Abstract
Objective
To determine visual outcomes and prevalence of amblyogenic risk factors in children with Apert, Crouzon, Pfeiffer and Saethre-Chotzen syndromes.
Methods
We conducted a single-centre, retrospective chart review of patients assessed at our unit between October 2000 and May 2017. Our outcome measures were as follows: age at first and last examination, refraction, horizontal ocular alignment, alphabet pattern deviations, anterior segment appearance, fundus examination findings, visual evoked potentials (VEPs) and genetics. The study’s primary endpoint was the proportion of children achieving best-corrected visual acuity (BCVA) ≥ 6/12 in the better eye at final visit, as per UK driving standards.
Results
165 patients were included in this study. Breakdown of diagnoses was as follows: Crouzon (n = 60), Apert (n = 57), Pfeiffer (n = 14) and Saethre-Chotzen (n = 34). 98 patients were male. Of 133 patients with full BCVA data available, 76.7% achieved BCVA ≥ 6/12 in the better eye. Of 122 patients, anisometropia >1.00 dioptre sphere (DS) affected 18.9% and astigmatism ≥1.00DS in at least one eye affected 67.2%. Of 246 eyes, 48.4% had oblique astigmatism. Of 165 patients, 60 had exotropia and 12 had esotropia. 48 of 99 patients demonstrated ‘V’ pattern. On multivariable logistic regression, nystagmus (p = 0.009) and ON involvement (p = 0.001) were associated with decreased vision in the worse eye. Normal VEPs were associated with better BCVA (p = 0.036).
Conclusion
There was a high prevalence of amblyogenic factors, however, the majority achieved BCVA ≥ 6/12 in their better eye. Optic neuropathy and nystagmus had the most significant impact on vision. VEPs can help the in overall assessment of visual function.
Subject terms: Eye abnormalities, Paediatrics, Risk factors, Eye manifestations
Introduction
Craniosynostosis is characterised by the premature fusion of one or more cranial sutures. It is classified as syndromic when a primary defect in ossification results in other associated systemic bony anomalies. Apert, Crouzon, Pfeiffer and Saethre-Chotzen syndromes are four common craniosynostosis syndromes, all inherited in an autosomal dominant manner. The former three result from mutations in the genes encoding fibroblast growth factor receptors (FGFR) 1, 2 and 3, while Saethre-Chotzen syndrome is caused by a mutated TWIST1 gene [1–3].
Visual compromise can occur in these patients due to raised intracranial pressure and optic pathway pathology, exposure keratopathy and amblyopia secondary to strabismus, astigmatism, ametropia and anisometropia [1–3]. Thus, patients attending Great Ormond Street Hospital for Children (GOSH) undergo ophthalmological monitoring as part of a multidisciplinary approach involving neurosurgery, craniofacial surgery, otolaryngology, speech and language therapy, audiology, psychology and genetics.
A previous study was conducted by Khan et al. at this unit, evaluating results from 141 children with Apert, Crouzon, Pfeiffer and Saethre-Chotzen syndromes seen in the craniofacial unit between October 1979 and October 2000 [4]. Khan et al. found 39.8% of patients (45 of 113) having visual acuity of 6/12 or worse in their better eye [4].
Liasis et al. subsequently demonstrated the utility of visual electrodiagnostic testing (EDT) in detecting sustained raised intracranial pressure [5, 6]. Furthermore, Thompson et al. highlighted EDTs as a tool for monitoring visual function [7, 8].
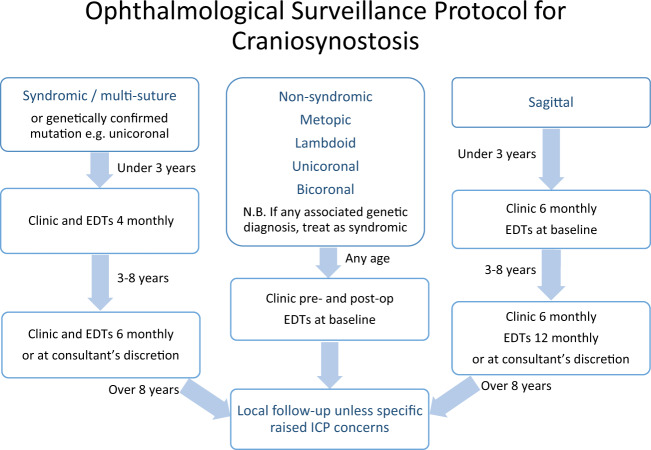
In light of these findings, our unit developed a formal surveillance protocol (Fig. 1), starting young and with regularly timed EDTs and ophthalmic examinations. This was coupled with aggressive corneal protection, squint surgery as needed and intensive amblyopia therapy.
Fig. 1. Ophthalmological surveillance protocol for craniosynostosis.
There are three separate streams for syndromic/multi-suture, non-syndromic and saggital craniosynostosis. EDTs = electrodiagnostic tests; ICP = intracranial pressure.
This study aimed to evaluate visual outcomes, electrophysiological outcomes and amblyogenic risk factors in children with syndromic craniosynostosis under our surveillance protocol.
Methods
Study design and participants
This study was a single-centre, retrospective chart review of 165 new patients with a confirmed diagnosis of Apert, Crouzon, Pfeiffer or Saethre-Chotzen syndrome seen in the GOSH Craniofacial Unit between October 2000 and May 2017. This study was approved as a retrospective chart review by the GOSH Clinical Audit Team.
Inclusion criteria were as follows: diagnosis of Apert, Crouzon, Pfeiffer or Saethre-Chotzen syndrome; age 0–16 at first presentation at referral to GOSH Craniofacial Unit; referral year 2000 onwards. Exclusion criteria were as follows: other diagnoses of syndromic craniosynostosis; non-syndromic craniosynostosis; age above 16 at referral to GOSH Craniofacial Unit; referral year 1999 or earlier. The GOSH craniofacial patient database was searched for all children with the above-mentioned diagnoses and data were extracted from the electronic patient records.
Outcome measures
Demographic data included age at first and last ophthalmology review and gender. Best corrected visual acuity (BCVA) at last visit (recorded using the Snellen system in metres), refraction, horizontal ocular alignment at first and last review, pattern deviations, anterior segment appearance and fundus examination findings at the last visit were recorded. Outcomes were compared with results from a previous study from the same unit, the study by Khan et al. [4]. Results of genetic testing and electrophysiological tests were also extracted.
The study’s primary endpoint was the proportion of children achieving BCVA better than 6/12 in the better eye at final visit, as per UK driving standards [9].
The definitions of oblique and rule obeying astigmatism were based on criteria from Denis et al. [10] (criterion 1): ‘rule obeying’ was defined as horizontal axis readings of between 175 and 5 degrees inclusively and vertical axis readings between 85 and 95 degrees inclusively. These were the criteria used by Khan et al. Another more common definition of oblique astigmatism from Abrahamsson et al. [11] was also used (criterion 2) where axes +/− 15 degrees from the main axes (90 and 180 degrees) were considered rule obeying.
Visual evoked potentials (VEP)
VEP, in the form of pattern reversal and flash VEP data, were recorded with both eyes open, representing bilateral pathway function. Pattern reversal visual evoked potential (pVEP) was qualitatively ranked according to the smallest check width that produced a consistent VEP. The ‘SALT’ analysis, which integrates VEP waveform Shape, Amplitude, Latency and Trans-occipital asymmetry, was used for each patient. The categories of vision level reported were good, good to moderate, moderate to good, moderate and poor to moderate. A pVEP to the ISCEV VEP standard large check 50’ which fell within the normal reference range was ranked as good. Macular pathway dysfunction was present when any of the measured parameters of a pattern VEP fell outside the normal reference range and this could include patients with any of the above grades of vision.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 2014. The Chi-squared test was used to assess whether the proportion of children achieving driving BCVA better than 6/12 in the better eye was significantly different to that previously reported in our unit by Khan et al. [4]. When looking at factors affecting visual acuity, better and worse eyes were analysed separately. Potential predictors of visual outcome including age, motility, refraction, nystagmus and optic nerve involvement were investigated pairwise and then significant predictors were entered into a multivariate model using VA as a linear outcome. For calculating percentages of eyes with greater than 6/12 acuity, non-quantitative acuity data such as ‘fixing and following’ (F + F), ‘not fixing and following’ and perception of light (PL) were treated as worse than 6/12. Non-quantitative data, including F + F and PL, were excluded from linear modelling calculations.
Results
Demographic data
The case notes of 165 children were analysed. Of these children, 98 were male (59.0%). Mean age at presentation was 17 months (S.D.: 25 months; range: 1–141 months). Mean follow up was 6 years (S.D.: 4 years; range: 1–15 years) and there was no significant difference among the groups (p = 0.63). Mean age at last follow-up was 95 months (S.D.: 51 months, range: 2–207 months). The distributions and differences between the four craniosynostotic syndromes are shown in Table 1.
Table 1.
Demographic and clinical data per syndrome.
| Diagnosis (n) | Apert (57) | Crouzon (60) | Pfeiffer (14) | Saethre-Chotzen (34) |
|---|---|---|---|---|
| Mean age at presentation, months (95% CI) | 10 (5–15) | 26 (19–34) | 9 (4–15) | 17 (9–25) |
| Mean final VA better eye (95% CI) | 0.30 (0.22–0.38) | 0.12 (0.05–0.18) | 0.19 (0.02–0.36) | 0.14 (−0.04–0.33) |
| Mean final VA worse eye (95% CI) | 0.54 (0.41–0.67) | 0.21 (0.13–0.30) | 0.39 (0.00–0.79) | 0.24 (0.01–0.47) |
| Astigmatism ≥ 1 dioptre | 31 (54%) | 21 (35%) | 5 (36%) | 13 (38%) |
| Exotropia at presentation | 27/57 (47%) | 21/60 (35%) | 5/14 (36%) | 7/34 (21%) |
| Esotropia at presentation | 2/57 (3%) | 4/60 (7%) | 0/14 (0%) | 6/34 (18%) |
| Exotropia final follow up | 25/57 (44%) | 31/60 (52%) | 8/14 (57%) | 10/34 (29%) |
| Esotropia final follow up | 21/57 (37%) | 8/60 (13%) | 0/14 (0%) | 13/34 (38%) |
| Optic neuropathy | 15/57 (26%) | 10/60 (17%) | 3/14 (21%) | 2/34 (6%) |
CI confidence intervals.
Visual acuity
133 children had quantitative BCVA data recorded at final follow up visit. Of these, 76.7% had final BCVA better than 6/12 in the better eye, thereby meeting the UK vision standard for driving [9]. Predictors of visual outcome are shown in Table 2. Mean BCVA per syndrome were as follows: Apert: 0.59 (95% CI: 0.43–0.75); Crouzon: 0.23 (95% CI 0.14–0.32); Pfeiffer: 0.39 (95% CI: 0.00–0.79); Saethre-Chotzen: 0.22 (95% CI: 0.03–0.42).
Table 2.
Factors affecting vision in better and worse eyes.
| Factor | Better eye | Worse eye |
|---|---|---|
| Anisometropia | N | P = 0.015 |
| Magnitude of cylinder in same eye | P < 0.0005 | P < 0.0005 |
| Age last exam (years) | P = 0.001a | P = 0.022 |
| Nystagmus | P = 0.01 | P = 0.009a |
| Exotropia at presentation | N | P = 0.03 |
| Clinical optic nerve involvement | P = 0.02 | P = 0.001a |
| Type of syndrome | N | P = 0.004 |
N no significant association.
aRetained significance in multivariate model.
There was a 16.5% difference between the proportion achieving driving standard vision in this cohort (76.7%) compared to the cohort reported by reported by Khan et al. (60.2%; October 1979–October 2000; n = 141 children). This difference was statistically significant improvement (Chi-squared test, p < 0.01). However, a direct comparison of factors in clinical management is not possible due to the difference in ages at time of presentation and final follow-up.
Refraction
Refraction data were available for 122 patients. Twenty-three (18.9%) had anisometropia ≥1.00DS. Eighty-two (67.2%) had astigmatism ≥1.00DS in at least one eye.
Of 98 patients with astigmatism of any magnitude in at least 1 eye, 74 (75.5%) had oblique astigmatism (criterion 1). In all, 48.4% of 246 eyes had oblique astigmatism. This figure fell to 31.3% using criterion 2.
Ocular alignment
Of the 165 children at presentation, 60 (36%) displayed exotropia and 12 (7%) displayed esotropia. Of 99 patients for whom data on alphabet patterns were available, 51 (51.5%) had no pattern and all of the remainder demonstrated ‘V’ pattern. Of the 165 children at final follow up, 74 (45%) displayed exotropia and 42 displayed (25%) for esotropia. Of 127 children at final follow up, 45 (35.4%) had no pattern and all of the remainder demonstrated ‘V’ pattern.
Optic nerve
Out of 148 patients (296 eyes) with recorded fundus examination findings from their last visit, 30 (20.3%) patients and 54 (18.2%) eyes had clinical signs of disc swelling and/or pallor.
Cornea
280 eyes of 140 patients had data on anterior segment findings. Signs of corneal exposure were documented in 27 eyes (9.6%) of 16 patients. Eight of these had corneal scarring (2.9% overall), 5 of which involved the visual axis.
Electrophysiology
Electrophysiology data were available for 147 (89.1%) patients and are summarised in Table 3. Patients with Apert syndrome had the highest prevalence of bilateral VEP macular pathway dysfunction (83%) and patients with Saethre–Chotzen syndrome had the least (31%). Patients with Apert and Pfeiffer syndromes had the highest prevalence of bilateral poor VEP vision outcome, 47% and 43% respectively. VEP deterioration over time was noted in 11% and 12% of patients tested with Crouzon and Apert syndromes, respectively, but Crouzon patients were twice as likely to preserve better VEP visual pathway function (82% with Crouzon syndrome compared to 46% with Apert syndrome). There was correlation between final VEP results and final visual acuity such that children with normal VEPs (no macular pathway dysfunction) had significantly better VA than those with any abnormality (p = 0.036). There was a statistically significant difference between the poor to moderate group and the good group (p = 0.000), as well as between the good to moderate group (p = 0.001) and the moderate group (p = 0.001). There were 12 cases where the VEP report indicated deterioration: six with Crouzon and six with Apert syndrome. Two cases with reported deterioration had optic nerve pallor recorded at the last visit. One case had ‘blurry margins but no frank papilloedema’ recorded. Another case had ‘congested not swollen’ recorded. All others had clinically normal optic nerves on fundoscopy.
Table 3.
Visual evoked potentials data per syndrome.
| Diagnosis | Apert | Crouzon | Pfeiffer | Saethre-Chotzen | All |
|---|---|---|---|---|---|
| Proportion who underwent VEP testing (%) | 52/57 (91%) | 57/60 (95%) | 7/14 (50%) | 29/34 (85%) | 145/165 (88%) |
| Proportion with normal bilateral pathway function (%) | 9/52 (17%) | 30/57 (53%) | 4/7 (57%) | 20/29 (69%) | 63/145 (43%) |
| Bilateral pathway dysfunction (%) | 43/52 (83%) | 27/57 (47%) | 3/7 (43%) | 9/29 (31%) | 82/145 (57%) |
| Bilateral pathway dysfunction with poor vision levels (%) | 23/43 (54%) | 5/27 (19%) | 3/3 (100%) | 1/9 (11%) | 32/82 (39%) |
| Bilateral pathway dysfunction with good vision levels likely under high contrast (%) | 20/43 (46%) | 22/27 (81%) | 0/3 (0%) | 8/9 (89%) | 50/82 (61%) |
| Evidence of VEP deterioration (%) | 6/52 (12%) | 6/57 (11%) | 0/7 (0%) | 0/29 (0%) | 10/145 7% |
VEP visual evoked potentials.
Genetics
Results were available for 57 of 69 patients who had their diagnosis confirmed by genetic testing (21 Apert, 26 Crouzon and 10 Saethre–Chotzen). In the remaining 12, testing had been done at another hospital and results were not available to our unit, or the parent had a confirmed diagnosis and therefore no testing was performed on the child.
Subgroup analysis revealed that Apert patients with the p.Ser252Trp were more likely to have oblique astigmatism (p = 0.003) and trended toward being more likely to have VA worse than or equal to 6/12 in the better eye (p = 0.057).
Each of the Saethre–Chotzen patients had a different mutation in the TWIST gene.
Factors affecting vision
Magnitude of astigmatism, anisometropia, nystagmus, exotropia, younger age at last follow-up, clinical optic nerve involvement and syndrome were all significantly associated with poorer BCVA in the worse eye (Table 2). All of the same factors except anisometropia, exotropia and optic nerve involvement were significantly associated with poorer BCVA in the better eye. On multivariable logistic regression, nystagmus (p = 0.32) and optic nerve involvement (p = 0.13) remained significantly associated with poorer BCVA in the worse eye (R = 0.56, F = 4.86, p = 0.000), while younger age at last follow-up was significantly associated with poorer BCVA in the better eye (R = 0.44, F = 4.75, p = 0.002).
Discussion
To the best of our knowledge, this is the largest study of visual outcomes in children with syndromic craniosynostosis. Of 133 children, 76.7% had final BCVA better than 6/12 in the better eye, thereby meeting the United Kingdom vision standard for driving. Normal VEPs were associated with better final BCVA and abnormal VEPs have been defined per diagnostic subgroup. Amblyogenic risk factors have also been defined per diagnostic subgroup.
Comparison with other studies
The proportion of children with driving standard vision was 76.7% in this study, as compared to 60.2% reported by Khan et al. [4]. However, this difference is likely multifactorial and it is not possible to conclude that the new surveillance protocol is solely responsible for this change. For instance, mean age at final follow-up in the study by Khan et al. was 76.3 months (S.D.: 56.3; range: 5.8–287.6), compared to 95 months in the current study (S.D.: 51 months, range: 2–207 months). It may be that BCVA tested in the current study is higher as the children were older and became more adept at performing the test. On the contrary, some children may have produced higher BCVA scores when younger if visually impairing condition(s) had not yet progressed, or if they performed better using picture cards in the earlier visit compared to letters in the later visit, for example. Moreover, the mean age at first presentation in the study by Khan et al. was 23.3 months (SD 38.3; range 0.6–278.3; median 8.9) compared to 17 months in the current study (S.D.: 25 months; range: 1–141 months). Under our new surveillance protocol, these children may have afforded an enhanced ability to have earlier intervention of the various causes of visual impairment in this population.
Although this study has revealed better final BCVA, these data show that vision remains a significant cause of morbidity in these children. We found a similar or higher prevalence of amblyogenic factors such as astigmatism, anisometropia and strabismus compared with the paper by Khan et al. [4]. After identifying a mean age at first review of 23 months, Khan et al. had put forward several reasons for this delay and recommended early ophthalmology referral regardless of ophthalmic signs. The younger age at first ophthalmological review in our cohort is likely a direct result of this recommendation. Our current practice also places increased emphasis on aggressive correction of ambylogenic factors and treatment of amblyopia, if present.
Khan et al. were unable to comment on what proportion of visual loss in their cohort was from amblyopia or optic neuropathy. A key difference in the present study is that VEPs are routinely applied in younger patients, as per our surveillance protocol (Fig. 1). Thus, our ability to pick up optic neuropathy early using VEPs [4, 7] has improved since Khan’s study and may be another factor leading to the difference in visual outcomes.
A study from Australia [12] (which also included craniofrontonasal dysplasia) reported final VA worse than 6/12 in the better eye in 34.5% of 55 patients. The commonest cause of visual impairment was ametropia followed by amblyopia and optic atrophy, then exposure keratopathy and infantile nystagmus. A Dutch study [13] reported ‘severe visual loss’ found in 3% patients overall, though the definition of severe visual loss was not clear. They reported impaired sight in 61% with ‘a high prevalence in all syndromes’ but did not report specific VA acuity outcomes.
The Apert cohort in the paper by Khan et al. had a higher prevalence of esotropia than exotropia at first examination. We found the opposite, but there was a notable shift towards esotropia during follow up which might suggest merit in delaying strabismus surgery for these children. Esotropic shift has been reported following craniofacial surgery [14, 15].
Relative impact of various factors on visual acuity
Refractive factors were more prevalent than optic pathway neuropathy or scarring from exposure keratopathy but, being more readily treatable, the associated visual decrement was less likely to be persistent. Also, these factors vary in their degree of amblyogenic potential. Based on Sjostrand’s paper [16], these were, in decreasing order: form deprivation from central corneal scar, strabismus, oblique astigmatism, hypermetropia ≥3.50 DS at 1 year of age, anisometropia at 1 year of age, with-the-rule astigmatism, anisometropia at 4 years of age, astigmatism ≥ 2.00 DS and against-the-rule astigmatism. We found, instead, that the factors with the most significant association were age at last follow-up, nystagmus and optic nerve involvement – factors outside of the scope of the Sjostrand paper. However, amblyopia remains a major consideration for these children. Moreover, it may have been that the older children were more motivated or better at chart vision testing, which is particularly relevant in a population where cognitive delay is common.
It was surprising that optic neuropathy was not independently associated with poorer vision in the better eye. Generally, both optic nerves should be equally impacted by elevation in intracranial pressure. However, loss of visual acuity may lag behind clinical or electrophysiological evidence of optic neuropathy. Also, though rare, there are reports of asymmetrical disc swelling in raised intracranial pressure and this is thought to be related to compartmentation of the perioptic subarachnoid spaces [17]. Six patients in this study had unilateral optic nerve swelling or atrophy.
Limitations of our model include inability to account for which patients had patching or atropine penalisation treatment and how compliant they were with these. Neither could we account for compliance with glasses wear which may be affected by parental belief, behavioural issues, facial dysmorphism and cranio-surgical apparatus. This model used cross-sectional data looking at absolute values of refractive error at a single time point. However, changes in refractive error over time have also been shown to carry some risk of amblyopia [16] and may account for some of the unexplained variance in our model.
As might be expected, the trend was generally toward worse mean BCVA for worsening VEP level of vision. However, it is important to note that the spatial threshold of a VEP does not measure the same element as high contrast, static, recognition visual acuity. The physiological processes differ so the two are commonly discrepant if there is optic atrophy. If the remaining functioning fibres are at the macula then high contrast VA can be good, but with few fibres, the VEP will be low amplitude.
Genetics
Genotype-phenotype correlations indicate that in Apert Syndrome, p.Ser252Trp is more frequently found with cleft palate and more severe facial anomalies, whereas p.Pro253Arg is more frequently associated with severe syndactyly [1]. The former mutation also appears to be associated with more severe ophthalmic features. Jadico et al reported a significantly higher frequency of astigmatism, nasolacrimal duct obstruction (NLDO) and superior rectus under-action in S252T patients but they also had a small sample size [17]. Khong et al. found that visual impairment rates were higher in the S252T group [18]. Our S252T patients were significantly less likely to meet the driving standard.
Increasing incidence of craniosynostosis over time
In the nearly 17 years from October 2000, 165 children with these 4 craniosynostosis syndromes were seen, compared with 141 in 21 years up to October 2000. This may represent improved diagnosis of these syndromes, or a true increase in incidence, or a combination of the two. With respect to the former theory, Saethre-Chotzen syndrome in particular can have a very variable phenotype [2]. Cases of unicoronal synostosis with only mild extra-cranial features may not be recognized as syndromic, especially in the absence of molecular diagnostics [19]. However, other studies have demonstrated increasing incidence of syndromic and non-syndromic craniosynostosis over time [20, 21].
This paper carries the usual limitations expected with a retrospective study. Not all data were available for all children. Visual acuity testing methods were tailored to age and cognitive ability such that in some cases only qualitative data, such as fixing and following, were available. This paper uses scientific methods and clinical judgement to attempt to identify the most likely cause of visual impairment but this cannot be determined with certainty from retrospective methods.
Conclusion
In conclusion, there remains a high prevalence of amblyogenic factors among patients with Apert, Crouzon, Pfeiffer and Saethre-Chotzen syndromes. However, using our current intensive screening and treatment protocol, a majority of these children demonstrated better than 6/12 BCVA in their better eye, thereby meeting the UK driving standard. Normal VEPs were associated with good BCVA at final visit. Based on our model, clinical optic neuropathy, nystagmus and age at the last follow-up had the most significant association with recorded BCVA.
Summary
What was known before
Syndromic craniosynostosis is often associated with poor visual acuity.
There is a significant prevalence of amblyogenic risk factors in syndromic craniosynostosis.
What this study adds
The majority of children with syndromic craniosynostosis can achieve driving standard vision, although a significant proportion still experience visual compromise.
Optic neuropathy and nystagmus are associated with poorer visual outcomes in syndromic craniosynostosis.
Normal electrophysiology is associated with better visual outcomes in syndromic craniosynostosis.
Acknowledgements
The authors would like to thank Andrea White for assistance with case identification and data collection. SRR’s post is funded by a National Institute for Health Research (NIHR) Doctoral Fellowship. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author contributions
AMH: methodology, formal analysis, investigation, data curation, writing—original draft, visualization. DAT: methodology, formal analysis, investigation, writing—original draft, writing—review and editing, visualization. SRR: formal analysis, writing—review and editing, visualization. KW: investigation, writing—review and editing. KS: investigation, writing—review and editing. VP: investigation. GJ: writing—review and editing. RB: Conceptualization, methodology, formal analysis, investigation, writing—review and editing, supervision, project administration.
Compliance with ethical standards
Conflict of interest
The authors declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen MM Jr. Apert, crouzon, and pfeiffer syndromes. In: Muenke M, Kress W, Collmann H, Solomon BD, editors. Monographs in human genetics. Basel: Karger; 2011. vol. 19, pp 67–88. 10.1159/isbn.978-3-8055-9595-7
- 2.Kressa W, Collmann H. Saethre-chotzen syndrome: clinical and molecular genetic aspects. In: Muenke M, Kress W, Collmann H, Solomon BD, editors. Monographs in human genetics. Basel: Karger; 2011. vol. 19, pp. 98–106. 10.1159/isbn.978-3-8055-9595-7
- 3.Muenke M, Kress W, Collman H, Solomon BD. Craniosynostoses: molecular genetics, principles of diagnosis, and treatment. Basel, Switzerland: S. Karger; 2011
- 4.Khan SH, Nischal KK, Dean F, et al. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003;87:999–1003. doi: 10.1136/bjo.87.8.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liasis A, Thompson DA, Hayward R, et al. Sustained raised intracranial pressure implicated only by pattern reversal visual evoked potentials after cranial vault expansion surgery. Pediatr Neurosurg. 2003;39:75–80. doi: 10.1159/000071318. [DOI] [PubMed] [Google Scholar]
- 6.Liasis A, Nischal KK, Leighton S, et al. Adenoid-tonsillectomy to treat visual dysfunction in a child with craniosynostosis. Pediatr Neurosurg. 2005;4:197–200. doi: 10.1159/000086561. [DOI] [PubMed] [Google Scholar]
- 7.Thompson DA, Liasis A, Hardy S, et al. Prevalence of abnormal pattern reversal visual evoked potentials in craniosynostosis. Plast Reconstr Surg. 2006;118:184–92. doi: 10.1097/01.prs.0000220873.72953.3e. [DOI] [PubMed] [Google Scholar]
- 8.Liasis A, Nischal KK, Walters B, et al. Monitoring visual function in children with syndromic craniosynostosis: a comparison of 3 methods. Arch Ophthalmol. 2006;124:1119–26. doi: 10.1001/archopht.124.8.1119. [DOI] [PubMed] [Google Scholar]
- 9.GOV.UK. Driving eyesight rules. 2020. https://www.gov.uk/driving-eyesight-rules. Accessed 12 Feb 2020.
- 10.Denis D, Genitori L, Bolufer A, et al. Refractive error and ocular motility in plagiocephaly. Child’s Nerv Syst. 1994;10:210–16. doi: 10.1007/BF00301156. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsson M, Sjostrand J. Astigmatic axis and amblyopia in childhood. Acta Ophthalmologica Scandinavica. 2003;81:33–37. doi: 10.1034/j.1600-0420.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 12.Tien Tay M, Martin F, Rowe N, et al. Prevalence and causes of visual impairment in craniosynostotic syndromes. Clin Exp Ophthalmol. 2006;34:434–40. doi: 10.1111/j.1442-9071.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.de Jong T, Bannink N, Bredero-Boelhouwer HH, et al. Long-term functional outcome in 167 patients with syndromic craniosynostosis; defining a syndrome-specific risk profile. J Plast Reconstr Aesthet Surg. 2010;63:1635–41. doi: 10.1016/j.bjps.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Khong JJ, Anderson P, Gray TL, et al. Ophthalmic findings in Apert’s syndrome prior to craniofacial surgery. Am J Ophthalmol. 2006;142:328–30. doi: 10.1016/j.ajo.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Khong JJ, Anderson P, Gray TL, et al. Ophthalmic findings in Apert’s syndrome after craniofacial surgery twenty-nine years’ experience. Ophthalmology. 2006;113:347–52. doi: 10.1016/j.ophtha.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Sjostrand J, Abrahamsson M. Risk factors in amblyopia. Eye. 1990;4:787–93. doi: 10.1038/eye.1990.124. [DOI] [PubMed] [Google Scholar]
- 17.Jadico SK, Young DA, Huebner A, et al. Ocular abnormalities in Apert syndrome: genotype/phenotype correlations with fibroblast growth factor receptor type 2 mutations. J Aapos. 2006;10:521–7. doi: 10.1016/j.jaapos.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Khong JJ, Anderson PJ, Hammerton M, et al. Differential effects of FGFR2 mutation in ophthalmic findings in apert syndrome. J Craniofac Surg. 2007;18:39–42. doi: 10.1097/01.scs.0000249358.74343.70. [DOI] [PubMed] [Google Scholar]
- 19.Mathijssen IMJ. Guideline for care of patients with the diagnoses of craniosynostosis: working group on craniosynostosis. J Craniofacial Surg. 2015;26:1735–807. doi: 10.1097/SCS.0000000000002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelissen M, Ottelander BD, Rizopoulos D, et al. Increase of prevalence of craniosynostosis. J Craniomaxillofac Surg. 2016;44:1273–9. doi: 10.1016/j.jcms.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Di Rocco F, Arnaud E, Renier D. Evolution in the frequency of nonsyndromic craniosynostosis. J Neurosurg Pediatrics. 2009;4:21–25. doi: 10.3171/2009.3.PEDS08355. [DOI] [PubMed] [Google Scholar]