Abstract
Objectives
The COVID-19 pandemic increases healthcare worker (HCW) absenteeism. The bacillus Calmette-Guérin (BCG) vaccine may provide non-specific protection against respiratory infections through enhancement of trained immunity. We investigated the impact of BCG vaccination on HCW absenteeism during the COVID-19 pandemic.
Methods
HCWs exposed to COVID-19 patients in nine Dutch hospitals were randomized to BCG vaccine or placebo in a 1:1 ratio, and followed for one year using a mobile phone application. The primary endpoint was the self-reported number of days of unplanned absenteeism for any reason. Secondary endpoints included documented COVID-19, acute respiratory symptoms or fever. This was an investigator-funded study, registered at ClinicalTrials.gov (NCT03987919).
Results
In March/April 2020, 1511 HCWs were enrolled. The median duration of follow-up was 357 person-days (interquartile range [IQR], 351 to 361). Unplanned absenteeism for any reason was observed in 2.8% of planned working days in the BCG group and 2.7% in the placebo group (adjusted relative risk 0.94; 95% credible interval, 0.78–1.15). Cumulative incidences of documented COVID-19 were 14.2% in the BCG and 15.2% in the placebo group (adjusted hazard ratio (aHR) 0.94; 95% confidence interval (CI), 0.72–1.24). First episodes of self-reported acute respiratory symptoms or fever occurred in 490 (66.2%) and 443 (60.2%) participants, respectively (aHR: 1.13; 95% CI, 0.99–1.28). Thirty-one serious adverse events were reported (13 after BCG, 18 after placebo), none considered related to study medication.
Conclusions
During the COVID-19 pandemic, BCG-vaccination of HCW exposed to COVID-19 patients did not reduce unplanned absenteeism nor documented COVID-19.
Keywords: BCG, COVID-19, Health care workers, Randomized controlled trial, Trained immunity
Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), represents an important cause of morbidity in healthcare workers (HCWs) caring for COVID-19 patients. Moreover, the burden of COVID-19 patients puts enormous pressures on healthcare system capacities worldwide [1]. Early in the pandemic, when the effectiveness and timely availability of effective SARS-CoV-2-specific vaccines was not yet defined, preventing HCW absenteeism by other means was imperative to safeguard patient care. Non-specific vaccines were suggested as a means to reduce the incidence and severity of COVID-19.
Bacillus Calmette-Guérin (BCG) vaccine, widely used to prevent tuberculosis, also provides non-specific effects against other infectious diseases [2]. These non-specific effects lead to a faster and more efficient innate immune response to heterologous pathogens, a process termed ‘trained immunity’ [3]. Both in vitro and in vivo beneficial effects have been observed for distinct viral pathogens, such as respiratory syncytial virus, influenza virus, and attenuated yellow fever vaccine virus [4].
We hypothesized that trained immunity induced by BCG vaccination may offer protection against COVID-19. We conducted a randomized trial to determine the efficacy of BCG vaccine to protect against all-cause unplanned absenteeism and absenteeism due to COVID-19 in HCWs during the COVID-19 epidemic in the Netherlands.
Methods
Trial design and oversight
BCG-CORONA was a parallel, double-blind, placebo-controlled randomized trial conducted in nine hospitals in the Netherlands. The study protocol was approved by the institutional review board of the University Medical Centre (UMC) Utrecht, registered at clinicaltrials.gov (Identifier: NCT04328441), and published [5]. All participants provided written informed consent. The trial was investigator-initiated and funded by the UMC Utrecht and Radboud UMC Nijmegen, the Netherlands.
Trial participants
Participants were adult (≥18 years) HCWs working in the participating hospitals or their affiliated ambulance services, with expected exposure to COVID-19 patients as part of their clinical duties. Four participating hospitals were university hospitals and five were non-university teaching hospitals. Primary exclusion criteria were known allergy to BCG, active or latent Mycobacterium tuberculosis infection, any other active infection, immunocompromised state, and current or planned pregnancy. The diagnosis of latent M. tuberculosis infection was judged by the principal investigator. A positive tuberculin skin test after previous BCG vaccination or a positive interferon gamma release assay test or tuberculin skin test after a fully treated latent or active M. tuberculosis infection were excluded from this definition. A full list of eligibility criteria is provided in Table S1.
Procedures
Participants were randomized to BCG or placebo (1:1) using a computer-generated dynamic randomization algorithm, stratified for participating hospital in random blocks of 2, 4, or 6 sequences. Participants received either 0.1 mL of the Danish strain 1331, Statens Serum Institut (SSI), Denmark, equivalent to 0.075 mg attenuated Mycobacterium bovis, or 0.1 mL of normal saline solution as an intradermal injection in the left upper arm.
Participants and study personnel conducting participant follow-up were blinded to treatment allocation. Study personnel preparing and administering the study vaccines were not blinded but could not influence treatment allocations or data collection. The trial statistician that conducted the interim and final analyses was not blinded throughout the trial but was not otherwise involved in trial conduct.
Participants used a mobile phone application (Research Follow App, Your Research BV, Huizen, The Netherlands) to complete daily questionnaires regarding respiratory and systemic infection symptoms, adverse events, absenteeism, and weekly questionnaires regarding SARS-CoV-2 exposures, testing and healthcare visits. Push notifications and emails were sent if participants did not complete the questionnaire for seven consecutive days. If someone did not respond to this, study personnel contacted them by telephone. After 6 months of follow-up, the daily questionnaire was integrated into a weekly questionnaire to improve user convenience.
Efficacy assessments
The primary endpoint was the number of days of unplanned absenteeism for any reason, reported as the average proportion of sick-days out of planned work days. Secondary endpoints are listed in Table S2.
As part of routine hospital infection prevention procedures, HCWs were urged to stay at home in case of symptoms (e.g. respiratory symptoms or fever), were offered nasopharyngeal polymerase chain reaction (PCR) testing for SARS-CoV-2 free-of-charge at the hospital where they worked, and were allowed to return to work when cleared by a hospital-affiliated occupational physician. HCWs also had access to PCR tests or rapid antigen test free-of-charge at public health service test sites. In symptomatic HCWs, negative antigen tests were confirmed with a PCR test. From 1 December 2020 onwards, HCWs identified as a close contact of an index case were also tested.
Documented COVID-19 required self-reporting of any respiratory tract symptom (cough, dyspnea, nasal cold, sore throat, or loss of smell or taste of any reported severity) or fever (body temperature ≥38.0°C) within 7 days before or up to 14 days after a positive SARS-CoV-2 PCR or rapid antigen test. From participants in the UMC Utrecht, we verified the self-reported COVID-19 with the COVID-19 PCR results in the hospital-affiliated laboratory. Unplanned absenteeism because of documented COVID-19 was counted from the first day that the participant reported symptoms of any severity prior to the test date to the first day at work after the test date. If the HCW had reported symptoms for more than 7 days prior to the test date, the start date was fixed at 7 days prior to the test date. Presence of fever required a self-measured body temperature of ≥38.0°C and respiratory symptoms required the presence of cough or dyspnea with a severity score of ≥2 (mild symptoms), or the presence of nasal congestion or sore throat with a severity score of ≥3 (moderate symptoms).
Safety assessments
Participants were instructed throughout to contact the study team in case of (serious) adverse events. Participants that reported a hospital admission in the mobile phone application were contacted by the research team. Known systemic and local injection site side effects of BCG vaccination were solicited during the first 7 days after injection, and local side effects again at the end of the trial.
Statistical analysis
The primary endpoint was analyzed as total counts (i.e. one total count per participant) using a Bayesian negative binomial regression and corrected for participant baseline characteristics. Secondary count endpoints (e.g. number of days reporting symptoms) were analyzed using maximum likelihood estimation (frequentist model) and are reported as relative risk (RR) with 95% confidence interval (CI), and secondary time-to-event endpoints (e.g. incidence of COVID-19) using Cox-proportional hazard models reporting hazard rations (HR) and 95% CIs, with adjustment for the same baseline participant characteristics as the primary analysis. COVID-19 related absenteeism was analyzed using the same method as the primary endpoint.
Interim analyses were performed as described for the primary outcome and were performed biweekly from weeks 4 to 26 and monthly from week 26 to study end (March 2021). Unblinded results of the interim analysis and incidence of (serious) adverse events were reported to the data safety monitoring board, once per month from week 4 to 26 and once per two months from week 26 to study end.
Data were analyzed using the R statistical package v3.6.3 [6]. The full statistical analysis plan and sample size calculation are provided in Table S3.
Results
Participants
From 24 March 2020 to 23 April 2020, 1511 participants were randomized (Fig. S1). Follow-up ended on 27 March 2021, and the database was locked on 18 April 2021. The median duration of follow-up was 358 person-days (interquartile range [IQR] 351–361) in the BCG group and 355 (IQR 351–361) in the placebo group. The proportions of follow-up days missing were 2% (IQR 0–7) and 1% (IQR 0–9), respectively.
The BCG and placebo groups were well balanced (Table 1 , Table S4–S5). During follow-up, 578 participants in the placebo group vs. 593 in the BCG group reported having been tested for COVID-19 for a total of 1648 and 1680 tests, respectively. The median number of days between testing and reporting was 5 (IQR 3æ8) in both intervention groups and not different between the first and second 6-month period. From January 2021 onwards, 439 participants reported to have received at least one dose of a COVID-19 vaccine, mostly the BioNtech/Pfizer vaccine, which was equally balanced between groups (Fig. S2, Table S6).
Table 1.
Baseline characteristics of participants receiving placebo or BCG
| Placebo | BCG | Missing (%) | |
|---|---|---|---|
| N | 758 | 753 | — |
| Age (y), mean (SD) | 42.8 (12.7) | 41.3 (12.6) | 0.0 |
| Female sex, n (%) | 550 (72.6) | 572 (76.0) | 0.0 |
| History of BCG vaccination, n (%) | 127 (16.8) | 129 (17.1) | 0.1 |
| Age (y) at BCG vaccination, median (IQR) | 19.0 [17.0, 22.0] | 19.0 [18.0, 23.0] | 83.2 |
| Time (y) since BCG vaccination, mean (SD) | 34.0 (13.9) | 32.5 (13.8) | 83.2 |
| History of positive tuberculin skin test, n (%) | 4.4 | ||
| No; never tested | 139 (19.1) | 158 (22.0) | — |
| No; at least one negative test | 510 (70.2) | 502 (70.0) | — |
| Yes | 78 (10.7) | 57 (7.9) | — |
| History of positive interferon gamma release assay test, n (%) | 2.5 | ||
| No; never tested | 680 (91.5) | 667 (91.4) | — |
| No; at least one negative test | 58 (7.8) | 59 (8.1) | — |
| Yes | 5 (0.7) | 4 (0.5) | — |
| Use of anti-hypertensive medication, n (%) | 50 (6.6) | 49 (6.5) | 0.0 |
| History of cardiovascular disease, n (%) | 19 (2.5) | 15 (2.0) | 0.0 |
| Use of anti-diabetic medication, n (%) | 6 (0.8) | 3 (0.4) | 0.0 |
| History of asthma, n (%) | 47 (6.2) | 54 (7.2) | 0.0 |
| History of other pulmonary diseases, n (%)b | 18 (2.4) | 14 (1.9) | 0.0 |
| Department of employment, n (%) | 0.0 | ||
| Internal medicine | 128 (16.9) | 125 (16.6) | — |
| Surgery | 108 (14.2) | 94 (12.5) | — |
| Intensive care | 67 (8.8) | 57 (7.6) | — |
| Radiology | 49 (6.5) | 47 (6.2) | — |
| Pulmonology | 40 (5.3) | 49 (6.5) | — |
| Emergency unit | 47 (6.2) | 40 (5.3) | — |
| Othera | 319 (42.1) | 341 (45.3) | — |
| (Planned) work on Corona-dedicated department, n (%) | — | — | 0.0 |
| Yes | 481 (63.5) | 481 (63.9) | — |
| No | 219 (28.9) | 226 (30.0) | — |
| Unknown | 58 (7.7) | 46 (6.1) | — |
| Position at department of employment, n (%) | — | — | 0.0 |
| Nurse | 367 (48.4) | 363 (48.2) | — |
| Medical doctor | 185 (24.4) | 172 (22.8) | — |
| Paramedical | 107 (14.1) | 123 (16.3) | — |
| Supportive personnel | 60 (7.9) | 66 (8.8) | — |
| Secretary | 39 (5.1) | 29 (3.9) | — |
| Average number of working days per week in the hospital, median (IQR) | 4.0 [3.5, 4.5] | 4.0 [3.5, 4.5] | 0.0 |
| Previously tested for COVID-19, n (%) | — | — | 0.0 |
| No | 621 (81.9) | 587 (78.0) | — |
| Yes, negative test result | 136 (17.9) | 165 (21.9) | — |
| Yes, positive test result | 1 (0.1) | 1 (0.1) | — |
BCG, bacillus Calmette-Guérin; IQR, interquartile range; SD, standard deviation.
Other departments: Pediatrics (72), Cardiology (69), Facility service (64), Miscellaneous other (61), Anesthesiology (51), Dialysis (42), Gynaecology (38), NA (38), Ambulance service (31), Acute care ward (28), Neurology (20), Medium care (20), Mental health care (19), Ophthalmology (17), Gastro-enterology (16), Revalidation (16), Infectious diseases (14), Endoscopy (13), Pharmacy (12), Dermatology (10), Day treatment (6), Physiotherapy (3).
Obstructive pulmonary disease, Interstitial pulmonary disease, Bronchiectasis, Pulmonary embolism, OSAS.
Efficacy
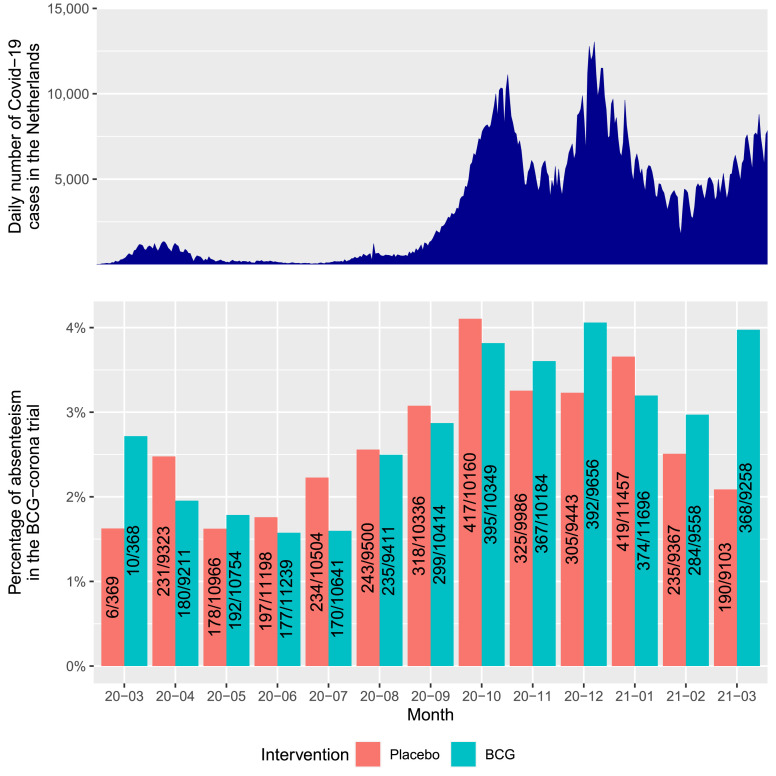
Participants reported 6741 days of unplanned absenteeism due to illness out of a total of 244 451 planned workdays (2.8%). Absenteeism was 3443/122 739 days (2.8%) in the BCG group and 3298/121 712 days (2.7%) in the placebo group (adjusted RR [aRR] 0.94; 95% credible interval (CrI), 0.78–1.15) (Table 2 , Fig. 1 ).
Table 2.
Self-reported primary and secondary outcomes
| Placebo (n = 758) | BCG (n = 753) | Crude effect | Adjusted effecta | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Days of unplanned absenteeism for any reason/planned workdays, work loss/planned work days (%) | 3298/121 712 (2.7) | 3443/122 739 (2.8) | RR 1.04 (0.85–1.25)b | RR 0.94 (0.78–1.15)b |
| Sensitivity analysis excluding observations after COVID-19 vaccination | 3161/114 226 (2.8) | 3216/115 177 (2.8) | RR 1.01 (0.83–1.23) | RR 0.92 (0.76–1.13) |
| Secondary endpoints | ||||
| Documented COVID-19, n (cum inc) | 108 (15.2%) | 102 (14.2%) | HR 0.94 (0.7221.23) | HR 0.94 (0.72–1.24) |
| COVID-19-related hospitalization | 2 | 1 | HR (95% CI) | HR (95% CI) |
| Cumulative incidence | 0.3% | 0.1% | 0.50 (0.05–5.48) | 0.32 (0.03–3.59) |
| Acute respiratory symptoms and/or fever | 443 | 490 | HR (95% CI) | HR (95% CI) |
| Cumulative incidence | 60.2% | 66.2% | 1.17 (1.03–1.33) | 1.13 (0.99–1.28) |
| Days of unplanned absenteeism because of documented COVID-19, n | 939/121 712 | 834/122 739 | RR (95% CI) | RR (95% CI) |
| Percentage | 0.8% | 0.7% | 0.89 (0.55–1.44) | 0.70 (0.47–1.05) |
Effect estimates are reported with 95% CrI (primary endpoint) or CI (secondary endpoints). CI, confidence interval; CrI, credible interval; cum inc, cumulative incidence, assuming uninformative censoring; HR, hazard ratio; RR, relative risk.
Adjusted for hospital, enrolment week (categorical), age, whether the participant was scheduled to work on a COVID-19 ward, having taken sick-leave between 1 January and 15 March, 2020 (the period just prior to trial commencement), and presence of at least one of four comorbidities (cardiovascular disease, diabetes, asthma, pulmonary diseases). Age and prior sick leave (as a percentage of planned work days) were modelled using a spline function.
The 99% CrI was 0.72–1.22; posterior probability for superiority: 0.7197; posterior probability for futility: 0.9493. The posterior probability for superiority had to be > 0.995 to declare superiority. The posterior probability for futility had to be > 0.990 to declare futility. # Robust standard errors were used for the endpoint COVID-19 related work loss because of violation of the distribution assumption.
Fig. 1.
Overview of unplanned absenteeism over the duration of the study in comparison to the number of reported positive severe acute respiratory syndrome coronavirus 2 tests per day in the Netherlands. The x-axis shows the year and month, with the red and green bars showing the overall percentage of unplanned absenteeism for the placebo and Bacillus Calmette-Guérin groups on the y-axis. The numbers in the bars represent the days of unplanned absenteeism and the total workdays planned for all participants in the respective month. The above graph provides the number of positive severe acute respiratory syndrome coronavirus 2 tests per day in the Netherlands between 01-Mar-2020 and 31-Mar-2021 (source: https://ourworldindata.org/coronavirus/country/netherlands, date assessed 20 May 2022). Please note that testing was not yet widely available in the community during the first epidemic wave in the Netherlands in March/April 2020, and the figures provided in the top graph for those months are therefore not reliable.
Unplanned absenteeism due to documented COVID-19 occurred in 816/122 739 planned workdays (0.7%) in the BCG group and 899/121 712 days (0.7%) in the placebo group (aRR 0.70; 95% CrI, 0.42–1.17). Of the total number of unplanned absenteeism days, 1715/6741 (25.4%) were attributable to documented COVID-19 (816 [12.1%]) in the BCG group and 899 [13.3%] in the placebo group). And 1776/6741 unplanned absenteeism days (26.3%) were due to quarantine with the participant reporting being physically capable of working, not including isolation due to COVID-19—867/3443 (25.2%) in the BCG group and 909/3298 (27.6%) in the placebo group.
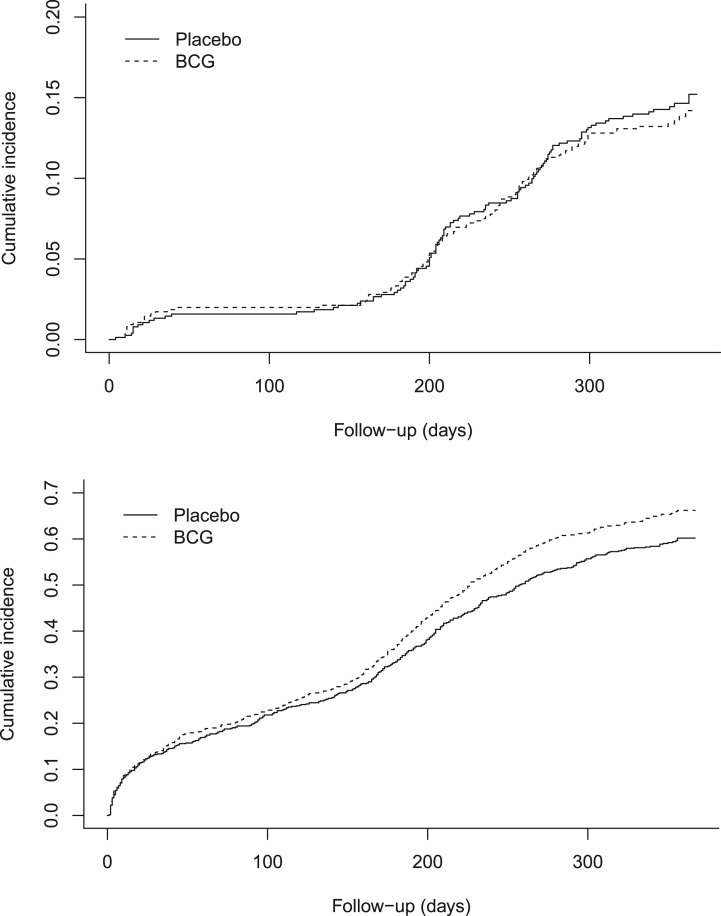
Documented COVID-19 during the follow-up period was reported by 108 (14.2%) participants in the BCG group and 102 (15.2%) in the placebo group (aHR 0.94; 95% confidence interval (CI), 0.72–1.24). In a sensitivity analysis with censoring at first COVID-19 vaccine, 101 (15.4%) participants in the BCG group and 92 (13.3%) in the placebo group had documented COVID-19 (aHR 0.90; 95% CI, 0.68–1.19). First episodes of self-reported acute respiratory symptoms or fever occurred in 490 (66.2%) participants in the BCG group and 443 (60.2%) participants in the placebo group (aHR 1.13; 95% CI, 0.99–1.28). Cumulative incidences over time are shown in Fig. 2 . Only three participants were hospitalized due to COVID-19, one in the BCG group and two in the placebo group.
Fig. 2.
Cumulative incidences of documented COVID-19 (above) and first episode self-reported acute respiratory symptoms or fever (below). COVID-19 is defined as PCR-based or rapid antigen based detection of severe acute respiratory syndrome coronavirus 2 in a respiratory sample in combination with respiratory tract symptoms (cough dyspnoea, nasal cold, sore throat, or loss of smell or taste of any reported severity) or fever (body temperature ≥38.0 °C) in the period 7 days before and up to 14 days after the positive test. Self-reported acute respiratory symptoms are defined as the presence of cough or dyspnoea with a severity score of 2 or higher, or the presence of nasal cold or sore throat with a severity score of 3 or higher. BCG, bacillus Calmette-Guérin.
In post-hoc analyses, results were consistent among subgroups of participants who had prior BCG-vaccination and/or a positive tuberculin skin reaction test, or in subgroups of participants aged below and above 50 years. Fig. S3 to S10 and Tables S7 to S12 provide the outcomes of the exploratory and post-hoc analyses results.
The cumulative incidence of documented COVID-19 and absenteeism rate were relatively low in HCWs affiliated to the intensive care unit (ICU) and high in HCWs affiliated to the internal medicine, pulmonology, and emergency ward (Table S13).
In the affiliated laboratories of the UMC Utrecht and the Noordwest Ziekenhuisgroep Alkmaar, covering 122 of 224 documented COVID-19 cases, 9 participants had positive COVID-19 PCR tests that were not reported in the mobile application and 3 participants had negative COVID-19 PCR tests, registered as positive in the mobile application. A sensitivity analysis of these laboratory-confirmed data did not lead to different results (Table S14).
Safety
Solicited (within 7 days) and unsolicited adverse events were higher in the BCG group than in the placebo group, and consisted mainly of pain and local skin reactions at the injection site (Table 3 ). At the end of the study, 18.0% of the participants in the placebo group had reported any complaints at the injection site, compared to 90.6% of the participants receiving BCG.
Table 3.
Adverse and serious adverse events
|
N |
Placebo |
BCG |
|---|---|---|
| 758 | 753 | |
| Unsolicited AE | ||
| Any AE, n (%) | 25 (3.3) | 83 (11.0) |
| Blood or lymphatic disorders, n (%) | 0 (0) | 0 (0) |
| Cardiac disorders, n (%) | 1 (0.1) | 0 (0) |
| Endocrine disorders, n (%) | 1 (0.1) | 1 (0.1) |
| Gastrointestinal disorders, n (%) | 5 (0.7) | 3 (0.4) |
| General disorders and administration site conditions, n (%) | 3 (0.4) | 59 (7.8) |
| Infection, n (%) | 0 (0) | 0 (0) |
| Musculoskeletal and connective tissue disorders, n (%) | 0 (0) | 11 (1.5) |
| Nervous system disorders, n (%) | 4 (0.5) | 5 (0.7) |
| Respiratory disorders, n (%) | 4 (0.5) | 2 (0.3) |
| Urologic and gynecological disorders, n (%) | 7 (0.9) | 1 (0.1) |
| Solicited adverse events at seven days | ||
| Fever, n (%) | 4 (0.5) | 8 (1.1) |
| Myalgia, n (%) | 46 (6.1) | 83 (11.0) |
| Fatigue, n (%) | 141 (18.6) | 180 (23.9) |
| Pain at injection sit, n (%) | 46 (6.4) | 655 (91.1) |
| Serious adverse events (SAE) | ||
| Any SAE, n (%) | 18 (2.4) | 13 (1.7) |
| Hospitalization for COVID-19, n (%) | 2 (0.3) | 1 (0.1) |
| Blood or lymphatic disorders, n (%) | 0 (0) | 1 (0.1) |
| Cardiac disorders, n (%) | 2 (0.3) | 2 (0.4) |
| Endocrine disorders, n (%) | 0 (0) | 0 (0) |
| Gastrointestinal disorders, n (%) | 3 (0.4) | 2 (0.3) |
| General disorders and administration site conditions, n (%) | 0 (0) | 0 (0) |
| Infection, n (%) | 1 (0.1) | 1 (0.1) |
| Musculoskeletal and connective tissue disorders, n (%) | 5 (0.7) | 0 |
| Nervous system disorders, n (%) | 2 (0.3) | 2 (0.3) |
| Respiratory disorders, n (%) | 1 (0.1) | 0 (0) |
| Urologic and gynecological disorders, n (%) | 2 (0.3) | 4 (0.5) |
| Outcomes of SAE | ||
| Congenital anomaly or birth defect, n (%) | 1 (0.1) | 0 (0) |
| Life threatening or required admission to ICU, n (%) | 0 (0) | 1 (0.1) |
| Required hospitalization or prolongation of hospitalization, n (%) | 17 (2.2) | 11 (1.5) |
| Resulted in death, n (%) | 0 (0) | 1 (0.1) |
AE, Adverse events; BCG, Bacillus Calmette-Guérin; ICU, intensive care unit; SAE, Serious Adverse Event.
In total, 31 serious adverse events occurred; 13 in the BCG and 18 in the placebo group, including one participant in the BCG group who died as a complication of chemotherapy. None of these were considered to be related to BCG vaccination. Tables S15 to S18 summarize all (serious) adverse events.
Discussion
In this multicenter double-blind placebo-controlled trial, BCG vaccination did not reduce unplanned absenteeism due to any cause in HCWs. In addition, BCG vaccination failed to reduce the incidence of documented COVID-19, or acute respiratory symptoms or fever. Our findings corroborate those from a similarly designed study in elderly in the Netherlands, also performed during the COVID-19 pandemic, described in an accompanying manuscript [5].
The trial started during the peak of the first epidemic wave in the Netherlands in March 2020 and was completed during the third epidemic wave in March 2021. The percentage of unplanned absenteeism ran parallel to the COVID-19 incidence. However, only a fraction of unplanned absenteeism (25.4%) was attributable to documented COVID-19. A similar fraction (26.3%) was a consequence of being quarantined or isolated while physically capable of working. The aim of the trial was to ensure the continuity of health care during the COVID-19 pandemic. Therefore, the primary endpoint was unplanned absenteeism by any cause which captured all potential beneficial effects of BCG vaccination, while secondary endpoints, such as documented COVID-19, were explanatory.
BCG vaccination has been associated with lower child mortality in low-income countries, and with a reduced incidence of respiratory infections, mainly from presumed viral origin, in patients who had been discharged from an intensive care unit in in Greece [[7], [8], [9]]. There was considerable in vitro evidence that BCG augments the innate immune response in a non-specific manner [10]. There was a worldwide interest in BCG potentially offering partial protection against COVID-19. Some retrospective and ecological studies from countries with differing BCG vaccination coverages suggested a protective effect of BCG [11], whereas others did not [12]. These are, however, inherently limited by confounding and other biases. Randomized controlled trials were required to resolve the question whether BCG protects against COVID-19 [13]. Unfortunately, our trial showed that BCG vaccination does not alter the incidence of COVID-19 in HCWs in a high-income European country.
Our trial had several study limitations. One of the inclusion criteria was ‘hospital personnel expected to provide care to patients with COVID-19’, while among 30% of the included participants did not expect to work on a COVID-19 department. Even if this hospital personnel does not work on a COVID-19 department, they may still have provided care for COVID-19 patients, e.g. radiology personnel involved in Computer Tomography (CT) scanning. No structural differences existed between the incidence of documented COVID-19 and the absenteeism rate between COVID-19 departments and other departments. The choice for self-reporting of symptoms and COVID-19 test results by participants carried a risk of under-detection and/or misclassifications of endpoints. However, COVID-19 PCR test results from two hospitals corresponded well with self-reported results in the mobile application. Sending push notifications and email alerts, and the adjustment to the weekly instead of daily self-reporting of symptoms, provided a risk of recall bias. This risk is probably lower for absenteeism and COVID-19 test results. Blinding was only partially possible due to the local injection site reactions that occur after BCG vaccination in most individuals. On the other hand, this did not result in a noticeable differential loss to follow-up, uptake of COVID-19 testing, or uptake of COVID-19 specific vaccination.
Our trial population of fit adults aged ≤65 years experienced few serious COVID-19 infections that resulted in hospitalization, let alone ICU admission or death. To determine the effect of BCG on severe endpoints, inclusion of older and more vulnerable participants is required. The ACTIVATE-2 randomized trial found a significantly reduced incidence of COVID-19 after BCG in frail elderly [14]. Parallel to the BCG-CORONA trial, we conducted two additional trials in elderly, the BCG-CORONA-ELDERLY trial [15], and the BCG-PRIME trial (NCT04537663). Several additional BCG trials with COVID-19 endpoints are ongoing around the world, including the BRACE study (NCT04327206), and a real-time individual data meta-analysis is being conducted to monitor the effect of BCG on severe endpoints (PROSPERO ID: CRD42021213069).
In conclusion, this trial found no effect of BCG vaccination on unplanned absenteeism or documented COVID-19 among HCWs during the SARS-CoV-2 pandemic in the Netherlands.
Transparency declaration
There were no conflicts of interest. No external funding was received.
Author contributions
TD is corresponding author. TD, TV, PD, ET, SM, JW, MN, MB and CW contributed to the design of the study. TD, TV, JW and HW did the data extraction and analysis. Writing – Original Draft: TD and TV. Writing – Review & Editing: TD, TV, PD, ET, SM, NP, WB, VK, AR, BR, AV, KV, AK, JO, RC, CN, JW, MN, MB and CW; Conceptualization: TD, TV and CW; All authors read and approved the final version. TD, TV and CW had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We would like to thank the health care workers for their participation. We gratefully acknowledge the help of all colleagues in the participating hospitals who helped carry out this randomized trial under difficult circumstances. A special thanks goes out to project manager Katina Kardamanidis, the data managers Frank Leus and Roxanne Schaakx, the members of the Data Safety Management Board, infectious diseases specialist dr. Jan-Jelrik Oosterheert, medical microbiologist dr. Miquel Ekkelenkamp and medical statistician Dr. Maarten van Smeden and to the independent expert of the trial, infectious disease specialist Prof. Dr. Andy Hoepelman.
Editor: L. Scudeller
Footnotes
Thijs ten Doesschate and Thomas W. van der Vaart contributed equally to this work.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.04.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/s2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Netea M.G., Joosten L.A.B., Latz E., Mills K.H.G., Natoli G., Stunnenberg H.G., et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Ten Doesschate T., Moorlag S.J.C.F.M., van der Vaart T.W., Taks E., Debisarun P., Oever J.T., et al. Two randomized controlled trials of Bacillus Calmette-Guérin Vaccination to reduce absenteeism among health care workers and hospital admission by elderly persons during the COVID-19 pandemic: a structured summary of the study protocols for two randomised. Trials. 2020;21:481. doi: 10.1186/s13063-020-04389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 7.Biering-Sørensen S., Aaby P., Napirna B.M., Roth A., Ravn H., Rodriguez A., et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guéerin vaccination at first health center contact. Pediatr Infect Dis J. 2012;31:306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen I., Aaby P., Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. Br Med J. 2000;321:1435–1438. doi: 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.D., et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 10.Netea M.G., Giamarellos-Bourboulis E.J., Dominguez-Andres J., Curtis N., van Crevel R., van de Veerdonk, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urashima M., Otani K., Hasegawa Y., Akutsu T. BCG vaccination and mortality of covid-19 across 173 countries: an ecological study. Int J Environ Res Public Health. 2020;17:5589. doi: 10.3390/ijerph17155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323:2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopalaswamy R., Ganesan N., Velmurugan K., Aravindhan V., Subbian S. The strange case of BCG and COVID-19: the verdict is still up in the air. Vaccines (Basel) 2020;8:612. doi: 10.3390/vaccines8040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID19 in individuals at risk. medRxiv. 2021 doi: 10.1101/2021.05.20.21257520. Epub ahead of print. Accessed 10 March 2022. [DOI] [Google Scholar]
- 15.Moorlag S., Taks E., Doesschate T.T., van der Vaart T.W., Janssen A.B., Muller L., et al. Efficacy of Bacillus Calmette-Guérin vaccination against respiratory tract infections in the elderly during the COVID-19 pandemic. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac182. Epub ahead of print. Accessed 10 Sept 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.