Abstract
Cadmium (Cd), though potentially beneficial at lower levels to some plant species, at higher levels is a toxic metal that is detrimental to plant growth and development. Cd is also a carcinogen to humans and other contaminated plant consumers, affecting the kidneys and reducing bone strength. In this study we investigated responses of growth, chlorophyll content, reactive oxygen species levels, and antioxidant responses to Cd in honeysuckle leaves (Lonicera japonica Thunb.), a potential Cd hyperaccumulator. Results indicated that plant height, dry weight, leaf area, and chlorophyll content increased when honeysuckle was exposed to 10 mg kg−1 or 30 mg kg−1 Cd (low concentration). However, in response to 150 mg kg−1 or 200 mg kg−1 Cd (high concentration) these growth parameters and chlorophyll content significantly decreased relative to untreated control plant groups. Higher levels of superoxide radical (O2·−) and hydrogen peroxide (H2O2) were observed in high concentration Cd groups. The activities of ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase were enhanced with exposure to increasing levels of Cd. Additionally, the Ascorbate–Glutathione (AsA–GSH) cycle was activated for the removal of H2O2 in honeysuckle in response to elevated Cd. The Pearson correlation analysis, a redundancy analysis, and a permutation test indicated that proline and APX were dominant antioxidants for removing O2·− and H2O2. The antioxidants GSH and non-protein thiols (NPTs) also increased as the concentration of Cd increased.
Subject terms: Plant physiology, Plant stress responses
Introduction
The ecological disruption caused by heavy metal soil contamination has been growing due to urbanization and industrialization as well as the increase in soil Cd content produced through commonly used phosphate soil fertilization1. Cd is the most common toxic contaminant found in soils2,3. It is readily absorbed by plant roots, transported to above-ground tissues, and absorbed by higher organisms when the contaminated plants are ingested even when the contaminant level is below the phytotoxicity threshold4,5.
Cd accumulation reduces growth and negatively affects metabolic factors in plants, thus impacting their basic developmental, physiological, and biochemical processes6,7. Research indicates that Cd alters photosynthesis, damages the internal structure of chloroplasts, inhibits the biosynthesis of chlorophyll, which as a result decreases chlorophyll content and causes leaf chlorosis8,9. Excessive Cd also has a negative impact on plant water relations which induces a water deficit, alters ion homeostasis, inhibits nutrient uptake of essential minerals such as Fe and Ca, all of which have a negative effect on plant growth10–12. Therefore, high levels of Cd in plants result in wilting and eventually in plant mortality13,14.
Cd is not a redox metal. It cannot directly participate in Fenton and Haber–Weiss reactions to produce reactive oxygen species (ROS)15,16. However, Cd impairs electron transport in mitochondria and chloroplasts, alters enzyme activity, and induces the production of ROS17. Excessive production and accumulation of ROS, such as superoxide anion (O2·−) and hydrogen peroxide (H2O2), alter the redox status of cells, resulting in oxidative injury, evidenced as an increase in ion leakage, lipid peroxidation, and DNA-strand cleavage18. Some plants have evolved a defense response against Cd and ROS accumulation, which includes activation of an antioxidant system and the production of osmoprotectants12,19. Enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and glutathione-S-transferase (GST), while non-enzymatic antioxidants include molecules such as ascorbic acid (AsA) and glutathione (GSH)20. Enzymatic and non-enzymatic antioxidants are associated with the ascorbate–glutathione pathway which is one of the effective ways to scavenge H2O2 in plant cells21. AsA acts as an electron donor to process H2O2 into water and oxygen through APX catalysis reducing oxidative damage. In addition, DHAR and MDHAR are responsible for AsA regeneration22. GSH and NADPH also act as electron donors and are involved in H2O2 degradation22.
Glutathione can indirectly scavenge Cd-induced ROS. The change in the ratio of GSH and its oxidized form (GSSG) acts as a redox pair to modulate the signaling of antioxidant mechanisms in cells20,23. When the GSH/GSSG ratio is balanced, plants can reduce or eliminate the oxidative stress caused by Cd. Once the balance is disturbed as a result of high levels of ROS, plants will experience oxidative damage24. NPTs are molecular compounds in plant cells such as cysteine (Cys), GSH, phytochelatins (PCs), metallothioneins (MTs)25. These small molecules combine with free Cd to form complexes such as Cd-GSH and Cd-PC. The complexes are then compartmentalized in vacuoles, and thus no longer participate in Cd toxic reactions26,27. Proline, a signaling molecule, is an important osmoprotectant and antioxidant28 which functions to maintain the redox balance in cells acting as a free radical scavenger, metal chelator, cell membrane stabilizer, and activator of the ROS detoxification pathway28–30. The accumulation of proline was shown to be related to Cd-induced iron deficiency and the inhibition of electron transport activity31,32.
Honeysuckle (Lonicera japonica Thunb.) is a twining, semi-evergreen vine, distributed widely in temperate and tropical regions33,34. It is a popular landscape plant with high environmental adaptability35. Honeysuckle has a high Cd tolerance and a strong tendency to accumulate Cd. Its shoot Cd concentration reached 286.12 μg g−1 dry weight when exposed to 25 mg L−1 Cd for 20 days36. Honeysuckle is regarded as a potential Cd hyperaccumulator36, however, the biological and chemical mechanisms of Cd enrichment have not been sufficiently revealed in previous studies. In the present study, the morphological and physiological responses of honeysuckle to elevated levels of Cd were explored. The non-enzymatic antioxidant contents and antioxidant enzyme activities were monitored along with ROS levels in honeysuckle in response to various levels of Cd. Redundancy analysis (RDA) and a permutation test were used to identify the crucial components of the antioxidant system.
Results
Effects of Cd levels on plant growth and chlorophyll content
Several growth parameters in honeysuckle cuttings were measured to determine their response to different levels of Cd in the soil including plant height, dry weight, and leaf area. Relative to the control, 10 and 30 mg kg−1 Cd promoted honeysuckle growth as measured by plant height, dry weight, and leaf area, all of which were significantly greater (P < 0.05) after 90 days of Cd-exposure. In soils containing 80 mg kg−1 Cd, plant height and leaf area were not significantly different (P > 0.05) from the control. In contrast, both plant height and leaf area were significantly lower (P < 0.05) in cuttings exposed to 150 and 200 mg kg−1 Cd (Table 1).
Table 1.
Effects of different concentrations of cadmium on growth parameters and chlorophyll content in Lonicera japonica Thunb. after 90 days.
| Cd concentration (mg kg−1) | Height (cm) | Dry weight (g) | Leaf area (cm2) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total chlorophyll (mg g−1 FW) |
|---|---|---|---|---|---|---|
| 0 | 44.67 ± 0.58 b | 6.74 ± 0.10 d | 13.20 ± 0.13 b | 6.50 ± 0.05 d | 2.45 ± 0.12 d | 8.95 ± 0.13 d |
| 10 | 46.67 ± 0.58 a | 8.69 ± 0.11 b | 14.18 ± 0.60 a | 7.69 ± 0.12 c | 3.27 ± 0.41 ab | 10.95 ± 0.29 c |
| 30 | 47.33 ± 1.15 a | 11.31 ± 0.50 a | 14.63 ± 0.23 a | 10.15 ± 0.02 a | 3.17 ± 0.16 abc | 13.32 ± 0.19 a |
| 80 | 43.83 ± 0.76 b | 7.09 ± 0.18 c | 13.13 ± 0.34 b | 8.56 ± 0.17 b | 2.75 ± 0.11 cd | 11.32 ± 0.15 b |
| 150 | 41.33 ± 1.53 c | 6.54 ± 0.14 d | 11.32 ± 0.14 c | 5.51 ± 0.15 e | 3.50 ± 0.34 a | 9.01 ± 0.18 d |
| 200 | 40.83 ± 0.29 c | 5.79 ± 0.18 e | 11.25 ± 0.23 c | 5.43 ± 0.12 e | 2.94 ± 0.07 bc | 8.37 ± 0.18 e |
Different letters indicate a significant difference (P < 0.05) based on Duncan’s multiple range test at 5% level. Values are the mean ± SD (n = 3).
The contents of chlorophyll a, chlorophyll b and total chlorophyll increased significantly (P < 0.05) within 10, 30 mg kg−1 Cd after 90 days of Cd-exposure. The chlorophyll a content decreased significantly in leaves of cuttings from plants exposed to 150 mg kg−1 Cd (P < 0.05), In soils containing 200 mg kg−1 Cd, the content of chlorophyll a and total chlorophyll decreased significantly (P < 0.05) (Table 1).
Effects of Cd levels on ROS levels
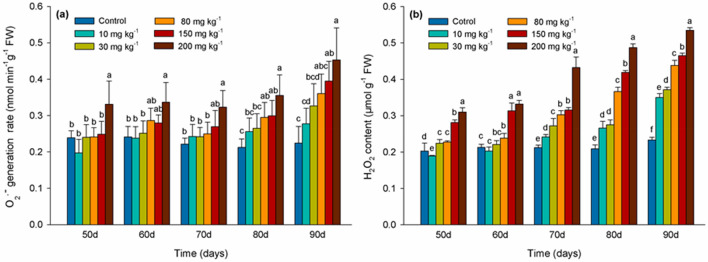
The rate of O2·− generation in honeysuckle leaves compared to the control was not significantly different (P > 0.05) within the 10, 30, 80 mg kg−1 Cd treated samples before the 80 days of Cd exposure. In contrast, the rate of O2·− generation was significantly higher in leaves of cuttings treated with 200 mg kg−1 Cd than that in control (Fig. 1a). H2O2 levels were significantly higher (P < 0.05) than in the control during the entire duration of the experiment in leaves of cuttings from plants exposed to 80, 150, and 200 mg kg−1 Cd. Notably, H2O2 levels were markedly increased (P < 0.05) at 90 days in leaves of cuttings exposed to all concentrations of Cd (Fig. 1b).
Figure 1.
Rate of superoxide radical (O2·−) generation (a) and hydrogen peroxide (H2O2) levels (b) in leaves of Lonicera japonica Thunb. cuttings exposed to different concentrations of Cd. Different letters indicate a significant difference (P < 0.05) based on Duncan’s multiple range test at 5% level. Values are the mean ± SD (n = 3).
Effects of Cd levels on antioxidant enzyme activity
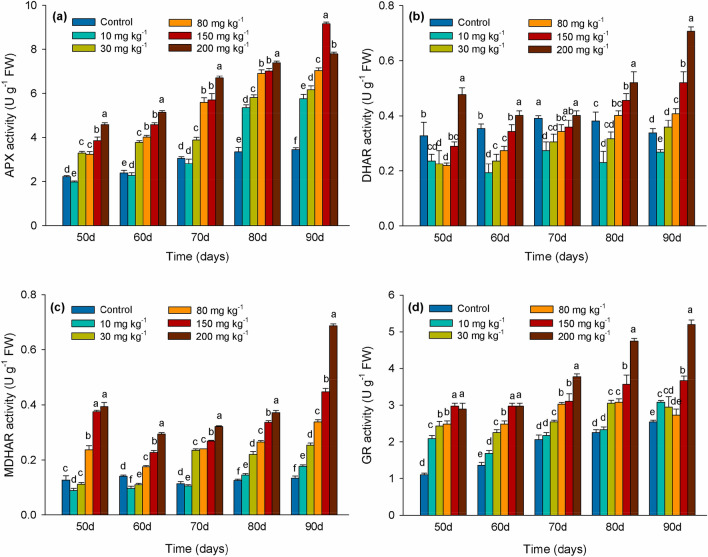
APX activity compared to the control decreased by 11% by day 50 in leaves of cuttings exposed to 10 mg kg−1 Cd. In sharp contrast, it was significantly higher than that in control after day 80. APX activity steadily increased in leaves of cuttings exposed to 30, 50, 80, 150, or 200 mg kg−1 Cd over the entire duration of the experiment (Fig. 2a). During the duration of the experiment, DHAR activity in leaves exposed to 10 mg kg−1 Cd was significantly lower (P < 0.05) than in the control group. In contrast, DHAR activity was significantly increased (P < 0.05) compared to the control group leaves in response to the 150 mg kg−1 Cd treatment (Fig. 2b). MDHAR activity consistently maintained an increased level compared to the control in leaves of cuttings exposed to 80, 150 and 200 mg kg−1. Prior to day 70, MDHAR activity was raised in leaves of cuttings exposed to 10 and 30 mg kg−1 Cd (Fig. 2c). GR activity exhibited an increasing trend over the entire duration of the experiment and reached its highest level in leaves of cuttings treated with 200 mg kg−1 Cd at day 90 (Fig. 2d).
Figure 2.
APX (ascorbate peroxidase) (a), DHAR (dehydroascorbate reductase) (b), MDHAR (monodehydroascorbate reductase) (c) and GR (glutathione reductase) (d) activities in leaves of Lonicera japonica Thunb. cuttings exposed to different concentrations of Cd. Different letters indicate a significant difference (P < 0.05) based on Duncan’s multiple range test at 5% level. Values are the mean ± SD (n = 3).
Effects of Cd levels on GSH pool and NPTs content
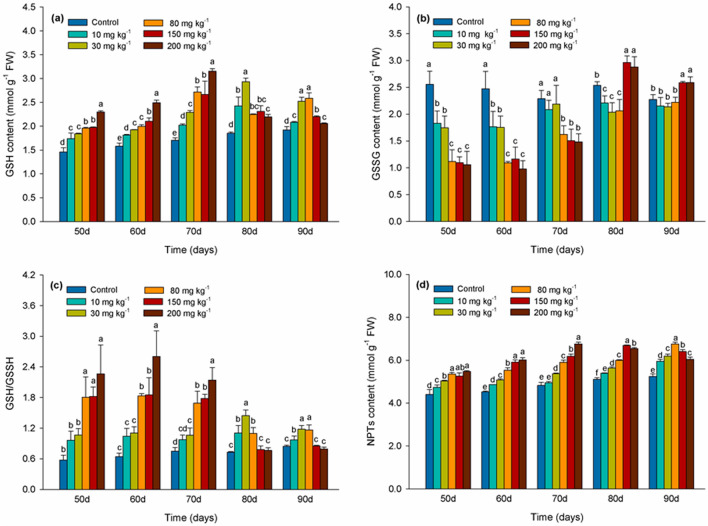
As illustrated in Fig. 3a, the level of GSH significantly increased over time compared with the control in response to all Cd treatments through the 70 days. GSH levels reached a maximum on day 70 in response to the 80, 150, and 200 mg kg−1 Cd treatments, increasing by 69%, 65%, and 99%, respectively. Glutathione levels gradually decreased after day 80 in leaves of cuttings exposed to 150 and 200 mg kg−1 Cd. The level of GSSG was significantly higher (P < 0.05) than in the control in the 150 and 200 mg kg−1 Cd treatments after 80 days (Fig. 3b). The ratio of GSH/GSSG decreased after 80 days in leaves treated with 150 and 200 mg kg−1 Cd resulting in no significant (P > 0.05) difference with the control at this time interval (Fig. 3c). As indicated in Fig. 3d, NPTs levels increased in response to the different Cd treatments and were significantly higher than in the control (P < 0.05). NPTs content was highest at day 90 in the 80 mg kg−1 Cd treatment, but was much lower in the 150 and 200 mg kg−1 Cd treatments (Fig. 3d).
Figure 3.
GSH (reduced glutathione) (a), GSSG (oxidized glutathione) (b), GSH/GSSG (c) and NPTs (non-protein thiols) (d) in leaves of Lonicera japonica Thunb. cuttings exposed to different concentrations of cadmium. Different letters indicate a significant difference (P < 0.05) based on Duncan’s multiple range test at 5% level. Values are the mean ± SD (n = 3).
Effects of Cd levels on proline content
The proline content in leaves of the 10 mg kg−1 Cd treatment markedly increased after 70 days suggesting an adaptation to higher Cd saturation levels accumulated over time. Proline content in leaves continually increased over the duration of the experiment in leaves of cuttings from plants exposed to 30, 50, 80, 150, and 200 mg kg−1 Cd. On day 90, proline content was 98% and 157% higher in leaves exposed to 150, and 200 mg kg−1 Cd respectively than in control (Fig. 4).
Figure 4.
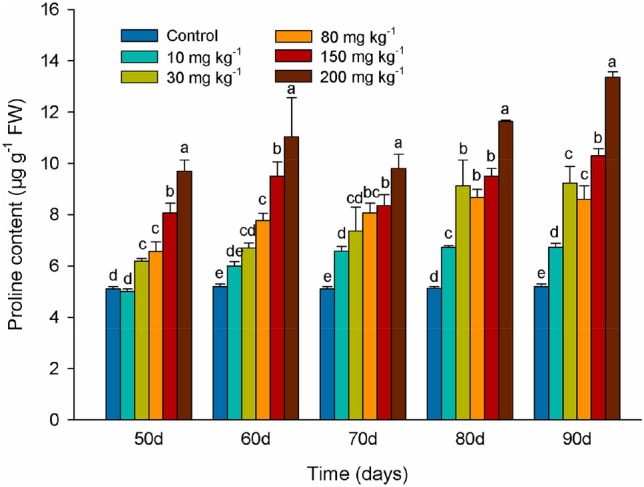
Proline levels in leaves of Lonicera japonica Thunb. cuttings exposed to different concentrations of Cd. Different letters indicate a significant difference (P < 0.05) based on Duncan’s multiple range test at 5% level. Values are the mean ± SD (n = 3).
Correlation between Cd levels and measurement indexes
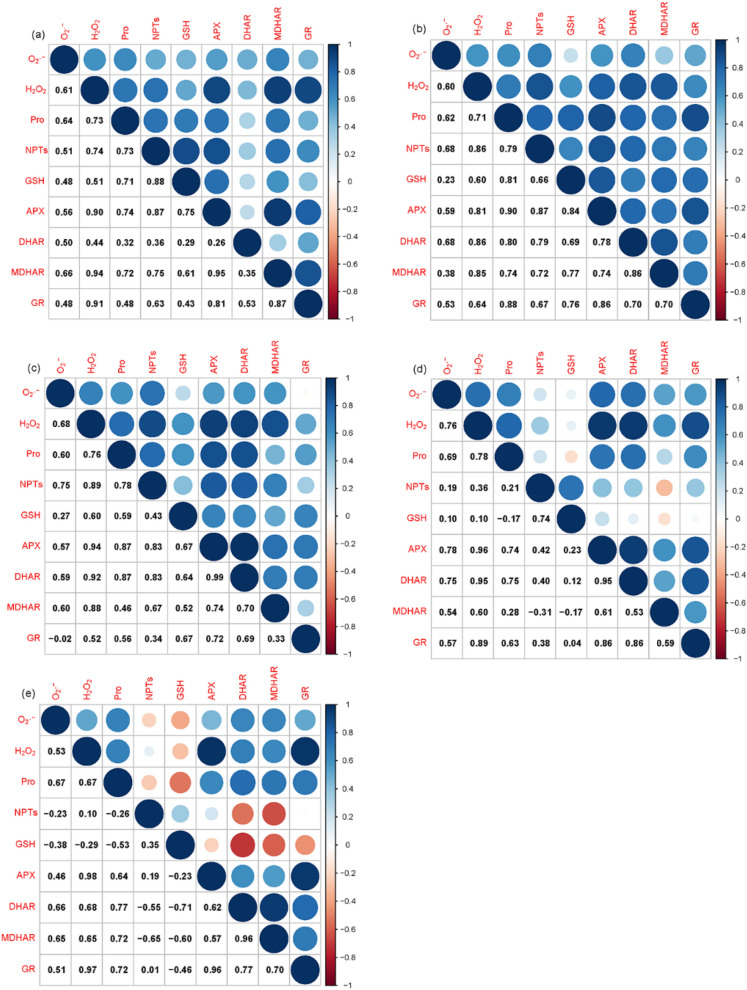
As shown in Fig. 5, APX and GR had a significant positive correlation with H2O2 (P < 0.05, rAPX = 0.90, 0.81, 0.94, 0.96 and 0.98, rGR = 0.91, 0.64, 0.52, 0.89 and 0.97, respectively) in the 10, 30, 80, 150 and 200 mg kg−1 Cd treatment. MDHAR had a significant positive correlation with H2O2 (P < 0.05, rMDHAR = 0.94, 0.85, 0.88, and 0.60, respectively) in 10, 30, 80, and 150 mg kg−1 Cd exposure. GSH had a significant positive correlation with H2O2 (P < 0.05, rGSH = 0.51, and 0.60, respectively) in 10 and 30 mg kg−1 Cd exposure. NPTs had a significant positive correlation with H2O2 (P < 0.05, rNPTs = 0.74, 0.86 and 0.89, respectively) in 10, 30, and 80 mg kg−1 Cd exposure. Proline had a significant positive correlation with H2O2 (P < 0.05, rPro = 0.71, 0.76 and 0.78, respectively) in 30, 80, 150 mg kg−1 Cd exposure. O2·− had a significant positive correlation with proline, NPTs, APX, and DHAR (P < 0.05, r = 0.60, 0.75, 0.57, and 0.59, respectively) in 80 mg kg−1 Cd exposure.
Figure 5.
The Pearson correlation coefficients among the measured variables in leaves of Lonicera japonica Thunb. cuttings exposed to different Cd treatments. (a) 10 mg kg−1 Cd; (b) 30 mg kg−1 Cd; (c) 80 mg kg−1 Cd; (d) 150 mg kg−1 Cd; (e) 200 mg kg−1 Cd.
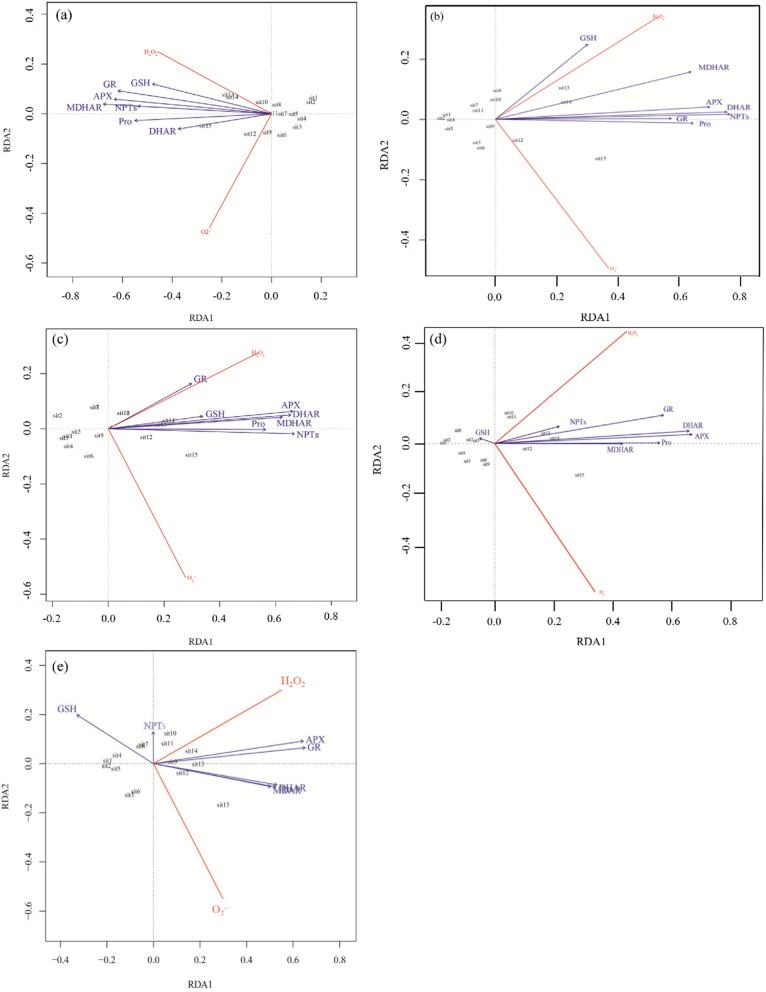
RDA and permutation tests of the measurement indexes with Cd levels
As showed in Fig. 6, proline, GSH, NPTs, APX, DHAR, MDHAR, and GR were positively correlated with H2O2 and O2·− generation rate in leaves of honeysuckle cuttings exposed to 10 mg kg−1 Cd (Fig. 6a). Notably, Proline, GSH, and APX were significant predictor variables at the 0.05 probability level (FPro = 25.2, FGSH = 4.56, FAPX = 6.54). Proline, GSH, NPTs, APX, DHAR, MDHAR, and GR were positively correlated with H2O2 and O2·− in leaves of honeysuckle cuttings exposed to 30 mg kg−1 Cd (Fig. 6b). Proline, GSH, and NPTs were significant predictor variables at the 0.05 probability level (FPro = 20.1, FGSH = 4.44, FNPTs = 6.54). Proline, GSH, NPTs, APX, DHAR, MDHAR, and GR were positively correlated with H2O2 and O2·− in leaves of honeysuckle cuttings exposed to 80 mg kg−1 Cd (Fig. 6c). Proline, NPTs, APX, and MDHAR were significant predictor variables at the 0.05 probability level (FPro = 47.69, FNPTs = 18.20, FAPX = 9.15, FMDHAR = 6.93). Proline, NPTs, APX, DHAR, MDHAR, and GR were positively correlated with H2O2 and O2·− in leaves of honeysuckle cuttings exposed to 150 mg kg−1 Cd. In contrast, GSH in the same treatment was negatively correlated with H2O2 and O2·− (Fig. 6d). Proline and APX were significant predictor variables at the 0.05 probability level (FPro = 31.27, FAPX = 12.14). Proline, APX, DHAR, MDHAR, and GR were positively correlated with H2O2 and O2·− in leaves of honeysuckle cuttings exposed to 200 mg kg−1 Cd. In the same treatment, GSH was negatively correlated with H2O2 and O2·− (Fig. 6e). Proline and APX were significant predictor variables at the 0.05 probability level (FPro = 51.08, FAPX = 9.42).
Figure 6.
Triplot of the redundancy analysis (RDA) of H2O2 and O2·− generation rate in leaves of Lonicera japonica Thunb. cuttings exposed to different Cd treatments. (a) 10 mg kg−1 Cd; (b) 30 mg kg−1 Cd; (c) 80 mg kg−1 Cd; (d) 150 mg kg−1 Cd; (e) 200 mg kg−1 Cd.
Discussion
Cd is a water-soluble element and is non-essential to plant growth7. Cd can bind to transporters used by plants to transfer certain essential elements17. This reduces the capacity of the plant to absorb, transport, and utilize essential elements and thus affects the growth of plants to varying degrees10. Reduction of these elements can be detrimental to the growth of the plant as well as reducing the nutrition provided to humans and livestock when consumed. This may due to shared root transporters through genetic expressions between Cd and Zn2+ transporter gene, and Fe2+ through transporter genes, which also transport Cd ions to the roots of common agricultural products such as rice causing micronutrient deficiency in supported populations37. In the current study, chlorophyll a, b and total chlorophyll content, plant height, dry weight, and leaf area increased in honeysuckle leaves of cuttings exposed to 10 and 30 mg kg−1 Cd. Our findings reinforce previous research showing that honeysuckle has a strong tolerance to moderate Cd exposure36. Cd has previously been reported to have a stimulating effect on plant growth at low concentrations38. In general, mild stress may stimulate plants to initiate a stress response that accelerates growth. The growth of cotton callus was stimulated by exposure to 0.55 mmol L−1 and 0.7 mmol L−1 (low concentration) Cd and inhibited by 1 mmol L−1 (high concentration) Cd39. Our experiment shows that honeysuckle has rapid biomass accumulation and acts as a Cd hyperaccumulator, which could be used to remediate Cd pollution levels. When plants are exposed to excessive amounts of Cd, reductions in plant growth, mineral nutrients, and biomass appear to be attributable to toxic effects of Cd40. In our study, the growth and chlorophyll content of honeysuckle decreased when the Cd concentration exceeded 80 mg kg−1, which was especially evident at 200 mg kg−1, where chlorophyll a, b and total chlorophyll contents were severely impacted (Table 1). Decrease in chlorophyll content may also be related to degradation of the chlorophyll structure caused by the accumulation of ROS in response to Cd stress41.
When plants are exposed to excessive levels of metals, they produce high levels of ROS, a phenomenon that is considered as one of the earliest biochemical changes exhibited by plants in response to metal induced stress18. The H2O2 content in leaves of honeysuckle increased when cuttings were exposed to increasing concentrations of Cd (Fig. 1). Low concentrations of Cd have been reported to induce low ROS levels that act as signal molecules in the induction of defense genes against Cd toxicity42. However, high concentrations of Cd induce high ROS levels generally causing a serious imbalance to occur in ROS synthesis and degradation. Plants are subjected to oxidative stress when high ROS levels are present posing a physiological challenge. In our study O2·− and H2O2 levels in honeysuckle leaves increased significantly in response to the 150 and 200 mg kg−1 Cd treatments. Similar results were observed in a study of wheat roots showing that O2·− generation rate and H2O2 content were also increased in response to elevated Cd exposure43. Elevated ROS levels can result in the inhibition of enzyme activity, protein oxidation44,45, and an inability to manage the higher levels of oxidative damage induced by ROS levels43.
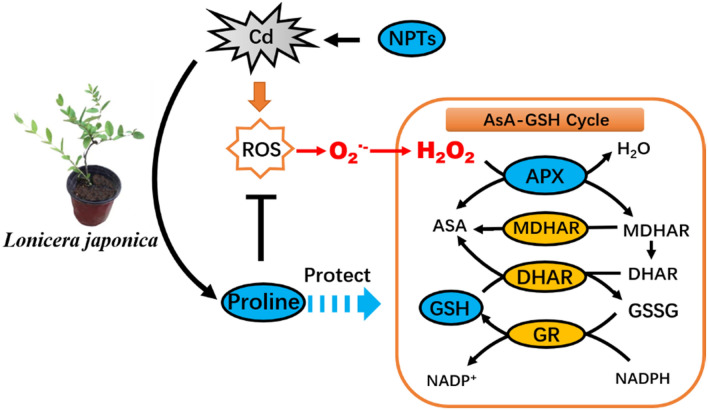
Measuring SOD over the duration of the experiment did not provide statistically significant data. After repeated tests the decision was made to use the main antioxidant enzymes in the ascorbic acid glutathione cycle to illustrate the important role of antioxidant enzymes in resisting Cd stress. An important antioxidant system involved in Cd detoxification is the AsA-GSH cycle composed of several antioxidants such as GSH, AsA, and critical antioxidant enzymes APX, MDHAR, DHAR, and GR46. APX plays a vital role in the antioxidant defense response of plants by catalyzing the conversion of H2O2 to water at the expense of AsA24. In the present study APX activity was increased as the duration of Cd exposure increased and Cd concentration intensified. APX activity had a significant linear correlation with H2O2 content in all Cd-exposed treatment. This suggests that APX participates in detoxifying H2O2, and might be a crucial factor in eliminating ROS in elevated Cd stress. In the AsA-GSH cycle, AsA, as an electron donor of H2O2, produces dehydroascorbic acid (DHA) through the activity of APX and then converts it to AsA through the activity of DHAR when GSH, as a product of GR, is present as an electron donor47. In the present study, we also observed that MDHAR, DHAR and GR activity levels were elevated as exposure to levels of Cd increased (Fig. 2b–d). The enhanced activities of the AsA-GSH cycle may be attributed to the need to maintain a favorable redox status by maintaining sufficient levels of GSH and reduced AsA to overcome the physiological repercussions of oxidation46.
GSH functions to regulate H2O2 levels in plant cells and acts as an antioxidant, reducing oxidative stress caused by metal-induced ROS48. In our study, GSH content increased and the GSH/GSSG ratio was up-regulated in response to the 10 and 30 mg kg−1 Cd treatment. A lower level of ROS was also observed at this concentration of Cd (Fig. 1) which could be explained by the key role of GSH in scavenging of ROS. Change in the ratio of GSH/GSSG during the degradation of H2O2 plays an important role in some redox signaling pathways49. GSH content decreased at later testing periods in 150 and 200 mg kg−1 Cd treatment, while GSSG content continued to increase. Data shows that at 70 days glutathione GSH is highest (Fig. 3a), and the oxidized glutathione GSSG is decreasing (Fig. 3b). In addition, the level of hydrogen peroxide is continuing to rise (Fig. 1b), and the consumption of glutathione is low. During this period as the GSH/GSSG ratio decreased the ROS levels increased. A decrease in GSH in response to Cd stress suggests that the protective role of GSH against oxidative stress may be significantly reduced as Cd levels increase beyond a sustainable threshold50. NPTs molecules, including GSH, Cysteine, MTs (metallothioneins), and other related substances, contain a high percentage of cysteine sulfhydryl residues that play an important role in the detoxification of metals in plants51. In our study, the content of NPTs also increased significantly as the concentration of Cd increased, suggesting that NPTs might have participated in the detoxification of Cd (Fig. 3d) which also correlates with the study of Cd management in perennial ryegrass (Lolium perenne L.)52. The concentration of NPTs compounds also increased as the concentration of Cd increased in Brassica pekinensis and B. chinensis, which might have been due to the chelation of sulfhydryl compounds with Cd53.
In many plants, free proline accumulates in response to a wide range of biotic and abiotic stresses54. Proline has multiple functions in stress adaption55. Proline acts as an osmoprotectant and an antioxidant by scavenging hydroxyl radicals (OH.) and singlet oxygen (1O2) to alleviate ROS-induced cellular injury56. In the current study, proline content increased both as time of exposure to elevated Cd and as the concentration of Cd increased (Fig. 4). It is possible that an increase in the concentration of H2O2 and O2·− induced free proline accumulation as the need to eliminate ROS increased in honeysuckle leaves.
Previous studies have demonstrated that Cd can induce an increase in the level of ROS in cells, whereas enzymatic and non-enzymatic antioxidants play an essential role in reducing excess ROS levels20,57–59. To expand on this knowledge, we analyzed the relationship between H2O2, O2·−, and antioxidants in the leaves of honeysuckle cuttings exposed to various concentrations of Cd by measuring specific antioxidant-related variables and determining their correlations using the RDA and permutation tests. Results indicated that when the content of H2O2 and O2·− increased in honeysuckle leaves in response to increasing concentrations of Cd, proline levels also increased. A positive correlation between proline and H2O2 and O2·− levels, and significant predictor variables were at the 0.05 probability level in 10, 30, 80, 150, and 200 mg kg−1 Cd exposure (Fig. 6). We confirmed that proline in honeysuckle leaves served as the dominant antioxidant in all Cd treatments, and accumulated proline acted as a protective response against oxidative stress60. Pearson correlation coefficients and permutation tests analysis (Fig. 6) indicated that the level of APX had a significant correlation with H2O2 content, in addition to proline. APX was an important predictor of oxidative stress in the 10, 80, 150 and 200 mg kg−1 Cd treatments. These factors suggested that APX, as an antioxidant enzyme, also plays an important role in scavenging H2O2 in honeysuckle leaves exposed to high concentration of Cd.
GSH was correlated with H2O2 and O2·−, and significant predictor variables at the P < 0.05 probability level in honeysuckle leaves of cuttings exposed to 10 and 30 mg kg−1 Cd. This positive correlation, however decreased as Cd concentration increased, and was even a negative correlation in the 150 and 200 mg kg−1 Cd treatments. This response may occur at high concentrations of Cd when GSH mechanisms are overwhelmed and unable to act as an effective antioxidant. When GSH is consumed in higher quantities in plant cells, the increasing role of NPTs in scavenging ROS became increasingly evident. NPTs levels were significant predictor variables correlating with the content of H2O2 and O2·− generation rate in 80 mg kg−1 Cd treatments. Compounds containing cysteine sulfhydryl residues including GSH played a major role in alleviating potential oxidative damage (Fig. 7).
Figure 7.
The antioxidant defense response in Lonicera japonica to Cd-induced oxidative stress.
Materials and methods
Plant material
Cuttings selected for this study were healthy, 1 year-old honeysuckle (Lonicera japonica Thunb.) from a representative strain cultivated at Liaoning University which continues to be used in additional research in the university's Department of life sciences. The cuttings were obtained in accordance with all relevant institutional, national, and international guidelines and legislation and all appropriate permissions and licenses were obtained for the specimens and materials. The cuttings were cultivated in sterilized sand for 10 weeks and then transplanted to a mixture of soil to sand (3:1). The soil was a brown topsoil (0–20 cm, pH 7.06), and was obtained from Liaoning University. The level of basic nutrients in the soil was 0.21 mg kg−1 calcium, 97.29 mg kg−1 nitrogen, 8.84 mg kg−1 phosphorus, 216.98 mg kg−1 potassium, and 1.50% organic matter. Air-dried soil samples were filtered through a 4.0 mm sieve for use in the experiment.
Cd exposure
The experiments were conducted in a laboratory in Liaoning University 25/17 °C day/night temperature, 50–60% relative humidity, 16 h light/8 h dark) starting in March, 2017. Eighteen plastic pots (20 cm diameter × 30 cm height) containing 2.5 kg of air-dried, disinfected soil were prepared. A CdCl2·2.5H2O solution of different concentrations of Cd were randomly added to each pot. Six levels of Cd were administered to the pots, including 0 (control), 10, 30, 80, 150, and 200 Cd mg kg−1 soil (each treatment was replicated three times). The soil was regularly mixed and allowed to come to equilibrium over a period of 40 days. During this time, the soil was mixed and sprayed with water every week to maintain an 80% water content. Three honeysuckle cuttings with similar growth and height were planted in each pot after the 40 days equilibrium period. Leaves from the cuttings were harvested every ten days from the 50th through the 90th day after the cuttings were placed in the Cd-containing soils. Leaf samples were wrapped in tin foil, immediately frozen in liquid nitrogen, and stored at − 80 °C until subsequent analysis.
Measurement of growth parameters
Height (cm) and leaf area (A, cm2) of plants were measured after 90 days of Cd exposure. At that time, all plants were collected. Roots were washed with distilled water, submerged in EDTA-Na2 for 20 min to balance ion levels, then cleaned with deionized water and immediately dried on filter paper. Lastly, the whole above-ground portion of the plant was placed in a 105 °C oven to a constant dry weight for the determination of biomass.
Determination of chlorophyll content
Chlorophyll was extracted from 0.5 g fresh leaves with 80% acetone (centrifuging at 5000 rpm) and the absorbance of the supernatant was measured at 663 nm and 645 nm to determine the level of chlorophyll a, chlorophyll b, and total chlorophyll, respectively61.
Determination of ROS levels
The rate of O2·− generation in leaves was determined by the hydroxylamine hydrochloride method62 with minor modifications. Initially, 0.1 g leaves were ground in 3 ml 0.05 mol L−1 phosphate (K–P) buffer (pH 7.8), followed by centrifugation at 5000 rpm, 3 min at 4 °C. Subsequently, 0.5 ml supernatant was mixed with phosphate buffer (pH 7.8) and 1 mol L−1 hydroxylamine hydrochloride and incubated for 20 min at 25 °C. Then, 17 mmol L−1 p-aminobenzene sulfonic acid and 7 mmol L−1 1-naphthylamine were added to the solution and absorbance was measured at 530 nm.
Hydrogen peroxide (H2O2) levels were determined in an extract prepared from 0.5 g leaves in 2.5 ml propanone, which was then centrifuged at 12,000 rpm for 10 min at 4 °C. The resulting supernatant was added to a mixture of 0.1 ml 5% Ti (SO4)2 (titanium sulphate) and 0.2 ml NH3 (ammonia), and then centrifuged at 10,000 rpm for 10 min at 4 °C. The resulting precipitate was dissolved in 2 mol L−1 H2SO4 and then re-centrifuged. The absorbance of the supernatant was measured at a wavelength of 415 nm using an ultraviolet spectrophotometer (UV-2100, UNICO, Shanghai, China)63.
Determination of enzymatic and non-enzymatic antioxidant compounds
A total of 0.5 g of fresh leaves were ground in 3.5 ml 50 mmol L−1 phosphate buffer (K–P; pH 7.8) containing 1.0 mmol L−1 EDTA-Na2, 1.0 mmol L−1 ascorbate and 2% (v/v) polyvinylpyrrolidone (PVP), and 1.5 ml saturated ammonium sulfate. The mixture was then centrifuged at 5000 rpm for 10 min at 4 °C (GF16RXII, HITACHI, Tokyo, Japan). The resulting supernatant was used to measure enzyme activity.
Ascorbate peroxidase (APX; EC 1.11.1.11) activity: A 1 ml reaction mixture containing phosphate buffer (pH 7.0), 0.83 ml ascorbate, 0.13 ml H2O2, 0.04 ml crude enzyme was utilized. Ascorbate consumption was measured by the reduction in absorbance at 290 nm over 1 min. APX activity was calculated using an extinction coefficient of 2.8 (mmol L)−1 cm−164.
Dehydroascorbate reductase (DHAR; EC 1.8.5.1) activity: A 1 ml reaction mixture containing 0.7 ml phosphate buffer, 0.1 ml reduced glutathione (GSH), 0.1 ml dehydroascorbate (DHA), and 0.1 ml crude enzyme extract was utilized. DHAR activity was calculated from the changes of absorbance at 265 nm over 1 min, using an extinction coefficient of 14 (mmol L)−1 cm−164.
Monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) activity: A 1 ml reaction mixture containing phosphate buffer (pH 7.6), 0.9 ml ascorbate, 0.04 ml ascorbate oxidase, 0.03 ml NADPH, and 0.03 ml of crude enzyme extract was utilized. MDHAR activity was determined by measuring the consumption of NADPH as indicated by the change in absorbance at 340 nm over 1 min, using an extinction coefficient of 6.2 (mmol L)−1 cm−165.
Glutathione reductase (GR; EC 1.6.4.2) activity: A 1 ml reaction mixture containing 0.86 ml oxidized glutathione (GSSG), 0.1 ml NADPH, and 0.04 ml of crude enzyme extract was utilized. GR activity was calculated from the change in absorbance at 340 nm over 1 min, using an extinction coefficient of 2.8 (mmol L)−1 cm−164.
GSH was determined according to the method of Yu et al.66 with a few modifications. Fresh leaves (0.15 g) were extracted with 1.75 ml 5% (w/v) sulfosalicylic acid, followed by centrifugation at 12,000×g for 4 min at 4 °C. The supernatant was used to determine the reduced and total glutathione content. Initialy, a 0.6 ml 0.1 mol L−1 phosphate buffer (K–P; pH 7.0; containing 0.5 mol L−1 EDTA) and 50 μl 3 mmol L−1 DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) was added to the supernatant and the content of reduced glutathione was determined by measuring the absorbance of the solution at 412 nm for 5 min. Then, 0.5 ml phosphate buffer, 50 μl DTNB, 0.1 ml NADPH (0.4 mmol L−1), and 2 μl GR were added to the supernatant and the solution was incubated for 20 min. Total glutathione was determined by measuring absorbance at 412 nm. Oxidized glutathione (GSSG) content was determined based on the difference between the values of total glutathione content and reduced glutathione content (GSSG). The obtained values were used to determine the GSH/GSSG ratio.
Determination of non-protein thiols (NPTs) and proline content
NPTs were measured as previously described by Sharma et al.67. Initially, 0.1 g of sample was ground in 5 ml 1 mol L−1 HCl and 1 mol L−1 EDTA, and centrifuged at 10,000 rpm for 3 min at 4 °C. The supernatant was then added to 0.5 ml phosphate buffer (pH 7.8), and 0.5 ml 6 mmol L−1 DTNB, N levels were determined by measuring the change in absorbance at 412 nm.
Proline content was determined using the acid ninhydrin assay68. Initially, 0.1 g fresh leaves were ground in 5 mL 3% sulfosalicylic acid. The homogenate was centrifuged at 10,000 rpm for 3 min at 4 °C. The supernatant was mixed in a 1:1:1 ratio with glacial acetic acid, and 2.5% acid ninhydrin, boiled at 100 °C for 30 min and finally cooled. Then 6 ml of toluene was added, after thorough mixing, the chromophore-containing toluene was separated, absorbance was measured at 520 nm taking blank toluene as a control.
Statistical analysis
One-way ANOVA followed by a Duncan’s multiple range test at a 5% level was used to statistically analyze the effect of Cd on plant growth, chlorophyll content, O2·− production rate, H2O2 content, proline content, GSH levels, NPTs content, and antioxidant enzyme activity (APX, DHAR, MDHAR and GR). Each treatment was replicated three times. A Pearson correlation coefficient was calculated for each treatment to explore the relationship between O2·− production rate, H2O2 content, proline content, GSH, NPTs content, APX, DHAR, MDHAR, and GR. A redundancy analysis (RDA) and a permutation test were performed for each treatment to determine the key variables explaining changes in the O2·− production rate and H2O2 content. The predictor variables included proline content, GSH, NPTs content, APX, DHAR, MDHAR, and GR. A significance level of P < 0.05 was used in the Pearson correlation analysis, RDA, and Permutation tests. The Pearson correlation analysis, RDA, and permutation tests were carried out in R 3.5.269. The ANOVA was carried out using SPSS software.
Conclusion
In the current study, excessive Cd induces an increase of oxidants H2O2, O2·−, and the activities of APX, MDHAR, DHAR and GR were enhanced with exposure to increasing levels of Cd. Additionally, the AsA-GSH cycle was activated in honeysuckle in response to elevated Cd. According to the RAD and permutation tests, we confirmed proline and APX serve as the dominant antioxidant and antioxidant enzyme in scavenging ROS in elevated Cd. Collectively, GSH and NPTs also act as the secondary antioxidants and their levels increase in response to increasing concentration of Cd. In addition, the honeysuckle cutting growth was promoted by the addition of low concentrations (10 mg kg−1 and 30 mg kg−1) of Cd, but when honeysuckle cuttings were subjected to higher concentrations (150 mg kg−1 and 200 mg kg−1) of Cd, their capacity to tolerate Cd was exceeded and plant growth was inhibited. Due to honeysuckle's hyperaccumulator traits and relative resilience to moderate levels of Cd exposure, future studies should explore the genetic mechanism affecting Cd accumulation and determine the effectiveness of using honeysuckle as an active phytoremediation mechanism for areas contaminated with various levels of Cd.
Supplementary Information
Acknowledgements
This research was funded by National Nature Science Foundation of China (31670700, 31370601). Innovative talents support plan for colleges and universities in Liaoning province (LR2018003). Projects of Liaoning Province Science and Technology Department (2019ZD0197, 2021BS088).
Author contributions
C.L. and Y.R. Participated in the discussion and experimental designs, Y.H. prepared the experiment materials, F.G., Q.W. and W.Y. performed most of the experiments, Y.T. undertook laboratory analyses and drafted the manuscript. All authors read and approved the final manuscript.
Data availability
All data analysed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10912-7.
References
- 1.Guowei Q, et al. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere. 2021;267:129205. doi: 10.1016/j.chemosphere.2020.129205. [DOI] [PubMed] [Google Scholar]
- 2.Nahar K, et al. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016;126:245–255. doi: 10.1016/j.ecoenv.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Zou J, et al. Transcriptional, physiological and cytological analysis validated the roles of some key genes linked Cd stress in Salix matsudana Koidz. Environ. Exp. Bot. 2017;134:116–129. doi: 10.1016/j.envexpbot.2016.11.005. [DOI] [Google Scholar]
- 4.Shi X, Sun H, Chen Y, Pan H, Wang S. Transcriptome sequencing and expression analysis of cadmium (Cd) transport and detoxification related genes in Cd-accumulating Salix integra. Front. Plant Sci. 2017;7:1577. doi: 10.3389/fpls.2016.01577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meeûs CD, Eduljee GH, Hutton M. Assessment and management of risks arising from exposure to cadmium in fertilisers: I. Sci. Total Environ. 2002;291:167–187. doi: 10.1016/S0048-9697(01)01098-1. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, et al. Hormesis effects induced by cadmium on growth and photosynthetic performance in a hyperaccumulator, Lonicera japonica Thunb. J. Plant Growth Regul. 2015;34:13–21. doi: 10.1007/s00344-014-9433-1. [DOI] [Google Scholar]
- 7.Hasanuzzaman M, et al. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017;8:115. doi: 10.3389/fpls.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- 9.Lomaglio T, et al. Effect of short-term cadmium stress on Populus nigra L. detached leaves. J. Plant Physiol. 2015;182:40–48. doi: 10.1016/j.jplph.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Ram H, Kumar B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016;15:89–109. doi: 10.1007/s11157-016-9390-1. [DOI] [Google Scholar]
- 11.Anwar S, et al. Chelators induced uptake of cadmium and modulation of water relation, antioxidants, and photosynthetic traits of maize. Environ. Sci. Pollut. Res. 2019;26:17577–17590. doi: 10.1007/s11356-019-05170-6. [DOI] [PubMed] [Google Scholar]
- 12.Jan S, et al. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018;18:146. doi: 10.1186/s12870-018-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DalCorso G, Farinati S, Maistri S, Furini A. How plants cope with cadmium: Staking all on metabolism and gene expression. J. Integr. Plant Biol. 2008;50:1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong M, et al. The phosphoproteomic response of rice seedlings to cadmium stress. Int. J. Mol. Sci. 2017;18:2055. doi: 10.3390/ijms18102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YF, Aarts M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012;69:3187–3206. doi: 10.1007/s00018-012-1089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou L, et al. Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int. J. Mol. Sci. 2017;18:1628. doi: 10.3390/ijms18081628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyno E, Klose C, Krieger-Liszkay A. Origin of cadmium-induced reactive oxygen species production: Mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008;179:687–699. doi: 10.1111/j.1469-8137.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- 18.Shahid M, et al. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014;232:1–44. doi: 10.1007/978-3-319-06746-9_1. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q, et al. Antioxidative systems, metal ion homeostasis and cadmium distribution in Iris lactea exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2017;139:50–55. doi: 10.1016/j.ecoenv.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere. 2007;69:99–107. doi: 10.1016/j.chemosphere.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Foyer CH, Noctor G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding S, et al. Exogenous glutathione enhances cadmium accumulation and alleviates its toxicity in Populus × canescens. Tree Physiol. 2017;37:1697–1712. doi: 10.1093/treephys/tpx132. [DOI] [PubMed] [Google Scholar]
- 24.Bashri G, Prasad SM. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016;132:329–338. doi: 10.1016/j.ecoenv.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Harada E, Yamaguchi Y, Koizumi N, Hiroshi S. Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. J. Plant Physiol. 2002;159:445–448. doi: 10.1078/0176-1617-00733. [DOI] [Google Scholar]
- 26.Silvia K, et al. Production of phytochelatins and glutathione by marine phytoplankton in response to metal stress. J. Phycol. 2006;42:975–989. doi: 10.1111/j.1529-8817.2006.00265.x. [DOI] [Google Scholar]
- 27.Seth CS, et al. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2012;35:334–346. doi: 10.1111/j.1365-3040.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 28.Siddique A, Kandpal G, Kumar P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J Pure Appl Microbiol. 2018;12:1655–1659. doi: 10.22207/JPAM.12.3.73. [DOI] [Google Scholar]
- 29.María EA, Arnould S, László S. Proline metabolism as regulatory hub. Trends Plant Sci. 2022;27:39–55. doi: 10.1016/j.tplants.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Giberti S, Funck D, Forlani G. Δ1-pyrroline-5-carboxylate reductase from Arabidopsis thaliana: Stimulation or inhibition by chloride ions and feedback regulation by proline depend on whether NADPH or NADH acts as co-substrate. New Phytol. 2014;202:911–919. doi: 10.1111/nph.12701. [DOI] [PubMed] [Google Scholar]
- 31.Kavi Kishor PB, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell Environ. 2014;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- 32.Sharmila P, Kumari PK, Singh K, Prasad NVSRK, Pardha-Saradhi P. Cadmium toxicity-induced proline accumulation is coupled to iron depletion. Protoplasma. 2017;254:763–770. doi: 10.1007/s00709-016-0988-5. [DOI] [PubMed] [Google Scholar]
- 33.Nickelson JB, Holzmueller EJ, Groninger JW, Lesmeister DB. Previous land use and invasive species impacts on long-term afforestation success. Forests. 2015;6:3123–3135. doi: 10.3390/f6093123. [DOI] [Google Scholar]
- 34.Wang HH, Koralewski TE, McGrew EK, Grant WE, Byram TD. Species distribution model for management of an invasive vine in forestlands of eastern Texas. Forests. 2015;6:4374–4390. doi: 10.3390/f6124374. [DOI] [Google Scholar]
- 35.Yan K, Wu C, Zhang L, Chen X. Contrasting photosynthesis and photoinhibition in tetraploid and its autodiploid honeysuckle (Lonicera japonica thumb.) under salt stress. Front. Plant Sci. 2015;6:227. doi: 10.3389/fpls.2015.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, He X, Chen W. Effects of cadmium hyperaccumulation on the concentrations of four trace elements in Lonicera japonica Thunb. Ecotoxicology. 2011;20:698–705. doi: 10.1007/s10646-011-0609-1. [DOI] [PubMed] [Google Scholar]
- 37.Atabayeva SD, et al. Response of plants to cadmium stress. Int. J. Biol. Chem. 2020;13:109–117. doi: 10.26577/ijbch.2020.v13.i1.11. [DOI] [Google Scholar]
- 38.Sobkowiak R, Deckert J. Cadmium-induced changes in growth and cell cycle gene expression in suspension-culture cells of soybean. Plant Physiol. Biochem. 2003;41:767–772. doi: 10.1016/S0981-9428(03)00101-3. [DOI] [Google Scholar]
- 39.Daud MK, et al. In vitro cadmium-induced alterations in growth and oxidative metabolism of upland cotton (Gossypium Hirsutum L.) Sci. World J. 2014;2014:1–10. doi: 10.1155/2014/309409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizwan M, et al. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016;130:43–53. doi: 10.1016/j.ecoenv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Li KW, Chen KJ, Liang J, Cui LJ. Effects of cadmium stress on seedlings growth and active ingredients in Salvia miltiorrhiza. Plant Sci. J. 2013;31:583–589. doi: 10.3724/SP.J.1142.2013.60583. [DOI] [Google Scholar]
- 42.Romero-Puertas MC, et al. Cadmium-induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant Cell Environ. 2004;27:1122–1134. doi: 10.1111/j.1365-3040.2004.01217.x. [DOI] [Google Scholar]
- 43.Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma. 2014;251:1047–1065. doi: 10.1007/s00709-014-0614-3. [DOI] [PubMed] [Google Scholar]
- 44.Opdenakker K, Remans T, Keunen E, Vangronsveld J, Cuypers A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012;83:56–61. doi: 10.1016/j.envexpbot.2012.04.003. [DOI] [Google Scholar]
- 45.Iqbal N, et al. Sulfur in the alleviation of cadmium-induced oxidative stress in plants. Environ Adapt. Stress Toler. Plants Era Clim. Chang. 2012;20:429–446. doi: 10.1007/978-1-4614-0815-4_20. [DOI] [Google Scholar]
- 46.Wu Z, et al. Antioxidant enzyme systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere. 2015;138:526–536. doi: 10.1016/j.chemosphere.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 47.Singh S, Singh A, Srivastava PK, Prasad SM. Cadmium toxicity and its amelioration by kinetin in tomato seedlings vis-à-vis ascorbate-glutathione cycle. J. Photochem. Photobiol. B Biol. 2018;178:76–84. doi: 10.1016/j.jphotobiol.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Sahoo S, Awasthi JP, Sunkar R, Panda SK. Determining glutathione levels in plants. Methods Mol. Biol. 2017;1631:273–277. doi: 10.1007/978-1-4939-7136-7_16. [DOI] [PubMed] [Google Scholar]
- 49.Li X, et al. Glycolate oxidase-dependent H2O2 production regulates IAA biosynthesis in rice. BMC Plant Biol. 2021;21:326. doi: 10.1186/s12870-021-03112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akinyemi AJ, Faboya OL, Olayide I, Faboya OA, Ijabadeniyi T. Effect of cadmium stress on non-enzymatic antioxidant and nitric oxide levels in two varieties of maize (Zea mays) Bull. Environ. Contam. Toxicol. 2017;98:845–849. doi: 10.1007/s00128-017-2069-7. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, et al. Contribution of cell walls, nonprotein thiols, and organic acids to cadmium resistance in two cabbage varieties. Arch. Environ. Contam. Toxicol. 2013;64:243–252. doi: 10.1007/s00244-012-9824-x. [DOI] [PubMed] [Google Scholar]
- 52.Jia H, et al. Exogenous phosphorus treatment facilitates chelation-mediated cadmium detoxification in perennial ryegrass (Lolium perenne L.) J Hazar Mater. 2020;389:121849. doi: 10.1016/j.jhazmat.2019.121849. [DOI] [PubMed] [Google Scholar]
- 53.Liu CP, Shen ZG, Li XD. Accumulation and detoxification of cadmium in Brassica pekinensis and B. chinensis. Biol. Plant. 2007;51:116–120. doi: 10.1007/s10535-007-0023-y. [DOI] [Google Scholar]
- 54.Ben Rejeb K, Abdelly C, Savouré A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014;80:278–284. doi: 10.1016/j.plaphy.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Szabados L, Savouré A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz DD, Parlak KU. Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol. Indic. 2011;11:417–423. doi: 10.1016/j.ecolind.2010.06.012. [DOI] [Google Scholar]
- 57.Paola I, Gianfranco S. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants. 2021;10:579. doi: 10.3390/antiox10040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qamer Z, Chaudhary MT, Du X, Hinze L, Azhar MT. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021;4:9. doi: 10.1186/s42397-021-00086-4. [DOI] [Google Scholar]
- 59.Hojati M, et al. Cadmium and copper induced changes in growth, oxidative metabolism and terpenoids of Tanacetum parthenium. Environ. Sci. Pollut. Res. 2017;24:1–12. doi: 10.1007/s11356-017-8846-3. [DOI] [PubMed] [Google Scholar]
- 60.Natarajan SK, et al. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic. Biol. Med. 2012;53:1181–1191. doi: 10.1016/j.freeradbiomed.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnon DI. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H, Wu F, Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127:1237–1242. doi: 10.1016/j.foodchem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 63.Patterson BD, MacRae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium (IV) Anal. Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- 64.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 65.Duarte B, Caetano M, Almeida PR, Vale C, Caçador I. Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal) Environ. Pollut. 2010;158:1661–1668. doi: 10.1016/j.envpol.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Yu CW, Murphy TM, Lin CH. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003;30:955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- 67.Sharma SS, et al. Cadmium toxicity to barley (Hordeum vulgare) as affected by varying Fe nutritional status. Plant Sci. 2004;166:1287–1295. doi: 10.1016/j.plantsci.2004.01.006. [DOI] [Google Scholar]
- 68.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 69.R Development Core Team, R. R: A Language and Environment for Statistical Computing. Vienna, Austria, ISBN 3900051070 (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this published article and its supplementary information files.