Abstract
Purpose
To retrospectively report a case of Weill-Marchesani syndrome 4 (WMS4) with compound heterozygous variants of ADAMTS17 gene.
Observations
The patient was a 7-year-old boy with progressively worsening eyesight and intermittent elevated intraocular pressure (IOP) for two years. His IOPs were temporarily controlled using anti-glaucoma drugs. At presentation he had a shallow anterior chamber, lens subluxation, spherophakia and extensive synechial angle closure with high myopia in both eyes. Ultrasound biomicroscopy (UBM) identified thickened zonule fibers and anteriorly rotated, flat and slender ciliary processes, both of which worsened and were accompanied by obvious iris bombe after miosis. Gene testing showed compound heterozygosity of a maternal submicroscopic deletion on chromosome 15q26.3 (0.774 Mb) affecting the sequences of ADAMTS17, LYSMD4 and CERS3 as well as a paternal nonsense variant (c.1051_1053delAAGinsTAA, P.K351X) in the ADAMTS17 gene in the proband. The diagnosis of WMS4 was confirmed by genetic testing. Phacoemulsification (Phaco), intraocular lens (IOL) implantation, and irido-zonulo-hyaloid-vitrectomy (IZHV) combined with Ahmed Glaucoma Valve (AGV) implantation as a staged or one-stage surgery effectively lowered IOP, deepened ACD, improved visual acuity, and resolved the configuration of the ciliary processes in both eyes.
Conclusion and Importance
Recessive ADAMTS17 variants are associated with WMS4. We report here compound heterozygous variants in ADAMTS17 causing WMS4, and anatomically highlighted the possible pathophysiology for its clinical phenotype. A modified surgical approach with Phaco, IOL implantation, and IZHV combined with AGV implantation could be used to treat these complicated cases.
Keywords: Weill-Marchesani syndrome 4, Angle-closure glaucoma, Recessive ADAMTS17 variants
1. Introduction
Weill-Marchesani syndrome (WMS) is a rare connective tissue disorder characterized by microspherophakia, ectopia of the lenses, severe myopia, glaucoma, short stature, brachydactyly, joint stiffness, and, occasionally, heart defects.1 In clinic, patients with incomplete WMS characteristics are diagnosed with Weill-Marchesani syndrome 4 (WMS4). To date, two modes of inheritance have been reported for WMS: autosomal dominant (AD), which is caused by variants in the FBN1 gene (gene ID 2200, OMIM 608328),2 and autosomal recessive (AR), which is caused by variants in ADAMTS10 gene (gene ID 81794, OMIM 608990)3 and LTBP2 gene (gene ID 4053, OMIM 602091).4 Variants in the FBN1, LTBP2 and ADAMTS17 gene (gene ID 170691, OMIM 607511) result in a WMS4.4, 5, 6
In this study, genetic analysis and clinical observations of a Chinese trio (the proband and his/her unaffected parents) affected with AR WMS4 are reported. Through whole exome sequencing (WES), we found compound heterozygous variants of a maternal submicroscopic deletion on chromosome 15q26.3 (0.774 Mb) affecting the sequences of ADAMTS17, LYSMD4 and CERS3 and a paternal nonsense variant (c.1051_1053delAAGinsTAA, P.K351X) in the ADAMTS17 gene in the proband.
2. Case report
A 7-year-old boy presented with progressive bilateral blurred vision and ocular pain for the preceding 2 years. He was initially diagnosed with “angle-closure glaucoma” in both eyes at a local community hospital with elevated intraocular pressures (IOP, the highest up to 37 mmHg) two years prior. He was prescribed anti-glaucoma drugs, including carteolol hydrochloride (2%) and brinzolamide eye drops (1%). No other treatment was provided.
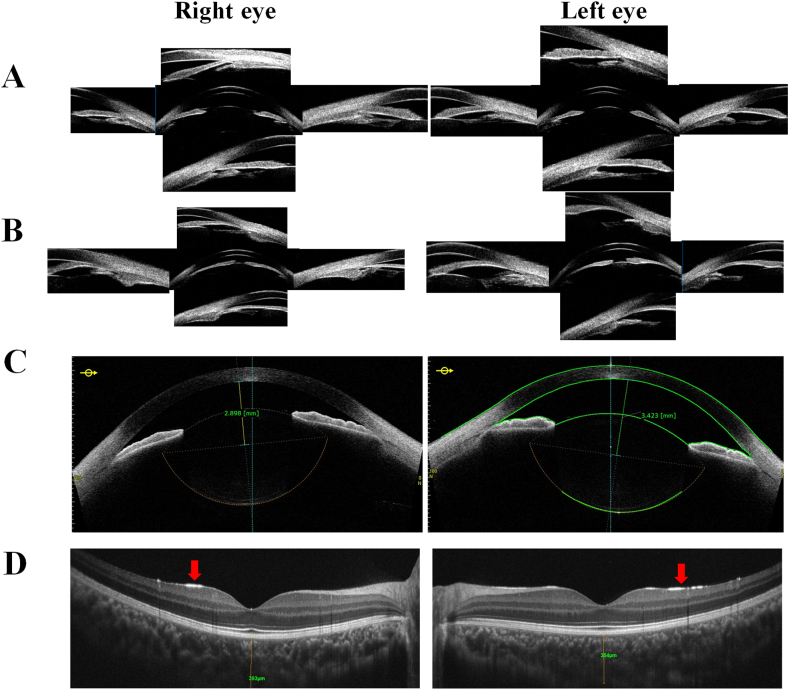
When the patient presented to us, his IOP was controlled (right eye, 14 mmHg; left eye, 19 mmHg) on anti-glaucoma drugs. His visual acuity was 10/200 in the right eye and 20/100 in the left eye, which could be improved to 30/100 and 70/100 after myopia correction with −9.0D and −8.5D, respectively. On slit-lamp examination, he was found to have bilateral narrow-angle glaucoma with the following findings: very shallow anterior chamber, lens subluxation, spherophakia in both eyes, and peripheral anterior synechia of the iris in the supratemporal quadrant of the right eye (Fig. 1). Static gonioscopy demonstrated a circumferential grade IV narrow angle by Scheie grading7 with 3–4 clock hours open in each eye on dynamic gonioscopy. Axial length measured by the IOL Master was slightly longer than peers (21.85 mm, OD; 21.60 mm, OS) and symmetric anterior chamber depth (ACD) was found (right eye: 1.25 mm; left eye: 1.58 mm) using anterior segment optical coherence tomography (AS-OCT), suggesting the possibility of lens subluxation. AS-OCT further showed that anterior-posterior lens thickness was 4.38 mm in the right eye and 4.37 mm in the left eye with large posterior curvature in the bilateral lens (Fig. 2C). Ultrasound biomicroscopy (UBM) confirmed angle closure in the setting of iridotrabecular contact with peripheral anterior synechiae at the iris root. It also showed anteriorly rotated, flat and slender ciliary processes, which worsened after miosis with pilocarpine. Pupillary block was not the main mechanism of angle closure since iris bombe was not obviously present under UBM at the resting state but became distinct after miosis (Fig. 2A and B). Concurrently, the ACD decreased and the lens thickened in the left eye after miosis but not in the right eye as measured by AS-OCT (ACD after miosis: right eye, 1.26 mm and left eye, 1.18 mm; lens thickness after miosis: right eye, 4.49 mm and left eye, 4.80 mm). Notably, thickened zonule fibers were found under UBM. Spectral domain optical coherence tomography (SD-OCT) revealed a distinct, flat, hyper-reflective layer at the vitreoretinal interface in both eyes (Fig. 2D), which had been presumed previously to be activated retinal astrocytes and Muller cells (ARAM).8 Subfoveal choroid thickness was greater in the right eye (393 μm) than the left eye (354 μm), but both were thicker than the reported normal range in Chinese children of similar age and with similar axial lengths.9 Optic nerve cupping was central and round, measuring approximately 0.2 in both eyes. No evidence of glaucomatous optic neuropathy was detected under fundus photography, OCT or Humphrey visual field testing. There was no history of eye diseases in the family and no history of ocular trauma or systemic diseases in the patient. He was 120 cm (−0.70SD below the median10) at the age of 7, and now he is 123 cm (−1.01SD below the median) at the age of 8. No other systemic dysmorphic features (such as brachydactyly and joint stiffness) were found.
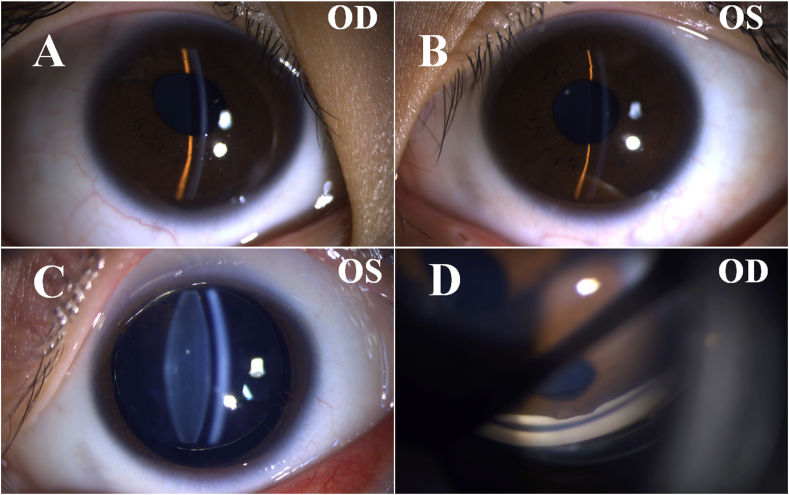
Fig. 1.
Clinical presentation pictures obtained using slit-lamp examination. (A–B) A shallow anterior chamber can be appreciated in the right eye (OD) and left eye (OS). (C) Bilateral spherophakia was present but only the left eye is shown here because only the left pupil could be fully dilated. (D) Representative narrow angle in the right eye.
Fig. 2.
Ocular imaging of the patient. (A) Ultrasound biomicroscope (UBM) images before miosis by pilocarpine showing angle closure in the setting of iridotrabecular contact with peripheral anterior synechiae of the iris root. An anteriorly rotated, flat and slender ciliary body without obvious iris bombe is also present. (B) Ultrasound biomicroscope (UBM) images after miosis by pilocarpine showing worsening iridotrabecular contact due to iris bombe. The ciliary process also became more anteriorly rotated, flatter, and slenderer. (C) Anterior segment optical coherence tomography (AS-OCT) showing the thickened lens and large posterior curvature in the bilateral lens. (D) Spectral domain optical coherence tomography (SD-OCT) showing a hyper-reflective line (labeled with red arrows) at the internal limiting membranes (ILM). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
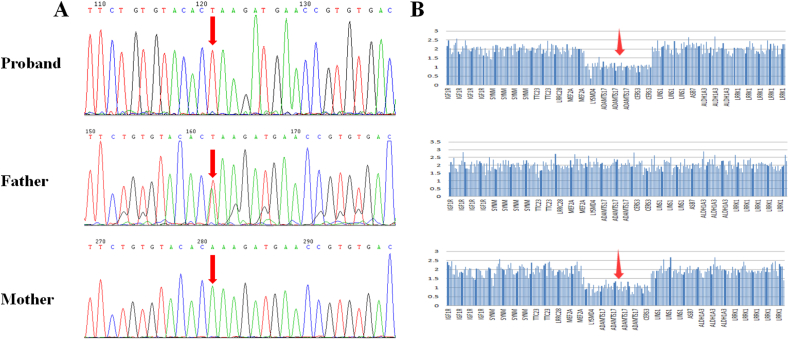
Genetic tests were performed by WES analysis using Novaseq 6000 sequencer (MyGenostics Inc., Beijing, China). WES analysis in the proband found a maternal submicroscopic deletion on chromosome 15q26.3 (0.774 Mb) affecting the sequences of ADAMTS17 (exon 1–22), LYSMD4 (exon 3–6) and CERS3 (exon 4–13) and a paternal nonsense variant (c.1051_1053delAAGinsTAA, p.K351X) in the ADAMTS17 gene, which was suspected as the causative agent. Co-segregation of the nonsense variant in the ADAMTS17 gene was determined with Sanger sequencing on family members (Fig. 3A) and the submicroscopic deletion is presented in Fig. 3B. The frequencies of the nonsense variant in 1000G, GnomAD and ExAC were less than 2% and predicted to be “damaging” by in silico predictions from SIFT, REVEL and CADD. According to the American College of Medical Genetics and Genomics (ACMG) guidelines, the submicroscopic deletion on chromosome 15q26.3 (0.774Mb) was classified as “Uncertain” and the nonsense variant in the ADAMTS17 gene was classified as “Likely pathogenic”. No causative variants of the other WMS and WMS4 genes (including ADAMTS10, LTBP2, and FBN1) were found in the proband or his parents. Thus, based on the clinical manifestations including ocular abnormalities and a slightly short stature and the compound heterozygous variant in ADAMTS17, the proband was diagnosed with WMS4. The genotypic results of the trio members are presented in Fig. 3.
Fig. 3.
Sequence analysis of ADAMTS17 in trios. A. Identification of the ADAMTS17 nonsense variant (c.1051_1053delAAGinsTAA, p.K351X) by Sanger sequencing. The proband: hemizygous variant; father: heterozygous variant; mother: no variant. B. Schematic diagram of the submicroscopic deletion on chromosome 15q26.3 (0.774 Mb) affecting the sequences of ADAMTS17, LYSMD4 and CERS3. The proband: heterozygous variant; father: no variant; mother: heterozygous variant.
Given intermittent elevated IOP on medical therapy, shallow anterior chamber, lens subluxation, spherophakia, and extensive synechial angle closure, the decision was made to proceed with phacoemulsification (Phaco), intraocular lens (IOL) implantation, and goniosynechialysis (GSL) combined with irido-zonulo-hyaloid-vitrectomy (IZHV) in the right eye. During continuous curvilinear capsulorrhexis (CCC), circumferential loosening of the zonule was noted. However, two weeks after surgery, IOP increased to 30 mmHg with obvious deepening of the anterior chamber (ACD: 3.489 mm), although the angle did not open further (remained 3 clock hours). Thus, Ahmed glaucoma valve (AGV) implantation was subsequently performed to lower the IOP in the setting of a deep anterior chamber after the first stage of surgery. Phaco, IOL implantation, and IZHV combined with AGV implantation in the left eye were performed uneventfully, although the circumferential zonule was also loose during CCC. Three months following TCP treatment, the patient's vision improved to 80/100 in each eye with IOP between 14 and 16 mmHg in both eyes without anti-glaucoma medications. The ACD remained stable around 3.2 mm and there was no change in fundus examination. At the visit of three months after surgery, the anteriorly rotated, flat and slender ciliary body had resolved in both eyes (Fig. 4). No recurrent IOP spike, anterior chamber shallowing or severe complications occurred between surgeries or during a follow-up of three months. Informed consent was obtained from the parents for research and publication purposes.
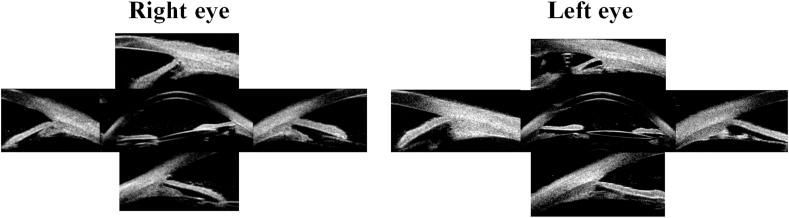
Fig. 4.
Ultrasound biomicroscope (UBM) images of both eyes at the last visit. The anteriorly rotated, flat and slender ciliary body resolved in both eyes. The previously dynamically opened angle at 9 o'clock in the right eye and 6 o'clock in the left eye opened widely.
3. Discussion
Here we reported compound heterozygous variants of a maternal submicroscopic deletion on chromosome 15q26.3 (0.774 Mb) affecting the sequences of ADAMTS17, LYSMD4 and CERS3 and a paternal nonsense variant (c.1051_1053delAAGinsTAA, P.K351X) in the ADAMTS17 gene associated with WMS4. Studies have identified that homozygous variants in CERS3 (ceramide synthase 3) caused autosomal recessive congenital ichthyosis (ARCI) in humans presenting with abnormal desquamation over the whole body, including skin, heart, and skeletal anomalies.11 These anomalies were not either reported in previous literatures of cases with heterozygous variants of CERS3 or in our case. In addition, no disease associations have been described for LYSMD4 (LysM domain containing 4) so far. Thus, we think the heterozygous deletion of LYSMD4 and CERS3 was not relevant to this case and the compound heterozygous variant in ADAMTS17 might be the cause of this case. Patients with incomplete WMS characteristics are diagnosed with WMS4. This report provides further evidence that recessive ADAMTS17 variants are a cause of the phenotype of WMS4 that presents with lens subluxation, spherophakia, severe myopia, secondary angle-closure glaucoma and slightly short stature (as opposed to the phenotype of WMS syndrome, which in addition to lens subluxation, spherophakia, severe myopia, and secondary angle-closure glaucoma, also includes brachydactyly, stiff joints, and thickened skin1).
The ADAMTS protease family, comprising 19 homologous secreted metalloproteases, plays a vital role in organ development and tissue homeostasis by regulating extracellular matrix (ECM) formation, remodeling and homeostatic adaptation via their C-terminal thrombospondin (TS) repeats.12 Previous studies have shown that ADAMTS proteins involved in different cellular mechanisms could be responsible for different subtypes of the syndrome.13 In addition, ADAMTS17 and ADAMTS10, members of the ADAMTS protease family, have been implicated in connective-tissue disorders and ectopia lentis.6 In one study, diffuse expression of ADAMTS17 within the area of a future ciliary body was found in the eye sections from E14.5 mouse embryos, which suggested a role in zonule development.6 ADAMTS17 undergoes rapid autocatalytic processing in trans after its secretion from cells and binds fibrillin-2 (FBN2), but cannot cleave it, resulting in the formation of bigger and thicker fibers. Furthermore, recombinant ADAMTS17 binds to microfibrils in cultured fibroblasts with differential effects on FBN1 and FBN2, indicating its impact on microfibril assembly.14 However, the exact function of ADAMTS17 in eye disease is still unknown.
In another study, three WMS patients from a Chinese family with a homozygous nonsense variant (c.1051A > T, p.lys351Ter) in the ADAMTS17 gene had short stature, spherophakia, brachydactyly and visual impairment, but only two patients showed secondary glaucoma and lens dislocation.15 Though the nonsense variant (c.1051_1053delAAGinsTAA, P.K351X) found in our study is similar (resulted in the same truncated protein of 351 amino acids), the proband in ours only showed lens subluxation, spherophakia, severe myopia, secondary angle-closure glaucoma and slightly short stature without other systemic abnormalities. In fact, many other studies have suggested that patients with ADAMTS17 variants show inconsistent phenotypes with incomplete WMS characteristics.6,15,16 Furthermore, except for the heterozygous nonsense variant, a heterozygous deletion on chromosome 15q26.3 encompassing the complete sequence of ADAMTS17 was also found in the proband. A homozygous deletion on chromosome 15q26.3 (1.67 Mb, encompasses the first three exons of ADAMTS17 and exon 13 of CERS3) leads to two independent diseases, autosomal recessive congenital ichthyosis (ARCI) and WMS4, respectively.11 Thus, the compound heterozygous variants of ADAMTS17 gene might be the reason that the phenotype is not consistent with other studies. On the other hand, the proband was 120 cm (−0.70SD below the median) at the age of 7 and 123 cm (−1.01SD below the median) at the age of 8. Although at present the proband does not show significant short stature, as the body develops, short stature as well as other systemic abnormalities, may become more pronounced. Further follow up will be necessary to identify these abnormalities.
Several studies have advocated for removal of the spherophakic lens as the primary modality of treatment, since this could relieve pupillary block, markedly decrease angle crowding and thus widen the angle.17, 18, 19, 20 While the lens in this case was thicker than peers by less than 1 mm and not thicker than old cataract patients,21 no obvious iris bombe was detected under resting state, suggesting the major mechanism of angle closure might not be pupillary block with a thicker lens. In our study, the patient had an anteriorly rotated, flat and slender ciliary body, which is similar to those of WMS patients in other studies.22 Interestingly, in our case, all these findings worsened after miosis but resolved after surgery. Abnormal ciliary zonule with a smaller ciliary body and decreased ciliary processes have also been observed in WMS mice, which implies primary abnormalities of ciliary in WMS patients.23 Besides, larger and thicker microfibril bundles were seen to emerge from the ciliary body in WMS mice.23 We speculated that the dysplastic ciliary processes might be easily stretched out by the condensed and inelastic zonule, causing lens diameter to decrease, lens curvature to increase, and ultimately lead to a presentation of microspherophakia.24,25 Additionally, the configuration of the ciliary body could potentially be further altered by the forward positioned lens when the iridolenticular diaphragm was pushed forward by pressure from the posterior segment, leading to anterior chamber shallowing, angle closure and further myopia apart from increased curvature of lens. In this case, choroidal expansion and a thicker choroid were detected in both eyes, creating a high-pressure environment. The patient's intact and un-liquefied vitreous may have played an important role in conducting the force from the thickened choroid, and poor vitreous flow conductivity could have prevented aqueous outflow, further increasing pressure in the posterior segment as posited by Quigley et al.26 This could also explain why this patient's condition was worse in the right eye (shallower ACD, more severe synechiae and decreased space between the iris and ciliary body) and why his right eye had a thicker choroid than the left. The restoration of ciliary configuration after surgery further supports this idea as the pressure between the anterior and posterior segments was balanced by IZHV. A dysplastic ciliary body with a longer ciliary process has also been seen in primary congenital glaucoma (PCG), however the longer ciliary process may have been caused by the stretched globe.27 Although a short history of glaucoma and normal eyeball size ruled out a diagnosis of PCG in this case, the similar changes in the ciliary process further support that a dysplastic ciliary might be easily stretched out.
Above all, considering that the IOP was temporarily controlled by medication and the synechial angle closure could be reopened by goniosynechialysis since no evidence of glaucomatous optic neuropathy was detected – suggesting a short time of angle closure – Phaco, IOL implantation, and GSL with viscoelastic were chosen to control IOP. Meanwhile, the patient's younger age, thicker choroid, unliquefied vitreous,26,28 more anteriorly rotated ciliary body, and condensed zonule fibers were considered possible predisposing factors for malignant glaucoma or persistent shallow anterior chamber after surgery.29 Therefore, combined IZHV was performed in the right eye first. However, two weeks after surgery, IOP increased to 30 mmHg with obvious deepening of the anterior chamber (ACD: 3.489 mm), although the angle did not open further (remained 3 clock hours). This finding suggested that the synechiae angle closure caused by abnormal ciliary cannot be reopened by lens removal and GSL, and the remaining opened angle was unable to afford sufficient aqueous humor drainage. Thus, AGV was performed as a second surgery to ensure adequate trabecular function and IOP lowering. In the left eye, Phaco, IOL implantation, and IZHV combined with AGV implantation were performed as the first surgery. There were no severe complications between surgeries or during a follow-up of three months. The persistently low IOP and improvement in visual acuity indicated that the above surgical approach was adequate for this case.
4. Conclusions
In conclusion, we report compound heterozygous variants of a maternal submicroscopic deletion (0.774 Mb on chromosome 15q26.3) and a paternal nonsense variant (c.1051_1053delAAGinsTAA, P.K351X) in the ADAMTS17 gene causing WMS4. Anatomically, dysplasia of the ciliary body and condensed zonule fibers combined with a thicker choroid and intact unliquefied vitreous might be predisposing factors for the anatomical changes, namely spherophakia, lens subluxation, shallow anterior chamber, myopia and extensive synechial angle closure in this subset of eyes. Furthermore, a modified surgical approach with Phaco, IOL implantation, IZHV and AGV implantation could be used to treat these complicated cases.
Statement of ethics
Written informed consent was obtained from the patient prior to publication of his relevant de-identified medical information. This work was conducted in accordance with the World Medical Association Declaration of Helsinki.
Funding
This work was supported by Capital's Funds for Health Improvement and Research (No.2020-4-2059) and the National Natural Science Foundation of China (82171050).
Contributorship statement
Yan Shi and Zhigang Fan: Conceptualization, Methodology, Writing-Reviewing and Editing; Xiaowei Yu: Data curation, Writing-Original draft preparation; Yan Gao: Data curation. Brad Kline and Ying Han: Writing-Reviewing and Editing. All authors approved the final version of the manuscript.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
The patient consented in writing to the publication of this case and associated images.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgements
None.
Contributor Information
Zhigang Fan, Email: fanzhigang@mail.ccmu.edu.cn.
Yan Shi, Email: yansmile4433@163.com.
References
- 1.Wright K.W., Chrousos G.A. Weill-Marchesani syndrome with bilateral angle-closure glaucoma. J Pediatr Ophthalmol Strabismus. 1985;22:129–132. doi: 10.3928/0191-3913-19850701-04. [DOI] [PubMed] [Google Scholar]
- 2.Faivre L., Gorlin R.J., Wirtz M.K., et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagoneau N., Benoist-Lasselin C., Huber C., et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haji-Seyed-Javadi R., Jelodari-Mamaghani S., Paylakhi S.H., et al. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum Mutat. 2012;33:1182–1187. doi: 10.1002/humu.22105. [DOI] [PubMed] [Google Scholar]
- 5.Yang G.-Y., Huang X., Chen B.-J., Xu Z.-P. Weill-Marchesani-like syndrome caused by an FBN1 mutation with low-penetrance. Chin Med J (Engl) 2021;134:1359–1361. doi: 10.1097/CM9.0000000000001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales J., Al-Sharif L., Khalil D.S., et al. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am J Hum Genet. 2009;85:558–568. doi: 10.1016/j.ajhg.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheie H.G. Width and pigmentation of the angle of the anterior chamber; a system of grading by gonioscopy. AMA Arch Ophthalmol. 1957;58:510–512. doi: 10.1001/archopht.1957.00940010526005. [DOI] [PubMed] [Google Scholar]
- 8.Cheung H., King B.J., Gast T.J. Presumed activated retinal astrocytes and Müller cells in healthy and glaucomatous eyes detected by spectral domain optical coherence tomography. Ophthalmic Physiol Opt. 2020;40:738–751. doi: 10.1111/opo.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J.M., Wu J.F., Chen J.H., et al. Macular choroidal thickness in children: the Shandong children eye study. Invest Ophthalmol Vis Sci. 2015;56:7646–7652. doi: 10.1167/iovs.15-17137. [DOI] [PubMed] [Google Scholar]
- 10.Standard for Height Level Classification Among Children and Adolescents Aged 7∼18 Years: National Health Commission of the People's Republic of China. 2018. [Google Scholar]
- 11.Radner F.P.W., Marrakchi S., Kirchmeier P., et al. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose K.W.J., Taye N., Karoulias S.Z., Hubmacher D. Regulation of ADAMTS proteases. Front Mol Biosci. 2021;8:701959. doi: 10.3389/fmolb.2021.701959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubmacher D., Schneider M., Berardinelli S.J., et al. Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease. Sci Rep. 2017;7:41871. doi: 10.1038/srep41871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi H., Zha X., Zhu Y., et al. A novel nonsense mutation in ADAMTS17 caused autosomal recessive inheritance Weill-Marchesani syndrome from a Chinese family. J Hum Genet. 2019;64:681–687. doi: 10.1038/s10038-019-0608-2. [DOI] [PubMed] [Google Scholar]
- 16.Shah M.H., Bhat V., Shetty J.S., Kumar A. Whole exome sequencing identifies a novel splice-site mutation in ADAMTS17 in an Indian family with Weill-Marchesani syndrome. Mol Vis. 2014;20:790–796. [PMC free article] [PubMed] [Google Scholar]
- 17.Nonaka A., Kondo T., Kikuchi M., et al. Angle widening and alteration of ciliary process configuration after cataract surgery for primary angle closure. Ophthalmology. 2006;113:437–441. doi: 10.1016/j.ophtha.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Guo H., Wu X., Cai K., Qiao Z. Weill-Marchesani syndrome with advanced glaucoma and corneal endothelial dysfunction: a case report and literature review. BMC Ophthalmol. 2015;15:3. doi: 10.1186/1471-2415-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel N., Sharma R., Sawhney A., Mandal M., Choudhry R.M. Lensectomy, vitrectomy, and transvitreal ciliary body photocoagulation as primary treatment for glaucoma in microspherophakia. J AAPOS. 2015;19:366–368. doi: 10.1016/j.jaapos.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Harasymowycz P., Wilson R. Surgical treatment of advanced chronic angle closure glaucoma in Weill-Marchesani syndrome. J Pediatr Ophthalmol Strabismus. 2004;41:295–299. doi: 10.3928/01913913-20040901-08. [DOI] [PubMed] [Google Scholar]
- 21.Chen J.H., Jiang W.J., Sun Z.Y., et al. Lens thickness and associated factors in Chinese children: the shandong children eye study. Acta Ophthalmol. 2017;95:e521–e522. doi: 10.1111/aos.13431. [DOI] [PubMed] [Google Scholar]
- 22.Dietlein T.S., Jacobi P.C., Krieglstein G.K. Ciliary body is not hyperplastic in Weill-Marchesani syndrome. Acta Ophthalmol Scand. 1998;76:623–624. doi: 10.1034/j.1600-0420.1998.760524.x. [DOI] [PubMed] [Google Scholar]
- 23.Mularczyk E.J., Singh M., Godwin A.R.F., et al. ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome. Hum Mol Genet. 2018;27:3675–3687. doi: 10.1093/hmg/ddy276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinn's zonule Bassnett S. Prog Retin Eye Res. 2021;82:100902. doi: 10.1016/j.preteyeres.2020.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar B., Chandler H.L., Plageman T., Reilly M.A. Lens stretching modulates lens epithelial cell proliferation via YAP regulation. Invest Ophthalmol Vis Sci. 2019;60:3920–3929. doi: 10.1167/iovs.19-26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley H.A., Friedman D.S., Congdon N.G. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12:167–180. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y., Han Y., Xin C., et al. Disease-related and age-related changes of anterior chamber angle structures in patients with primary congenital glaucoma: an in vivo high-frequency ultrasound biomicroscopy-based study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Tian J., Han Y., Oatts J., Wang N. Pathogenic role of the vitreous in angle-closure glaucoma with autosomal recessive bestrophinopathy: a case report. BMC Ophthalmol. 2020;20:271. doi: 10.1186/s12886-020-01543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Huang J., Lin J., Liang X., Cai X., Ge J. Quantitative measurements of the ciliary body in eyes with malignant glaucoma after trabeculectomy using ultrasound biomicroscopy. Ophthalmology. 2014;121:862–869. doi: 10.1016/j.ophtha.2013.10.035. [DOI] [PubMed] [Google Scholar]