., Ramirez de Arellano A. Estimating the epidemiological and economic impact of implementing preoperative anaemia measures in the German healthcare system: the health economic footprint of patient blood management. Adv Ther. 2020;37(8):3515–3536. doi: 10.1007/s12325-020-01372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzby G.P., Mullen J.L., Matthews D.C., Hobbs C.L., Rosato E.F. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 26.Cummings J.L. Mini-mental state examination. Norms, normals, and numbers. JAMA J Am Med Assoc. 1993;269:2420–2421. [PubMed] [Google Scholar]
- 27.Yao Y., Jin X., Cao K., et al. Residential proximity to major roadways and cognitive function among Chinese adults 65 years and older. Sci Total Environ. 2021;766 doi: 10.1016/j.scitotenv.2020.142607. [DOI] [PubMed] [Google Scholar]
- 28.Tai P., Yang S., Liu W., et al. Association of anthropometric and nutrition status indicators with cognitive functions in centenarians. Clin Nutr. 2021;40(4):2252–2258. doi: 10.1016/j.clnu.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal E., Miller M., Yaxley A., Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76(4):296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Cabrerizo S., Cuadras D., Gomez-Busto F., Artaza-Artabe I., Marín-Ciancas F., Malafarina V. Serum albumin and health in older people: review and meta-analysis. Maturitas. 2015;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Miller M.D., Thomas J.M., Cameron I.D., et al. BMI: a simple, rapid and clinically meaningful index of under-nutrition in the oldest old? Br J Nutr. 2009;101(9):1300–1305. doi: 10.1017/s0007114508076289. [DOI] [PubMed] [Google Scholar]
- 32.Sergi G., Coin A., Enzi G., et al. Role of visceral proteins in detecting malnutrition in the elderly. Eur J Clin Nutr. 2006;60(2):203–209. doi: 10.1038/sj.ejcn.1602289. [DOI] [PubMed] [Google Scholar]
- 33.Fu S., Yao Y., Lv F., Zhang F., Zhao Y., Luan F. Associations of immunological factors with metabolic syndrome and its characteristic elements in Chinese centenarians. J Transl Med. 2018;16(1):315. doi: 10.1186/s12967-018-1691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corish C.A., Bardon L.A. Malnutrition in older adults: screening and determinants. Proc Nutr Soc. 2019;78(3):372–379. doi: 10.1017/S0029665118002628. [DOI] [PubMed] [Google Scholar]
- 35.Lipschitz D.A., Udupa K.B. Influence of ageing and protein deficiency on neutrophil function. J Gerontol. 1986;41:281–288. doi: 10.1093/geronj/41.6.690. [DOI] [PubMed] [Google Scholar]
- 36.Cederholm T., Jagren C., Hellstrom K. Outcome of protein-energy malnutrition in elderly medical patients. Am J Med. 1995;98:67–74. doi: 10.1016/S0002-9343(99)80082-5. [DOI] [PubMed] [Google Scholar]
- 37.Soto M.E., Secher M., Gillette-Guyonnet S., et al. Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer's disease. J Alzheimer's Dis. 2012;28:647–654. doi: 10.3233/JAD-2011-110713. [DOI] [PubMed] [Google Scholar]
- 38.Sugita Y., Miyazaki T., Shimada K., et al. Correlation of nutritional indices on admission to the coronary intensive care unit with the development of delirium. Nutrients. 2018;10(11):1712. doi: 10.3390/nu10111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura A., Sugimoto T., Kitamori K., et al. Malnutrition is associated with behavioral and psychiatric symptoms of dementia in older women with mild cognitive impairment and early-stage Alzheimer's Disease. Nutrients. 2019;11(8):1951. doi: 10.3390/nu11081951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poon L.W., Clayton G.M., Martin P., et al. The Georgia centenarian study. Int J Aging Hum Dev. 1992;34(1):1–17. doi: 10.2190/8M7H-CJL7-6K5T-UMFV. [DOI] [PubMed] [Google Scholar]
- 41.Spencer S.J., Korosi A., Layé S., Shukitt-Hale B., Barrientos R.M. Food for thought: how nutrition impacts cognition and emotion. NPJ Sci Food. 2017;1:7. doi: 10.1038/s41538-017-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panza F., et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J. Alzheimer's Dis. 2010;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- 43.Kim D.H., Lee B., Lee J., et al. FoxO6-mediated IL-1β induces hepatic insulin resistance and age-related inflammation via the TF/PAR2 pathway in aging and diabetic mice. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Titova O.E., Ax E., Samantha S.J., Sjögrenb P., et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol. 2013;48 doi: 10.1016/j.exger.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Panza F., Solfrizzi V., Colacicco A.M., et al. Mediterranean diet and cognitive decline. Public Health Nutr. 2004;7(7):959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- 46.Giuseppe S., De Rui M., Coin A., Inelmen E.M., Manzato E. Weight loss and Alzheimer' s disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72(1) doi: 10.1017/S0029665112002753. [DOI] [PubMed] [Google Scholar]
- 47.Lamport D.J., Saunders C., Butler L.T., Spencer J.P. Fruits, vegetables, 100% juices, and cognitive function. Nutr Rev. 2014;72:774–789. doi: 10.1111/nure.12149. [DOI] [PubMed] [Google Scholar]
- 48.Devore E.E., Kang J.H., Breteler M.M., Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kossioni A.E. The association of poor oral health parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients. 2018;10(11):1709. doi: 10.3390/nu10111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H., Iinuma M., Onozuka M., Kubo K.Y. Chewing maintains hippocampus-dependent cognitive function. Int J Med Sci. 2015;12:502–509. doi: 10.7150/ijms.11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarmeas N., Anastasiou C.A., Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. doi: 10.1016/S1474-4422(18)30338-7. [DOI] [PubMed] [Google Scholar]
- 52.Tyas S.L., Snowdon D.A., Desrosiers M.F., Riley K.P., Markesbery W.R. Healthy ageing in the Nun Study: definition and neuropathologic correlates. Age Ageing. 2007;36(6):650–655. doi: 10.1093/ageing/afm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kryscio R.J., Schmitt F.A., Salazar J.C., Mendiondo M.S., Markesbery W.R. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66(6):828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 54.Meyer A.M., Podolski N., Pickert L., Polidori M.C. Präventive geriatrie: kognitiven Abbau verhindern [Strategies to prevent age-related cognitive decline] Dtsch Med Wochenschr. 2020;145(3):146–150. doi: 10.1055/a-0955-9587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.