Abstract
Mycobacterium tuberculosis antigen 85 is induced in vitro by isoniazid (INH); its sustained induction in sputum during tuberculosis (TB) therapy predicts relapse. In this trial, rifampin or rifalazil inhibited the induction of sputum antigen 85 by INH in a dose-dependent fashion. This approach may facilitate the evaluation of new TB drugs.
Drugs for the therapy of tuberculosis (TB) differ substantially with respect to sterilizing activity, defined as the capacity to sterilize sputum rapidly and to prevent relapse (D. A. Mitchison, Letter, Am. Rev. Respir. Dis. 147:1062–1063, 1993). Rifampin and pyrazinamide are highly active; many other drugs, such as ethambutol, are only bacteriostatic (4). Isoniazid (INH) readily kills extracellular bacilli in sputum but has little ultimate influence on TB relapse. This characteristic appears to reflect its reduced activity against stationary-phase or semidormant organisms. These differential drug effects on specific mycobacterial subpopulations give rise to the independence of sterilizing activity from early bactericidal activity (EBA), defined as the rate of decline of sputum CFU counts during the first few days of therapy. EBA primarily reflects the killing of extracellular bacilli. INH has high EBA but low sterilizing activity; conversely, rifampin and pyrazinamide have low or no EBA despite high sterilizing activity (1, 2, 6). This discrepancy between EBA and sterilizing activity limits the power of EBA as a research tool and has slowed the evaluation of new drugs for TB.
We previously examined the role of sputum Mycobacterium tuberculosis antigen 85 as a surrogate marker in patients receiving standard short-course TB therapy (7, 8). The expression of this antigen is induced in vitro by INH (3). Its sustained induction in sputum shortly after the initiation of TB therapy predicts treatment failure or relapse, apparently indicating the lack of a sterilizing effect (7). In this study, we investigated the potential role of monitoring sputum antigen 85 in the evaluation of new TB drugs. We examined the effects of two dose levels of rifalazil (also known as KRM-1648 or benzoxazinorifamycin) on sputum M. tuberculosis antigen 85 and CFU counts in an EBA-type trial.
Patients with cough were prospectively recruited at a TB control clinic in Vitória, Brazil. The study protocol was approved by the institutional review boards of Case Western Reserve University and University Hospitals in Cleveland, Ohio, and Universidade Federal do Espírito Santo in Vitória, Brazil. TB was diagnosed by sputum smear, with subsequent confirmation by culturing. Subjects who were human immunodeficiency virus type 1 seropositive or whose isolates subsequently were found resistant to isoniazid, rifampin, pyrazinamide, or ethambutol were excluded.
After collection of two baseline sputum specimens, subjects were randomly assigned to one of four treatment arms: isoniazid at 300 mg daily, isoniazid plus rifampin at 600 mg daily, or daily isoniazid plus rifalazil at a dose of either 10 or 25 mg once weekly (on days 1 and 8). These treatments were administered for 2 weeks, during which time patients were hospitalized to facilitate frequent sputum collection. Pooled sputum specimens were collected at intervals during the protocol. Specimens were processed as previously described (7). Antigen 85 was measured by an enzyme-linked immunosorbent assay using monoclonal antibody TBC-27 for antigen capture (7). All subjects subsequently received standard short-course chemotherapy. No subjects failed to respond to therapy or showed relapse.
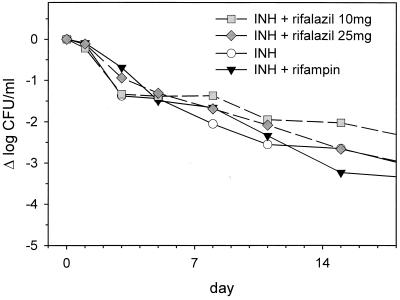
Sputum log CFU counts and antigen 85 values were equal at baseline among the four treatment arms (P = 0.8 and 0.9, respectively, as determined by analysis of variance [ANOVA]). Subjects in all four arms experienced a progressive reduction in log sputum CFU counts during the 2-week protocol (Fig. 1). The mean effect of INH alone during this entire interval was −2.65 log, or −0.19 log/day. Approximately half of this effect occurred during the first 2 days of treatment, with a reduced rate of decline thereafter. As shown in Fig. 1, neither rifampin nor rifalazil added significantly to the EBA of INH. A trend toward increased activity of INH-rifampin compared to INH alone did not appear until the end of the treatment period and even then did not reach statistical significance (P = 0.3, as determined by t test).
FIG. 1.
Sputum CFU counts during the first 2 weeks of treatment of sputum-smear-positive pulmonary TB in subjects randomly assigned to receive daily isoniazid (INH), daily INH-rifampin, or daily INH-weekly rifalazil (10 or 25 mg/week, administered on days 1 and 8).
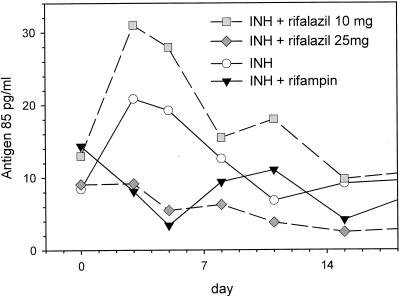
Sputum antigen 85 values changed significantly during the 2-week protocol, as determined by repeated-measures two-way ANOVA (P = 0.04) (Fig. 2). However, the power of this analytic approach to identify individual arms or days giving rise to the overall effect was limited by the small sample size and the inherent variability of sputum. Therefore, the differences among the four arms were determined by one-way ANOVA for each day of the study. A significant difference was found only on day 5, although a similar trend also was observed on day 3. Values in all groups returned to baseline by the end of the treatment interval. The timing of this effect was consistent with our prior observation that the induction of antigen 85 was maximal on day 4 of therapy and was transient in subjects who ultimately were cured (7).
FIG. 2.
Sputum M. tuberculosis antigen 85 values, measured by an enzyme-linked immunosorbent assay, during the first 2 weeks of treatment of sputum-smear-positive pulmonary TB. Rifalazil was administered on days 1 and 8; other drugs were administered daily.
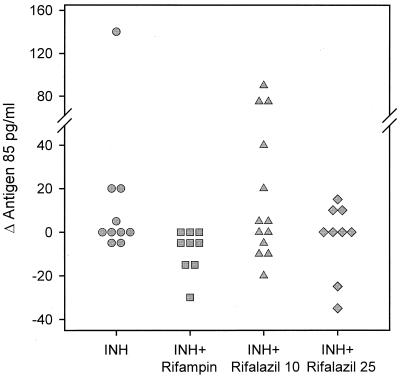
Data for individual subjects in each treatment arm, expressed as change from baseline to days 3 to 5, are indicated in Fig. 3. Values in subjects receiving INH-rifampin were significantly lower than values in those receiving INH alone and INH-rifalazil (10 mg) (P = 0.02 and 0.04, respectively, as determined by Mann-Whitney rank sum test). Values in subjects receiving INH-rifampin and INH-rifalazil (25 mg) did not differ (P = 0.13).
FIG. 3.
Sputum antigen 85 values of individual subjects, expressed as change from baseline (maximum value on days 3 to 5 minus maximum pretreatment value). Values for subjects receiving INH-rifampin were significantly lower than values for those receiving INH-rifalazil (10 mg) or INH alone but did not differ from values for subjects receiving INH-rifalazil (25 mg).
These data indicate that the administration of INH results in the transient induction of M. tuberculosis antigen 85 in sputum 3 to 5 days after the initiation of treatment and that this process can be inhibited by the concurrent administration of rifampin or rifalazil in a dose-dependent fashion. Monitoring of sputum antigen 85 may therefore represent an important extension to the evaluation of new TB drugs in EBA-type trials, particularly since its sustained expression is associated with relapse (7, 8). The strong activity of both rifampin and rifalazil on antigen 85 is in marked contrast to their weak EBA. This result may reflect their mechanism of action, interrupting new protein synthesis through inhibition of RNA polymerase. Alternatively, the ability to block INH-induced antigen 85 expression may be shared by other drugs with high sterilizing activity. Further studies of drug combinations such as INH-levofloxacin and INH-pyrazinamide may be helpful in dissecting this interaction.
In summary, the monitoring of sputum M. tuberculosis antigen 85 may offer an important new approach to evaluating the preliminary activity and dose-response relationship of new drugs for TB.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grants NO1-AI45244 and RO1-AI41911 and by PathoGenesis Corporation.
REFERENCES
- 1.Botha F J, Sirgel F A, Parkin D P, Van de Wal B W, Donald P R, Mitchison D A. Early bactericidal activity of ethambutol, pyrazinamide and the fixed combination of isoniazid, rifampicin and pyrazinamide (Rifater) in patients with pulmonary tuberculosis. S Afr Med J. 1996;86:155–158. [PubMed] [Google Scholar]
- 2.Donald P R, Sirgel F A, Botha F J, Seifart H I, Parkin D P, Vandenplas M L, Van de Wal B W, Maritz J S, Mitchison D A. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 3.Garbe T R, Hibler N S, Deretic V. Isoniazid induces expression of the antigen 85 complex in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1754–1756. doi: 10.1128/aac.40.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison D A. The action of antituberculosis drugs in short-course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 5.Mitchison D A, Nunn A J. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 6.Sirgel F A, Botha F J, Parkin D P, Van de Wal B W, Donald P R, Clark P K, Mitchison D A. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J Antimicrob Chemother. 1993;32:867–875. doi: 10.1093/jac/32.6.867. [DOI] [PubMed] [Google Scholar]
- 7.Wallis R S, Perkins M, Phillips M, Joloba M, Demchuk B, Namale A, Johnson J L, Williams D, Wolski K, Teixeira L, Dietze R, Mugerwa R D, Eisenach K D, Ellner J J. Induction of the antigen 85 complex of M. tuberculosis in sputum: a determinant of outcome in pulmonary tuberculosis. J Infect Dis. 1998;178:1115–1121. doi: 10.1086/515701. [DOI] [PubMed] [Google Scholar]
- 8.Wallis R S, Perkins M, Phillips M, Joloba M, Namale A, Whalen C C, Johnson J L, Teixeira L, Dietze R, Mugerwa R D, Eisenach K D, Ellner J J. Predicting the outcome of therapy for pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;161:1076–1080. doi: 10.1164/ajrccm.161.4.9903087. [DOI] [PMC free article] [PubMed] [Google Scholar]