Summary
Background
Whether meat consumption is related to risk of mortality in patients with inflammatory bowel disease (IBD) remains poorly understood.
Methods
In the UK Biobank, 5763 patients with IBD were recruited from 2007 to 2010 and finished a brief food frequency questionnaire at baseline. We followed them until March 13, 2021 to document all-cause death events. Cox proportional hazard models were used to estimate hazard ratios (HRs) for all-cause mortality associated with consumptions of fish, unprocessed poultry, unprocessed red meat, and processed meat among the patients.
Findings
During 67,095 person-years (mean follow-up 11·7 years, mean age 57·3, 52·5% female), we documented 590 death events. Higher consumption of processed meat was associated with an increased risk of all-cause mortality in patients with IBD (HR comparing >4·0 with 0–0·9 time/week=1·52, 95% confidence interval (CI) 1·05–2·19), but the P-trend for each 25 g increment was 0·075. This association remained significant in patients with Crohn's disease (HR 1·77, 95% CI 1·01–3·10) but not in patients with ulcerative colitis (HR 1·34, 95% CI 0·82–2·20). Consumptions of fish (HR 1·27, 95% CI 0·84–1·91), unprocessed poultry (HR 0·59, 95% CI 0·28–1·21), or unprocessed red meat (HR 0·87, 95% CI 0·60–1·26) were not significantly associated with the mortality of patients with IBD.
Interpretation
More frequent consumption of processed meat was associated with an increased risk of mortality in patients with IBD, while no associations were observed for consumption of other types of meat. Our exploratory and speculative findings should be cautiously interpreted and need further replication in other cohorts.
Funding
The National Natural Science Foundation of China (81,970,494); Key Project of Research and Development Plan of Hunan Province (2019SK2041).
Keywords: Inflammatory bowel disease, Crohn's disease, Ulcerative colitis, Nutrition, Meat consumption
Research in context.
Evidence before this study
We systematically searched in PubMed from the inception of the database to October 1, 2021 for relevant studies, using terms (‘Inflammatory bowel disease’ OR ‘Crohn's disease’ OR ‘Ulcerative colitis’) and (‘Meat’ OR ‘Diet’). Previous studies suggested that higher meat consumption was positively associated with disease relapse as well as short-term adverse events of patients with inflammatory bowel disease (IBD). The relation of meat consumption to long-term outcomes of IBD is less clear, which is crucial in developing dietary recommendations for patients with IBD.
Added value of this study
A retrospective cohort study in patients with IBD in the UK Biobank found that more frequent consumption of processed meat was associated with increased risk of all-cause mortality, while unprocessed red meat, poultry, or fish were not related to death. These exploratory findings could be a start point for future studies to further discover the underlying mechanisms and to inform the composing of dietary recommendations for patients with IBD.
Implications of all the available evidence
For patients with IBD, consuming more processed meat seems associated with higher risk of mortality. In contrast, whether it is necessary to restrict intake of unprocessed red meat, unprocessed poultry, and fish warrants further investigation.
Alt-text: Unlabelled box
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn's disease (CD), poses increasingly great burdens worldwide.1, 2, 3 Considering the intimacy of IBD with the digestive tract, dietary factors are regarded as one of the most crucial modifiable lifestyle factors for incident IBD and prognosis of patients with IBD.4,5
Previous studies have revealed the associations of high intakes of animal protein and meat with an increased risk of incident IBD,6,7 while there has been no consensus on whether it is necessary to restrict meat consumption for patients with IBD.8 On the one hand, meat is the major source of multiple nutrients, and nutrients that can be easily absorbed are essential for the prognosis of patients with IBD.9 On the other, the impaired digestive function of patients with IBD10 may make them more vulnerable to unhealthy meat intake.11 Emerging studies suggested that meat consumption, especially red meat consumption, was associated with the progression of IBD11, 12, 13, 14, 15 and highlighted the role of Trimethylamine-N-oxide (TMAO) in the effect of meat consumption. Epidemiology evidence and animal experiments demonstrated that higher TMAO level was associated with an increased risk of inflammation,16,17 colorectal cancer,18 cardiovascular disease as well as mortality.19 Given that patients with IBD have a higher mortality rate20 and risk of colorectal cancer,21 a healthy meat intake may play an essential role in preventing premature death among patients with IBD. However, there is limited evidence concerning the relation of meat consumption to long-term outcomes of IBD, which is crucial in the development of targeted dietary recommendations for patients with IBD. A previous study had linked a high-quality diet to reduced mortality risk in patients with IBD,22 but which types and frequencies of meat intake are associated with a particularly higher risk of mortality among patients with IBD remained unclear.
Therefore, we hypothesized that more frequent consumptions of red meat and processed meat is associated with higher risk of all-cause mortality in patients with IBD. To clarify their relations, we conducted a retrospective cohort study based on UK Biobank to investigate the associations of consumptions of different types of meat with all-cause mortality in patients with IBD.
Methods
Study population
The UK Biobank is a large-scale cohort that recruited over 500 000 participants aged 40–69 years from 2006 to 2010 across the United Kingdom.23 Participants attended one of the twenty-two assessment centres across England, Wales, and Scotland,23 where they electronically signed informed consent and completed a self-administered, touch-screen questionnaire, a face-to-face interview, physical measurement, and sample collection, as described in more detail elsewhere.24 All available resources are listed on the UK Biobank website (http://www.ukbiobank.ac.uk). Ethical approval was granted for the UK Biobank by the North West-Haydock Research Ethics Committee (REC reference: 16/NW/0274). This study followed the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement.
We leveraged data of UK Biobank participants who were diagnosed with IBD before recruitment. We extracted their disease information via self-report (collected from the verbal interview and converted to International Classification of Disease, 10th (ICD-10) code), primary care (documented in specific code form and mapped into ICD-10 code), and hospital inpatient data (recorded in ICD-9 or ICD-10 form). Baseline IBD was defined as being diagnosed before recruitment with the specific diagnostic code (ICD-10 code: K50, K51; ICD-9 code: K555, K556). We excluded participants (1) without IBD subtype information or diagnoses with UC and CD simultaneously (n = 167); (2) with missing dietary data for meat at baseline (n = 9); and (3) enrolled in pilot recruitment conducted in 2006 (n = 33) (Supplementary Figure S1). Finally, we included 5763 patients with IBD in the primary analyses. The prevalence of IBD (∼1%) in the UK Biobank was comparable with previous studies based on primary care data.25,26
Meat consumption measures
A validated brief food frequency questionnaire (FFQ) consisting of 47 items27 was contained in touchscreen questionnaire used in UK Biobank. We leveraged data on the consumption of meat and other major food groups of the patients. The main exposures in the current study were unprocessed fish (oily and non-oily), unprocessed poultry, unprocessed red meat (pork, beef, and lamb/mutton), and processed meat. Participants were asked how often they consumed each item (‘never’, ‘less than once a week’, ‘once a week’, ‘2–4 times a week’, ‘5–6 times a week’, or ‘once or more daily).
We categorized meat consumption groups in several steps according to previous studies.28,29 First, we assigned values for meat consumption according to the frequency per week (never eaten = 0, eaten <1 time/week = 0·5, 1 time/week = 1, 2–4 times/week = 3, 5–6 times/week = 5·5, and ≥1 time daily = 7). We combined oily and non-oily fish as fish consumption, and unprocessed red meat was defined as the combination of unprocessed beef, pork, and mutton. Finally, we categorized intake frequencies for each meat type into 4 groups as follows: 0–0·9 time/week, 1·0–1·9 times/week, 2·0–4·0 times/week, >4·0 times/week.
We used data from the Oxford WebQ questionnaire conducted in a subgroup of participants to estimate the mean intake of each meat category of the 5763 participants. The Oxford WebQ questionnaire28,30 was administrated online for a subgroup of participants in five rounds (Apr 2009-Sep 2010; Feb 2011-April 2011; June 2011-Aug 2011; Oct 2011-Dec 2011; April 2012-June 2012) to quantitatively assess meat consumption in the past 24 h. Each item was recorded in portions (e.g., how many rashers of bacon). The weight of each food (grams) for each participant was calculated by multiplying the number of portions with the standard portion size.27 For 2171 participants who had more than one round of valid WebQ, we calculated their mean values of daily intakes by the frequency category (0–0·9, 1·0–1·9, 2·0–4·0, >4·0 times/week, frequency from the touchscreen questionnaire) (Supplementary Table S1). These mean values were then assigned to the 5763 participants. Valid questionnaire here was defined as the questionnaire with typical diet and credible energy intake (>0 MJ and ≤20 MJ for male, >0 MJ and ≤18 MJ for female)31 to increase the reliability and representativeness of the data. The mean intake of each meat category was also used to estimate trends (per 25 g/d) in risk across categories, as is suggested by previous studies28,32,33 to minimize potential regression dilution bias in the FFQ.34
Ascertainment of death
The outcome of interest was death events among patients with IBD, obtained from the National Health Service (NHS) Digital (England and Wales), the NHS Central Register, and National Records of Scotland (Scotland). Details of the linkage procedure can be found at http://content.digital.nhs.uk/services. Death data was available up to March 13, 2021.
Other covariates
We incorporated multiple covariates according to a priori knowledge of factors associated with meat consumption and mortality risk, as well as previous studies,29,35 including sociodemographic factors (age at recruitment, sex, ethnicity, education level, Townsend deprivation index (TDI)), lifestyle factors (smoking status (never, previous or current), body mass index (BMI), alcohol drinking status (never, previous, current), physical activity level), and other major food groups (including vegetable in tablespoons/day, fruit in pieces/day, and grain product in slices or bowls/week) collected in the FFQ. Physical activities were classified into two categories (low, high) according to whether the participants met the criteria of more than 150 min per week of moderate-intensity physical activity, or 75 min per week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activities.36 To account for the quantity of alcohol consumption, we also incorporated information for alcohol consumption collected from the baseline questionnaire37 and further categorized participants were categorized into none, moderate, or high consumers. Moderate level of alcohol consumption was defined as <14 g/d for women and < 28 g/d for men,38 with participants exceeding this quantity being categorized as high consumers. We also collected baseline duration time of IBD and baseline comorbidities, including hypertension, cardiovascular disease (angina, myocardial infarction), stroke, and cancer from multiple sources (hospital inpatient, primary care, cancer registry, self-report).
Statistical analysis
Baseline characteristics were summarized for all patients with IBD and by subtypes (UC and CD). Continuous variables were presented as means (SDs) and categorical variables were displayed as numbers (percentages). Given the low rates of missing (0·1∼0·9%), we handled the missing data by single imputation,39 using means for continuous variables and the most populated categories for the categorical variables. The associations of meat consumptions with all-cause mortality in patients with IBD, UC, and CD were assessed using Cox proportional hazard regressions. Proportional hazard assumption was tested and verified using weighted residuals method.40 Survival time (in person-years) was calculated from baseline (date of recruitment) to death, loss-to-follow-up, or the end of linkage (March 13, 2021), whichever came first. We also plotted the Kaplan-Meier curves by meat consumptions to visualize these associations. The minimally-adjusted model estimated the hazard ratios (HRs) with 95% confidence intervals (CIs) adjusted for age (continuous), age-squared (continuous), sex (female or male), and ethnicity (white or others). The fully-adjusted model was further adjusted for TDI (low, moderate, or high deprivation), education level (college and above or high school and below), physical activity level (low or high), smoking status (never, previous, or current), alcohol drinking status (never, previous, or current), and other major food groups (intake of vegetable, fruit, and grain product, as continuous variables respectively).
To test potential effect modifications by major covariates, we re-run the fully-adjusted model stratified by sex (female or male), age (≤ 60 or >60 years), smoking status (never, previous or current), alcohol drinking status (never, previous, or current), education level (college and above or high school and below), physical activity level (low or high). The P-interaction was calculated by testing the change of goodness-of-fit before and after allowing a multiplication term of the meat consumption and the covariate.
We conducted several sensitivity analyses to verify the robustness of the results. Based on the fully-adjusted models, we further: (1) adjusted for BMI and baseline comorbidities considering their potential effects on meat consumption and mortality; (2) evaluated the associations of subtypes of red meat (i.e., mutton, beef, and pork) and fish (i.e., oily and non-oily) with mortality; (3) reprocessed the covariates using multiple imputations to address the potential influence of the imputing method41; (4) used no consumption (0 time/week) or 0·1–0·9 time per week consumption as the reference group; (5) adjusted the quantity of alcohol consumption instead of alcohol drinking status to further account for the effect of alcohol intake; (7) excluded patients with only self-report of IBD but no health system records; (8) excluded patients who died within the first 1, 2, and 3 years, respectively, to alleviate the potential of reverse causation.
Statistical analyses were performed using R 3.6.0, and two-sided P-values < 0·05 were considered indicators for statistical significance.
Role of funding sources
The funders had no role in data collection, analysis, interpretation, writing of the manuscript and the decision to submit.
Results
Baseline characteristics
Baseline characteristics of patients with IBD, CD, and UC were shown in Table 1. Of the 5763 patients with IBD, 3028 (52·5%) were female and 3929 (68·2%) were with UC. A total of 590 deaths were documented over 67,095 person-years (average follow-up = 11·7 years). The mean (SD) age was 57·3 (7·9) years in patients with IBD, 56·4 (8·1) years in patients with CD, and 57·7 (7·8) years in patients with UC. The proportions of participants who consumed fish, unprocessed poultry, unprocessed red meat, or processed meat 0–0·9 time/week were 8·1%, 14·7%, 10·0%, and 37·5%, respectively.
Table 1.
Baseline characteristics of patients with IBD stratified by IBD subtypes in the UK Biobank cohort study.1
| All patients (n = 5763) | Patients with CD (n = 1834) | Patients with UC (n = 3929) | |
|---|---|---|---|
| Sex (%) | |||
| Female | 3028 (52·5) | 1028 (56·1) | 2000 (50·9) |
| Male | 2735 (47·5) | 806 (43·9) | 1929 (49·1) |
| Age (mean (SD)), years | 57·3 (7·9) | 56·4 (8·1) | 57·7 (7·8) |
| TDI (%) | |||
| Low deprivation | 1919 (33·3) | 599 (32·7) | 1320 (33·6) |
| Moderate deprivation | 1918 (33·3) | 574 (31·3) | 1344 (34·2) |
| High deprivation | 1919 (33·3) | 657 (35·8) | 1262 (32·1) |
| Missing | 7 (0·1) | 4 (0·2) | 3 (0·1) |
| Education (%) | |||
| College and above | 1619 (28·1) | 491 (26·8) | 1128 (28·7) |
| High School and below | 4091 (71·0) | 1326 (72·3) | 2765 (70·4) |
| Missing | 53 (0·9) | 17 (0·9) | 36 (0·9) |
| Ethnic (%) | |||
| White | 5524 (95·9) | 1778 (96·9) | 3746 (95·3) |
| Others | 217 (3·8) | 51 (2·8) | 166 (4·2) |
| Missing | 22 (0·4) | 5 (0·3) | 17 (0·4) |
| Physical activity (%)2 | |||
| Low | 2197 (38·1) | 755 (41·2) | 1442 (36·7) |
| High | 3423 (59·4) | 1032 (56·3) | 2391 (60·9) |
| Missing | 143 (2·5) | 47 (2·6) | 96 (2·4) |
| Smoking status (%) | |||
| Previous or current smoker | 3021 (52·4) | 1010 (55·1) | 2011 (51·2) |
| Never smoked | 2723 (47·2) | 819 (44·7) | 1904 (48·5) |
| Missing | 19 (0·3) | 5 (0·3) | 14 (0·4) |
| Alcohol drinking status (%) | |||
| Never | 288 (5·0) | 100 (5·5) | 188 (4·8) |
| Previous drinker | 297 (5·2) | 108 (5·9) | 189 (4·8) |
| Current drinker | 5168 (89·7) | 1622 (88·4) | 3546 (90·3) |
| Missing | 10 (0·2) | 4 (0·2) | 6 (0·2) |
| BMI (%), kg/m2 | |||
| <18·5 | 45 (0·8) | 23 (1·3) | 22 (0·6) |
| ≥18·5–<25·0 | 1940 (33·7) | 657 (35·8) | 1283 (32·7) |
| ≥25 | 3742 (64·9) | 1145 (62·4) | 2597 (66·1) |
| Missing | 36 (0·6) | 9 (0·5) | 27 (0·7) |
| With coronary heart disease history (%)3 | 426 (7·6) | 116 (6·5) | 310 (8·1) |
| With stroke history (%) | 151 (2·7) | 45 (2·5) | 106 (2·8) |
| With hypertension history (%) | 1660 (29·5) | 521 (29·2) | 1139 (29·7) |
| With cancer history (%) | 622 (11·1) | 190 (10·6) | 432 (11·3) |
| Fish (%) | |||
| 0–0·9 time/week | 466 (8·1) | 154 (8·4) | 312 (7·9) |
| 1·0–1·9 times/week | 2309 (40·1) | 737 (40·2) | 1572 (40·0) |
| 2·0–4·0 times/week | 2612 (45·3) | 818 (44·6) | 1794 (45·7) |
| > 4·0 times/week | 376 (6·5) | 125 (6·8) | 251 (6·4) |
| Unprocessed poultry (%) | |||
| 0–0·9 time/week | 850 (14·7) | 265 (14·4) | 585 (14·9) |
| 1·0–1·9 times/week | 2076 (36·0) | 631 (34·4) | 1445 (36·8) |
| 2·0–4·0 times/week | 2703 (46·9) | 878 (47·9) | 1825 (46·4) |
| > 4·0 times/week | 134 (2·3) | 60 (3·3) | 74 (1·9) |
| Unprocessed red meat (%) | |||
| 0–0·9 time/week | 574 (10·0) | 169 (9·2) | 405 (10·3) |
| 1·0–1·9 times/week | 2182 (37·9) | 693 (37·8) | 1489 (37·9) |
| 2·0–4·0 times/week | 2478 (43·0) | 799 (43·6) | 1679 (42·7) |
| > 4·0 times/week | 529 (9·2) | 173 (9·4) | 356 (9·1) |
| Processed meat (%) | |||
| 0–0·9 time/week | 2161 (37·5) | 649 (35·4) | 1512 (38·5) |
| 1·0–1·9 times/week | 1670 (29·0) | 546 (29·8) | 1124 (28·6) |
| 2·0–4·0 times/week | 1679 (29·1) | 546 (29·8) | 1133 (28·8) |
| > 4·0 times/week | 253 (4·4) | 93 (5·1) | 160 (4·1) |
| Duration of IBD (%) | |||
| ≤10 years | 2114 (36·7) | 605 (33·0) | 1509 (38·4) |
| >10 years | 3649 (63·3) | 1229 (67·0) | 2420 (61·6) |
IBD, inflammatory bowel disease; CD, Crohn's disease; UC, ulcerative colitis; TDI, Townsend deprivation index; BMI, body mass index.
Continuous variable are displayed as means (SDs), and categorical variables are displayed as numbers (percentages).
Physical activity level ‘High’ was defined as 150 min per week of moderate-intensity physical activity, or 75 min per week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activities.
Coronary heart disease comprises of angina and myocardial infarction.
Meat consumption and all-cause mortality
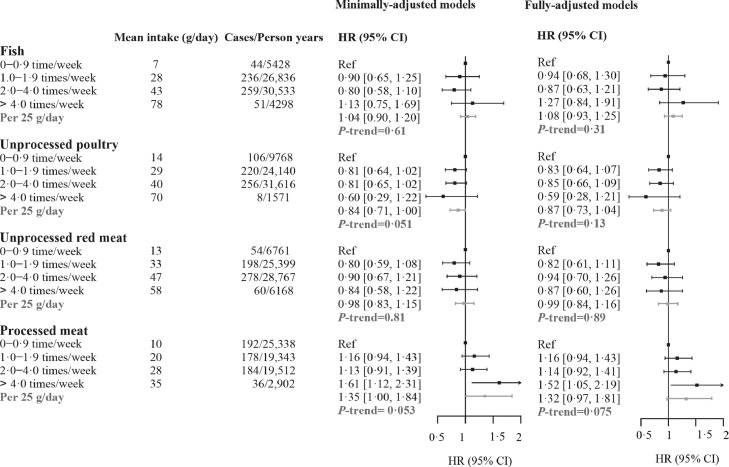
The associations between meat consumptions and all-cause mortality among patients with IBD were shown in Figure 1 and Supplementary Figure S2. All Cox models satisfied the proportional hazard assumptions (P-values for fish, 0·11; poultry, 0·48; unprocessed red meat, 0·58; processed meat, 0·25). Individuals with IBD who consumed processed meat more frequently were at an increased risk of mortality (HR comparing > 4·0 with 0–0·9 time/week, 1·52, 95% CI 1·05–2·19), but the trend was not significant (P-trend for each 25 g increment = 0·075). We observed no associations of other types of meat with mortality, including fish (HR 1·27, 95% CI 0·84–1·91; P-trend for each 25 g increment = 0·31), unprocessed poultry (HR 0·59, 95% CI 0·28–1·21; P-trend for each 25 g increment = 0·13), and unprocessed red meat (HR 0·87, 95% CI 0·60–1·26; P-trend for each 25 g increment = 0·89).
Figure 1.
Associations between meat consumption and all-cause mortality among patients with IBD in the UK Biobank cohort (n = 5763). HRs were calculated by Cox proportional hazard regression models, minimally-adjusted models adjusted for age, age-squared, sex and ethnicity. Fully-adjusted models further adjusted for Townsend deprivation index, education, physical activity level, smoking status, alcohol drinking status, and intake of grain product, vegetable and fruit based on minimally-adjusted model. Mean intake in each category is from the 24-h dietary assessments. HR, hazard ratio; CI, confidence interval; IBD, inflammatory bowel disease.
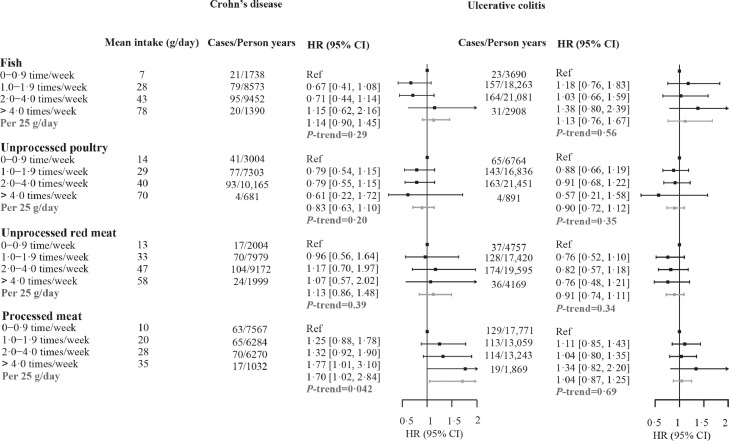
The association between processed meat (>4 times/week) with mortality remained robust in patients having IBD over 10 years (Table 2, HR 1·65, 95% CI 1·03–2·64). Furthermore, more frequent consumption of processed meat (>4 times/week) was significantly associated with increased mortality risk (Figure 2) in patients with CD (HR 1·77, 95% CI 1·01–3·10), with each 25 g increment being associated with approximately 70% increased risk (HR 1·70, 95% CI 1·02–2·84). On the contrary, we did not observe this association in patients with UC (HR 1·34, 95% CI 0·82–2·20).
Table 2.
HRs (95% CIs) for the associations between meat consumption and all-cause mortality among patients with IBD with baseline duration of disease survival >10 years or ≤10 years.1
| Patients with IBD ≤10 years (n=2,114) | P2 | Patients with IBD >10 years (n=3,469) | P | |
|---|---|---|---|---|
| Fish | ||||
| 0–0·9 time/week | Ref | Ref | ||
| 1·0–1·9 times/week | 0·86 [0·52, 1·42] | 0·57 | 1·01 [0·66, 1·55] | 0·95 |
| 2·0–4·0 times/week | 0·82 [0·50, 1·35] | 0·43 | 0·94 [0·61, 1·44] | 0·77 |
| > 4·0 times/week | 1·39 [0·75, 2·59] | 0·30 | 1·24 [0·72, 2·16] | 0·44 |
| Unprocessed poultry | ||||
| 0–0·9 time/week | Ref | Ref | ||
| 1·0–1·9 times/week | 0·77 [0·53, 1·12] | 0·17 | 0·90 [0·67, 1·21] | 0·49 |
| 2·0–4·0 times/week | 0·89 [0·62, 1·28] | 0·53 | 0·85 [0·63, 1·14] | 0·27 |
| > 4·0 times/week | 1·02 [0·43, 2·41] | 0·97 | 0·26 [0·06, 1·08] | 0·063 |
| Unprocessed red meat | ||||
| 0–0·9 time/week | Ref | Ref | ||
| 1·0–1·9 times/week | 0·86 [0·51, 1·43] | 0·56 | 0·81 [0·55, 1·18] | 0·27 |
| 2·0–4·0 times/week | 0·87 [0·53, 1·45] | 0·60 | 0·98 [0·68, 1·42] | 0·93 |
| > 4·0 times/week | 0·99 [0·54, 1·81] | 0·97 | 0·79 [0·49, 1·28] | 0·35 |
| Processed meat | ||||
| 0–0·9 time/week | Ref | Ref | ||
| 1·0–1·9 times/week | 1·10 [0·78, 1·54] | 0·59 | 1·21 [0·93, 1·57] | 0·16 |
| 2·0–4·0 times/week | 1·09 [0·78, 1·54] | 0·61 | 1·14 [0·87, 1·50] | 0·35 |
| > 4·0 times/week | 1·30 [0·72, 2·34] | 0·39 | 1·65 [1·03, 2·64] | 0·037 |
HR, hazard ratio; CI, confidence interval; IBD, inflammatory bowel disease.
HR was adjusted for age, age-squared, sex, ethnicity, Townsend deprivation index, education, physical activity level, smoking status, alcohol drinking status, and intake of grain product, vegetable, and fruit.
P values <0·05 were considered statistically significant.
Figure 2.
Associations between meat consumption and all-cause mortality among patients with Crohn's disease or ulcerative colitis. HRs were calculated by Cox proportional hazard regression models, adjusted for age, age-squared, sex and ethnicity, Townsend deprivation index, education, physical activity level, smoking status, alcohol drinking status, and intake of grain product, vegetable and fruit. Mean intake in each category is estimated from the 24-h dietary assessments. HR, hazard ratio; CI, confidence interval.
In most sensitivity analyses, the observed results maintained consistent, but was non-significant when we used no consumption (i.e., 0 time/week) as the reference group (HR comparing >4·0 with 0 time/week=1·57, 95% CI 0·99–2·48), potentially due to limited sample size in the reference group (Supplementary Table S2). Patients who consumed processed meat >4 times/week also showed an increased risk of mortality when we further adjusted the model for BMI and baseline comorbidities (HR 1·46, 95% CI 1·02–2·10). This association persisted when we excluded death cases that occurred in the first 1-year (HR 1·50, 95% CI 1·03–2·18), 2-year (HR 1·48, 95% CI 1·01–2·18), and 3-year (HR 1·52, 95% CI 1·02–2·27) periods, respectively (Supplementary Table S3–4). When separately assessing the relations of oily and non-oily fish, unprocessed pork, unprocessed beef, and unprocessed lamb/mutton to mortality, we did not observe any statistically significant association (Supplementary Table S5). For example, higher intake of non-oily fish was not associated with mortality risk (HR 1·72, 95% CI, 0·70–4·19, comparing >4·0 with 0–0·9 time/week). Since the confidence intervals were too wide to make any solid conclusion, further studies are needed to explore these associations. We also observed similar associations of more frequent consumption of processed meat with mortality (Supplementary Tables S6–9) when we rehandled covariates using multiple imputations (HR 1·47, 95% CI 1·01–2·15), adjusted the models for the quantity of alcohol consumption instead of alcohol drinking status (HR 1·51, 95% CI 1·05–2·18), excluded patients with only self-reports but no health system records (HR 1·58, 95% CI 1·08–2·31), and used 0·1–0·9 time per week as the reference group (HR 1·51, 95% CI 1·04–2·19).
We observed similar associations (Supplementary Tables S10, 11) in analyses stratified by age, sex, smoking status, alcohol drinking status, education level, and physical activity level (P-interactions > 0·05 for all).
Discussion
In this study, we discovered that more frequent intake of processed meat, but not fish, unprocessed poultry, or unprocessed red meat, was associated with an increased risk of mortality in patients with IBD. This association remained robust in patients with CD but was non-significant in patients with UC. The observed relations were consistent across major subgroups defined by age, sex, smoking status, alcohol drinking status, education level, and physical activity level. Such associations were not significant when we took no consumption as the reference group. Taken together, these findings provided additional evidence, but should be interpreted with caution.
Multiple studies have assessed the associations between processed meat and all-cause mortality in the general population or patients, most of which revealed an increased risk of all-cause mortality associated with processed meat.42, 43, 44 A meta-analysis indicated that each serving per day increment in processed meat intake was associated with an 15% increased risk of all-cause mortality (RR 1·15, 95% CI 1·11–1·19),45 and another reported that a reduction of 3 servings/week of processed meat was associated 8% decreased risk of all-cause mortality (RR 0·92, 95% CI 0·87–0·96).46 Our study, focused on patients with IBD, found a strong association (HR 1·51, 95% CI 1·04–2·19) between processed meat and all-cause mortality, and this estimate was higher than that of the general population reported by a previous study in 29,682 US adults (HR=1·09, 95% CI 1·01–1·18).42 Given that patients with IBD tended to have altered dietary habits,47, 48, 49 our results thus provided additional evidence for targeted nutrition recommendations. Our findings, if proven causal, suggested that for patients with IBD, especially CD, consuming processed meat >4 times/week conferred significantly increased (∼50%) risk of mortality. However, whether other types of meat, i.e., fish, unprocessed poultry, and unprocessed red meat, are necessarily associated with mortality risk needs further investigation.
We discovered notable differences in the associations between processed meat consumption and mortality among patients with CD and UC in the associations. The association remained strong in patients with CD but was non-significant in patients with UC, which corresponded to previous epidemiologic studies, showing that diet may be more closely related to the course of CD instead of UC.50 Another study also put forward that Crohn's Disease Exclusion Diet (CDED), which avoided or reduction of exposure to foods containing animal/dairy fat, protein, etc., plus partial enteral nutrition (PEN) induced sustained remission in patients with CD.51 A possible explanation might be the different sites of the lesions between patients with CD and UC. The entire gastrointestinal tract can be affected in patients with CD while the lesion is localized to the colon in patients with UC.52 The small intestine is regarded as the primary site of nutrient digestion and absorption. Moreover, researchers demonstrated that microbiota in the small intestine is extremely responsive to dietary stimuli and plays an important role in nutrient assimilation.53 However, other studies also found that processed meat was strongly associated with the risk of relapse of UC.11 Therefore, further studies are needed to explore the effects of diets on patients with CD and UC and the critical composition.
Our findings kept robust in almost all sensitivity analyses. When we used 0·1–0·9 time per week consumption as the reference group, the result was consistently significant. However, when we took participants with no consumption as the reference group, the HR (95% CI) (consuming > 4·0 times/week compared to 0 time/week) for associations between processed meat consumption and mortality was 1·57 (0·99–2·48). The direction of association was consistent, but due to the limited sample size, the confidence interval was widened and included the null. Considering the small number of participants with no consumption, the non-significant association should be interpreted with caution.
Although the underlying mechanism was unclear, several hypotheses can explain the increased risks of processed meat consumption on mortality in patients with IBD. First, it is well established that high salt and fat diet from processed meat increases the risk of cardiovascular disease54 while polycyclic aromatic hydrocarbons and nitrates may contribute to some of the adverse health effects that can lead to cancer.55 In addition, patients with IBD may be more vulnerable to the exposure of N-nitroso compounds (NOC) produced by processed meat, therefore leading to a higher risk of colorectal cancer and increased risk of all-cause death.56,57 Second, protein, fat, salt, glycan including in processed meat promoted inflammation in various ways, compromising the colonic intestinal epithelium structure.58, 59, 60 Third, the roles of TMAO can't be ignored as well. Higher intake of processed red meat could increase circulating TMAO levels under the transformation of gut microbiota,61 which was proven to be associated with inflammation and colorectal cancer,16, 17, 18 thus posing a higher risk of adverse outcomes for patients with IBD. Also, diet was proved to have potential effects on the composition and function of the intestinal microbiome, thus involved in the courses of patients with IBD, either directly or indirectly.62
Given the widespread consumption of processed meat, this study provides relevant and useful information for developing dietary recommendations for patients with IBD. To our knowledge, this is the first study examining the associations of meat consumption on the longevity of patients with IBD. There are several strengths in our study. First, we took advantage of the large sample size and well-administrated cohort from UK Biobank, which enabled a series of analyses on the specific population with IBD. Second, an adequate number of death cases defined based on the NHS death records allowed us to assess the associations with acceptable statistical power. Third, potential confounding effects were considered seriously and dealt with through multiple adjustments and sensitivity analyses.
Nevertheless, our study has several limitations. First, patients with IBD in this study were mainly of European ancestry, so the generalizability of our findings awaits exploration. Second, as the food frequency was only measured at baseline, measurement error might still exist despite our efforts in incorporating dietary data from the repeated 24-h dietary assessments.28,32,33 Since the 24-h dietary questionnaire used in UK Biobank was validated in general population but not in patients with IBD, the over- or underestimation of actual meat intake may also exist. Third, residual confounding and reverse causation could still exist, given the observational nature of this study. As the baseline touchscreen brief FFQ does not support the calculation of total energy, we could not account for the potential confounding by total energy. Instead, we adjusted for the major food groups (including vegetable, fruit, and grain product), BMI, and physical activity in the models to partially address this issue.28,29,33 Also, as we mainly considered the long-term outcome of IBD rather than focusing on the shorter-term outcomes such as relapse or hospital admissions, the developmental course and the underlying mechanism warrants further investigation.
In conclusion, we found that more frequent consumption of processed meat was associated with an increased risk of mortality in patients with IBD, especially in patients with CD, while no significant association was observed for other types of meat consumption. Although our findings are exploratory and speculative, they may serve as a start point for the attention of meat intake in patients with IBD. Further studies are needed to explore whether it is necessary to reduce intake of other types of meat.
Contributors
XW and JC made substantial contributions to conception and design. HC, TF, and LD were involved in data acquisition, data analysis, and manuscript drafting. XW, JC, HC, TF, and LD accessed and were responsible for the raw data associated with the study. The data was verified by YS and TH. JC, XC, YS and TH helped interpret the data and revise the manuscript. XW and JC took the decision to submit the manuscript for publication. All authors gave final approval of the version to be published.
Data sharing statement
Researchers can request the data we used upon approval from the UK Biobank (www.ukbiobank.ac.uk/).
Declaration of interests
XW: Grant funding from The National Natural Science Foundation of China (81970494); Key Project of Research and Development Plan of Hunan Province (2019SK2041).
All other authors report no conflicts.
Acknowledgments
Acknowledgments
This work was conducted using the UK Biobank Resource under application number 73595. We want to thank all UK Biobank participants and the management team for their participation and assistance.
Funding
The National Natural Science Foundation of China (81970494); Key Project of Research and Development Plan of Hunan Province (2019SK2041).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101406.
Contributor Information
Jie Chen, Email: med_chenjie@zju.edu.cn.
Xiaoyan Wang, Email: wangxiaoyan@csu.edu.cn.
Appendix. Supplementary materials
References
- 1.Molodecky N.A., Soon I.S., Rabi D.M., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan G.G., Ng S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152(2):313–321. doi: 10.1053/j.gastro.2016.10.020. e2. [DOI] [PubMed] [Google Scholar]
- 4.Lee D., Albenberg L., Compher C., et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148(6):1087–1106. doi: 10.1053/j.gastro.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzello F., Spisni E., Giovanardi E., et al. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11(5) doi: 10.3390/nu11051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jantchou P., Morois S., Clavel-Chapelon F., Boutron-Ruault M.C., Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol. 2010;105(10):2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 7.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 8.Levine A., Rhodes J.M., Lindsay J.O., et al. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(6):1381–1392. doi: 10.1016/j.cgh.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Hou J.K., Lee D., Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol. 2014;12(10):1592–1600. doi: 10.1016/j.cgh.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilby K., Mathias H., Boisvenue L., Heisler C., Jones J.L. Micronutrient absorption and related outcomes in people with inflammatory bowel disease: a review. Nutrients. 2019;11(6) doi: 10.3390/nu11061388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jowett S.L., Seal C.J., Pearce M.S., et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albenberg L., Brensinger C.M., Wu Q., et al. A diet low in red and processed meat does not reduce rate of Crohn's disease flares. Gastroenterology. 2019;157(1):128–136. doi: 10.1053/j.gastro.2019.03.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters V., Spooren C., Pierik M.J., et al. Dietary intake pattern is associated with occurrence of flares in IBD patients. J Crohns Colitis. 2021;15(8):1305–1315. doi: 10.1093/ecco-jcc/jjab008. [DOI] [PubMed] [Google Scholar]
- 14.Campmans-Kuijpers M.J.E., Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the groningen anti-inflammatory diet (GrAID) Nutrients. 2021;13(4) doi: 10.3390/nu13041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasson L., Canova C., Vettorato M.G., Savarino E., Zanotti R. Influence of diet on the course of inflammatory bowel disease. Dig Dis Sci. 2017;62(8):2087–2094. doi: 10.1007/s10620-017-4620-0. [DOI] [PubMed] [Google Scholar]
- 16.Farhangi M.A., Vajdi M. Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2020;60(16):2801–2823. doi: 10.1080/10408398.2020.1770199. [DOI] [PubMed] [Google Scholar]
- 17.Arias N., Arboleya S., Allison J., et al. The Relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. 2020;12(8) doi: 10.3390/nu12082340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aune D., Chan D.S., Vieira A.R., et al. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control. 2013;24(4):611–627. doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- 19.Tang W.H., Wang Z., Kennedy D.J., et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jess T., Frisch M., Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin Gastroenterol Hepatol. 2013;11(1):43–48. doi: 10.1016/j.cgh.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Nadeem M.S., Kumar V., Al-Abbasi F.A., Kamal M.A., Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol. 2020;64:51–60. doi: 10.1016/j.semcancer.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Lo C.H., Khalili H., Song M., et al. Healthy lifestyle is associated with reduced mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19(1):87–95. doi: 10.1016/j.cgh.2020.02.047. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 24.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers T.J., Weiner A.B., Graff R.E., et al. Association between inflammatory bowel disease and prostate cancer: a large-scale, prospective, population-based study. Int J Cancer. 2020;147(10):2735–2742. doi: 10.1002/ijc.33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King D., Reulen R.C., Thomas T., et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000-2018. Aliment Pharmacol Ther. 2020;51(10):922–934. doi: 10.1111/apt.15701. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury K.E., Young H.J., Guo W., Key T.J. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Greenwood D.C., Risch H.A., Bunce D., Hardie L.J., Cade J.E. Meat consumption and risk of incident dementia: cohort study of 493,888 UK Biobank participants. Am J Clin Nutr. 2021;114(1):175–184. doi: 10.1093/ajcn/nqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papier K., Fensom G.K., Knuppel A., et al. Meat consumption and risk of 25 common conditions: outcome-wide analyses in 475,000 men and women in the UK Biobank study. BMC Med. 2021;19(1):53. doi: 10.1186/s12916-021-01922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galante J., Adamska L., Young A., et al. The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: administration of the Oxford WebQ in UK biobank. Br J Nutr. 2016;115(4):681–686. doi: 10.1017/S0007114515004821. [DOI] [PubMed] [Google Scholar]
- 31.Bradbury K.E., Tong T.Y.N., Key T.J. Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK biobank. Nutrients. 2017;9(12):1317. doi: 10.3390/nu9121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradbury K.E., Murphy N., Key T.J. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246–258. doi: 10.1093/ije/dyz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei X., Zhu C., Ji M., et al. Diet and risk of incident lung cancer: a large prospective cohort study in UK biobank. Am J Clin Nutr. 2021;114(6):2043–2051. doi: 10.1093/ajcn/nqab298. [DOI] [PubMed] [Google Scholar]
- 34.MacMahon S., Peto R., Cutler J., et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y., Li Y., Satija A., et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. doi: 10.1136/bmj.l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 37.Lourida I., Hannon E., Littlejohns T.J., et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans 2020-2025.https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. Accessed 25 December 2021
- 39.Scheffer J. Dealing with missing data. 2002.
- 40.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 41.van Buuren S., Boshuizen H.C., Knook D.L. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Zhong V.W., Van Horn L., Greenland P., et al. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern Med. 2020;180(4):503–512. doi: 10.1001/jamainternmed.2019.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohrmann S., Overvad K., Bueno-de-Mesquita H.B., et al. Meat consumption and mortality-results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etemadi A., Sinha R., Ward M.H., et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Lin X., Ouyang Y.Y., et al. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. doi: 10.1017/S1368980015002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeraatkar D., Han M.A., Guyatt G.H., et al. Red and processed meat consumption and risk for all-cause mortality and cardiometabolic outcomes: a systematic review and meta-analysis of cohort studies. Ann Intern Med. 2019;171(10):703–710. doi: 10.7326/M19-0655. [DOI] [PubMed] [Google Scholar]
- 47.Marsh A., Kinneally J., Robertson T., Lord A., Young A., Radford-Smith G. Food avoidance in outpatients with inflammatory bowel disease - who, what and why. Clin Nutr ESPEN. 2019;31:10–16. doi: 10.1016/j.clnesp.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Casanova M.J., Chaparro M., Molina B., et al. Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J Crohns Colitis. 2017;11(12):1430–1439. doi: 10.1093/ecco-jcc/jjx102. [DOI] [PubMed] [Google Scholar]
- 49.Vagianos K., Clara I., Carr R., et al. What are adults with inflammatory bowel disease (IBD) eating? A closer look at the dietary habits of a population-based Canadian IBD cohort. JPEN J Parenter Enteral Nutr. 2016;40(3):405–411. doi: 10.1177/0148607114549254. [DOI] [PubMed] [Google Scholar]
- 50.Spooren C.E., Pierik M.J., Zeegers M.P., Feskens E.J., Masclee A.A., Jonkers D.M. Review article: the association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(10):1172–1187. doi: 10.1111/apt.12501. [DOI] [PubMed] [Google Scholar]
- 51.Levine A., Wine E., Assa A., et al. Crohn's disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440–450. doi: 10.1053/j.gastro.2019.04.021. e8. [DOI] [PubMed] [Google Scholar]
- 52.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Guryn K., Hubert N., Frazier K., et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23(4):458–469. doi: 10.1016/j.chom.2018.03.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grillo A., Salvi L., Coruzzi P., Salvi P., Parati G. Sodium intake and hypertension. Nutrients. 2019;11(9) doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demeyer D., Mertens B., De Smet S., Ulens M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: a review. Crit Rev Food Sci Nutr. 2016;56(16):2747–2766. doi: 10.1080/10408398.2013.873886. [DOI] [PubMed] [Google Scholar]
- 56.Bingham S.A., Pignatelli B., Pollock J.R., et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17(3):515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 57.de Kok T.M., Engels L.G., Moonen E.J., Kleinjans J.C. Inflammatory bowel disease stimulates formation of carcinogenic N-nitroso compounds. Gut. 2005;54(5):731. doi: 10.1136/gut.2004.057471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samraj A.N., Pearce O.M., Läubli H., et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015;112(2):542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esmaillzadeh A., Kimiagar M., Mehrabi Y., Azadbakht L., Hu F.B., Willett W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137(4):992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 60.Binger K.J., Gebhardt M., Heinig M., et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest. 2015;125(11):4223–4238. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z., Bergeron N., Levison B.S., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh R.K., Chang H.W., Yan D., et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.