Abstract
Teledermoscopy, or the utilization of dermatoscopic images in telemedicine, can help diagnose dermatologic disease remotely, triage lesions of concern (i.e., determine whether in-person consultation with a dermatologist is necessary, biopsy, or reassure the patient), and monitor dermatologic lesions over time. Handheld dermatoscopes, a magnifying apparatus, have become a commonly utilized tool for providers in many healthcare settings and professions and allows users to view microstructures of the epidermis and dermis. This Dermoscopy Practice Guideline reflects current knowledge in the field of telemedicine to demonstrate the correct capture, usage, and incorporation of dermoscopic images into everyday practice.
Subject terms: Health services, Physical examination, Education
Introduction
Teledermatology involves a remote consultation for diagnostic and/or therapeutic advice. Teledermatology can occur by real-time video consultation or store-and-forward (SAF) images1. SAF images are prepared by general practitioners/patients or other specialists and sent to a dermatologist via secure portal, who makes recommendations for diagnosis and/or treatment after image examination1. Teledermoscopy is defined as the use of teledermatology to transmit dermoscopic images for remote consultation1.
Dermoscopy is an essential diagnostic tool for dermatologists to visualize epidermal structures, pigment patterns, and vascular patterns to aid examination of lesions and clinical decision-making. Access to dermoscopy images in addition to conventional telemedicine photographs is shown to significantly increase dermatologist diagnostic confidence2. Furthermore, addition of dermoscopic images improves efficacy and cost-effectiveness when used for skin cancer screening3,4. However, appropriate dermoscopy training is vital to proper and consistent use. User expertise and training increases diagnostic accuracy, whereas lack of training can pose major barriers to providers5,6. Teledermatologic modalities of care dramatically increased during the recent SARS-CoV-2 pandemic, yet there were increased numbers of missed melanomas and non-melanoma skin cancers, further necessitating proper dermoscopic provider competency in teledermatology visits7,8.
Additionally, as use of dermoscopy increases in fields outside of dermatology, there is need for proper dermatoscopic education in the primary care setting. Dermatoscopes in the primary care setting can inform decisions for biopsy and timely referral to dermatology, improve diagnostic accuracy of pigmented lesions, and facilitate earlier detection of melanoma and basal cell carcinoma9–20. Marwaha et al. showed that there is up to 9% greater probability of cancer detection with use of dermatoscopes compared with direct referral21. When compared to clinical photographs alone, use of dermatoscopes by well-trained primary care providers can also improve access to dermatologic care for underserved populations, reduce wait times, allow for continuous monitoring of lesions over time, and improve detection of suspicious lesions22–25.
While diagnostic accuracy in teledermoscopy is improved with high-quality images, up to 36% of dermoscopic images obtained by general practitioners during everyday practice were of poor quality26. Dermoscopy adds additional value in primary care when used by an expert or trained user27–30. A sequential intervention trial showed that use of dermoscopy by trained primary care providers improved sensitivity in diagnosis of melanoma and reduced benign-to-malignant excision ratio by assessing lesion stability31,32. A study of thirty-four healthcare practitioners demonstrated that use of mobile dermatoscopes in their practice was helpful for lesion monitoring, but reported technical tissues (33%) and uncertainty to advocate teledermoscopy for direct-to-consumer use (36%)29.
Additional challenges present as clinicians incorporate teledermoscopy along with gross lesion imaging into daily practice. If a physician does not recognize structures or incorrectly interprets them, this decreases diagnostic accuracy33. Diagnostic accuracy can also be further compromised if lesions are diagnosed without clinical context and a physical exam (i.e., palpation, stretch of skin, tenderness)34. Baseline and ongoing education for practitioners and proper dermatoscopes with accessories should be available.
Further limitations of teledermoscopy include safety and security concerns regarding data collection and storage, technical challenges in obtaining dermoscopy photos on genitalia and other anatomical locations, high cost of dermatoscopes (although this cost is decreasing as consumer-friendly mobile dermatoscopes are further developed), medico-legal concerns, reimbursement, and use of teledermoscopy by patients, which poses a new set of challenges related to user competency. Technical challenges associated with teledermoscopy (image orientation, resolution, scale, lighting, focus, color) are addressed in these guidelines.
Formal guidelines and requirements for acquiring dermoscopy images is crucial in ensuring standardized high-quality images for routine use in primary care settings. Currently, there are no guidelines or protocols for use of dermoscopy in telemedicine. Guidelines presented herein incorporate current recommendations with the goal of informing proper and standardized dermoscopic utilization in telemedicine to ensure high-quality images. We will describe guidelines for physical environment, patient evaluation and examination, follow-up care and coordination, devices, and equipment, including mobile device use, image capture and quality, storage, and requirements for asynchronous imaging. These guidelines will not cover interpretation of dermoscopy images, and it will not discuss in detail recommendations specific to patient-acquired mobile teledermoscopy, which can be found in Koh et al. 202135.
The dermoscopy guidelines apply to individual practitioners, hospitals, practices, and any other healthcare teams who evaluate cutaneous lesions and rashes. Those who utilize these guidelines should comply with professional protocols set by his/her area of expertise on the diagnosis and treatment of skin lesions. These practical guidelines are for use within the United States (U.S.). Interactions, where either party resides outside of the United States, should consider local guidelines over the guidelines presented here, in accordance with the rules of prevailing jurisdictions.
The purpose of these guidelines is to provide practitioners a more comprehensive protocol for the use of dermatoscopes in telemedicine. The following expert-developed guidelines on the proper use of dermoscopy will help establish a standard of care to assist medical providers of diverse professions in the proper capture and storage of dermoscopic images for use in primary care, nursing, and more. These guidelines, protocols, and recommendations have been iteratively reviewed and consensus was reached by a panel of teledermatology experts of the American Telemedicine Association (ATA). Guidelines do not guarantee diagnostic accuracy, nor should they be followed in certain situations such as emergencies.
Refer to ATA Practice Guidelines for Teledermatology clinical practice guidelines, informed consent, physical environment, patient evaluation and examination, follow-up care and coordination, and documentation36.
Results
To ensure clinical and technical benefit, teledermoscopy should follow a standardized approach and guidelines set forth on dermatoscope devices, image orientation, resolution, scale, measurement, focus, depth of field, color, and field of view.
Devices
Dermatoscopes are available as independent devices, cellular device attachments, connections to a digital camera via coupling adaptors or direct lens attachments, and as a total dermoscopy system (pre-mounted dermoscopic lens on a high-quality camera linked directly to a computer). Use of digital cameras can prove beneficial to capture both regional, macroscopic, and dermoscopic images. Some digital cameras will require manual transfer of imaging, while those such as visioMed MicroDERM have wireless transfer22. A comprehensive list of devices can be found in Supplemental Tables 1 and 2.
Image orientation
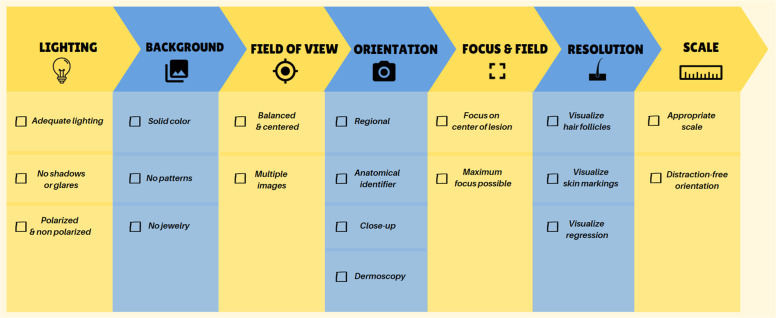
Image orientation shall be consistent to ensure accurate comparison over time. Regional images should have cephalic orientation, with the patient’s head toward the superior aspect of the image frame32. Vertical or horizontal orientation can be selected by discretion of the individual using the device and shall remain consistent for all follow-up images captured, including regional, close-up, and dermoscopic images. Regional images should include appropriate anatomical sites (i.e., a joint), to help orient and identify location of the lesion32. Orientation of dermoscopic images should be consistent with the corresponding gross image25. Finnane et al. and McKoy et al. provide additional complementary views and suggested image orientation based on body region; see Fig. 1 and Supplemental Fig. 232,37.
Fig. 1. Guidelines for acquisition of clinical images.
Follow this checklist to ensure proper lighting, background, field of view, orientation, focus, field, resolution, and scale when taking clinical images for store-and-forward utilization.
Resolution
Resolution is defined as the number of pixels in an image32,38. A device is considered to have acceptable resolution if hair follicles are visualized in regional images and skin markings are visualized in close-up images32. Dermoscopic images are considered to have appropriate resolution and magnification if dots and regression structures can be visualized25,32,39. These criteria will generally require a file size of at least 200KB32.
Scale and measurement
A diameter scale shall be used to consistently measure and monitor lesion dimensions and morphology over time25,32,39. The scale should not obscure when used in close-up images28. Digital scales incorporated into the device should be used over physical scales to avoid inaccuracies associated with physical scales (i.e., poor placement of scale and/or obscuring of surrounding skin or lesions)32,40.
Software measurement tools can automatically generate a distance measurement. However, accurate measurement relies on the area of interest being aligned precisely parallel to the device sensor and the scale placed in the same orientation of the camera, measuring the longest diameter of the lesion. Independent of physical or digit scale utilization, the scale shall be placed in the same orientation as the dermatoscope32.
Lighting
Selection of non-polarized light versus polarized light shall be left to the discretion of the individual using the device, guided by lesion characteristics32. However, polarized and nonpolarized dermoscopy can provide complementary information and it is generally recommended to capture at least one dermoscopic image with polarized light25,32,39,41,42.
Non-polarized light shall be used to visualize superficial structures and has higher specificity in diagnosing epidermal structures such as milia cysts, comedo-like openings in seborrheic keratoses, and blue-white veil associated with orthokeratosis42–44. Application of a gel or liquid such as ultrasound gel or alcohol-based hand sanitizer should be used to provide an interface between the skin surface and the device.
Polarized light eliminates superficial glare and should be used to visualize deep structures such as vasculature and collagen, and aid in identification of some malignant neoplasms42–45. Devices equipped with polarized-light do not allow for proper visualization of superficial structures, but can serve as an important diagnostic tool in assessing seborrheic keratoses45. Some dermatoscopes are capable of switching between non-polarized and polarized light and will blink if a lesion is visible in one mode and not in the other46.
Focus/Depth of field
Depth of field defines the distance between the nearest and farthest objects that are in sharp focus in front of or behind the point of interest that the camera is focused on. This is influenced by focal length, distance to the lesion, and aperture. The center of the area of interest should be in the center of the frame32,38. The camera shall be positioned perpendicular (90 degrees) to the skin surface using a lens with a deep depth of field, allowing for maximum area of the image to be in focus47. Auto-focus can be used, or the user can first depress the shutter half-way to focus, then adjust the camera to the center of the image, and finally depressing the shutter button to capture the image. Smartphone cameras may have an autofocus built in, but to optimize the photo it is helpful to manually focus. When utilizing an iPhone camera, the image can be focused by using a finger to tap the lesion of interest on the screen. A yellow box will appear indicating that the focus has been set. On an Android device, images can be focused by activating manual or pro mode in camera settings, activating the focus “MF” (manual focus) icon, and utilizing a slider to focus manually.
Color
Colors visualized by dermoscopy include yellow, brown, black, red, blue, gray, and white. The color of images taken over time shall be comparable to diagnose and monitor skin lesions correctly32. Based on recent ATA guidelines, an image color resolution of 24 bits is recommended for teledermatology applications36. Per manufacturer instruction, equipment shall be calibrated to prevent variability in color calibration and white balance between time points32. Standardization of color calibration for clinical or dermoscopic images has not been recommended to date48. We suggest utilization of Digital Imaging and Communications in Medicine (DICOM) for image transmission, processing, and storage given recent efforts being made by this platform to include metadata needed to derive standard colorimetry in medical imaging49.
Field of view
The lesion or area of interest shall be centered when positioning the device32. Close up images of rashes should include 25:75% ratio of normal looking skin to rash22. Multiple images shall be captured if the longest axis of the lesion or focal area of interest is larger than the field of view captured by the device. All edges of the lesion shall be visualized32. Images should be taken with a device less than 2 inches from the skin in non-contact mode or touching the skin after application of alcohol wipe to both skin and device in contact mode to improve luminance36. Dermoscopic images should be captured using the same orientation as the corresponding close-up39.
Quality
Providers obtaining dermoscopic images should be trained and technically competent dermatoscope utilization and have access to high-quality equipment. All clinicians should also have evidence of up-to-date nationally-accredited Continuing Professional Education (CPE) specific to dermoscopy and the clinical management of pigmented lesions in the five-year revalidation cycle50. Providers shall use a continuous quality improvement program, including a clinical oversight process. The quality improvement program includes:
Technical or administrative failures.
Appropriateness of virtual encounter.
Patient and/or provider satisfaction.
Patient outcomes.
Pathology or imaging results.
Recommendations for follow-up.
Follow-up feedback on quality of images to the imager.
Additionally, for patients with pigmented lesions, dermoscopic images are an essential supplement for any teledermatology referral that is used to replace face-to-face consultation50. Providers and organizations shall uphold regular maintenance and testing of devices to ensure proper functioning of equipment and connectivity. A system-wide firewall and antivirus software shall be kept up-to-date.
Store and forward consultation
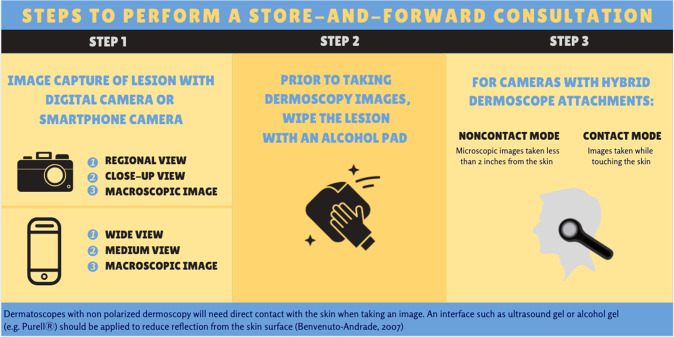
Steps to perform a SAF consultation as described by Walocko and Tejasvi17. Please refer to Fig. 2 for steps to perform an SAF consultation and ATA guidelines for instructions for taking these images36.
Fig. 2. Store-and-forward consultation steps.
Capture gross image of lesion. Wipe lesion with alcohol pad before taking dermoscopy photos.
Teledermatologists
Consultant dermatologists should have experience and/or training in dermoscopic interpretation, including an understanding of limitations as well as appropriate selection of patients suitable for teledermatology. Half of teledermatologists have subspeciality in dermoscopy, and 33% subspecialize in skin cancer2. In order to minimize risk of luminance decay, the dermatologist should review images on a display less than five years old. Decreasing ambient lighting and/or increasing the brightness of the display can minimize reflection. Furthermore, it can be helpful to use software that permits image rotation, panning, and zooming25.
Models of care
Teledermatology proves a valuable asset for diagnosis and management of dermatologic disease for underserved patients, including those in rural areas, medicaid populations, and the elderly. This being said, inherent reliance on and unequal access to internet connectivity and advanced devices could serve to worsen disparities. Further research is warranted in order to delineate the most effective ways to provide teledermatology for populations without access to in-person care, including the development of guidelines for non-physicians or non-providers collecting teledermoscopic images from remote locations51,52.
Discussion
Triage
Dermoscopy remains two-dimensional when viewing the images virtually or in person, which makes this an excellent tool for any teledermatology triage models. Patterns to differentiate benign from malignant have been very well described and so is the features for inflammatory dermatoses. For example, a pigmented lesion resembling seborrheic keratosis clinically could show concerning features on dermoscopy warranting a biopsy, Thus, the triage model could help in early detection of a malignant lesion. Similarly, if the lesion demonstrates dermoscopy patterns consistent with seborrheic keratosis, then reassurance is provided hence avoiding an unnecessary health visit. Inclusion of dermoscopy images may help discern, rashes, but evidence in literature is still evolving. Overall, utilizing dermoscopy in telemedicine for triage purposes improves access to expert consultation and dermatologic care when warranted.
Caveats
Teledermatology, including teledermoscopy, should be used only in the appropriate clinical context when the provider is fully comfortable deferring in-person evaluation. For example, literature is limited for dermoscopy utility in rashes, though dermoscopic images in combination with clinical images have been found to improve the tele-diagnostic accuracy of pityriasis rosea and discoid lupus erythematosus compared to clinical images alone53. Providers must take a conservative approach, for there are inherent risks involved, including inability to perform total body skin exams, biases towards focusing solely on the lesion of interest, and ultimately harm to patients with possible legal repercussions for missed or misdiagnosed lesions17.
Dermosopy has demonstrated efficacy in improving the accuracy of detecting nonpigmented skin cancers compared to the unaided eye and assists with selection of appropriate management54. It is also increasingly utilized for monitoring and follow-up for skin cancers, as well as triage and follow-up of lesions which are changing, concerning, or clinically different from other lesions. For pigmented lesions, a full skin examination is useful to determine if the dermoscopic lesion of interest appears different from others on a given individual. Dermoscopy is an especially useful tool for patients with a history of melanoma, increased risk for melanoma, or presence of many atypical nevi.
However, it is unclear if dermoscopy is helpful in assessing melanomas smaller than 6 mm33,55,56. Certain lesions, such as Spitzoid proliferations and atypical melanocytic nevi, may have challenging dermoscopy features rendering this tool less useful57. Dermoscopy may be less appropriate or useful if there are technical challenges in obtaining a quality image in areas such as the genitalia and other anatomic sites such as the inside of the external ear, medial canthi, and nares. Hypo- and non-pigmented lesions also pose a challenge since identification of structural elements is more difficult. For pigmented nail lesions, dermoscopy can be a useful adjunct for recognizing patterns consistent with benign (eg, melanonychia) or malignant (eg, melanoma) entities. However dermoscopy of the nail should not replace microscopic examination which is necessary to exclude malignancy. Finally, certain lesional characteristics are best visualized under particular types of light, so clinicians must be aware of benefits and limitations of light sources32.
Implications and call to action
Limited access to dermatology is a growing problem which is mitigated by implementation of teledermatology as an alternative to face-to-face visits. Incorporating teledermoscopy in primary care offers many benefits, including improved internet-based skin cancer screening, timely referral to dermatology, improved diagnostic accuracy of pigmented lesions, earlier detection of melanoma and basal cell carcinoma, remote evaluation for underserved populations, reduced wait times, cost effectiveness, reduced percentage of excisions, decreased malignant/benign excision ratio, post-biopsy review, educational opportunities, and continuous monitoring of lesions over time3,22,24,27,44,58–69. Given the multitude of benefits discussed, we recommend adoption of teledermoscopy with integration of these guidelines for appropriate image capture and assessment.
However, these benefits are only realized with proper user training, expertise, implementation, and workflow. Teledermoscopy is becoming more common in primary care. This set of guidelines and proposed framework directs clinicians on proper use to increase confidence, limit user errors, decrease under and misdiagnoses, and improve patient satisfaction with teledermoscopy. We recommend the development of and use of validated diagnostic criteria and characteristics when examining dermoscopic images of melanocytic and non-melanocytic lesions, and for determining whether skin lesions are suspicious. Although not discussed here, incorporation of computerized analyzing instruments or artificial intelligence (AI) can aid pattern recognition and facilitate dermoscopy use among primary care providers and dermatologists. These practice guidelines serve as a foundation for high-quality image capture and utilization in telemedicine and maximize the potential of telemedicine and artificial intelligence to benefit all patients.
Digital camera use, privacy, administration guidelines, security, licensing and credentialing, and liability should follow ATA guidelines.
Methods
Scope
These guidelines offer recommendations in consultation with experts in the field of teledermatology to delineate the proper use of teledermoscopy for all types of medical providers.
Data acquisition
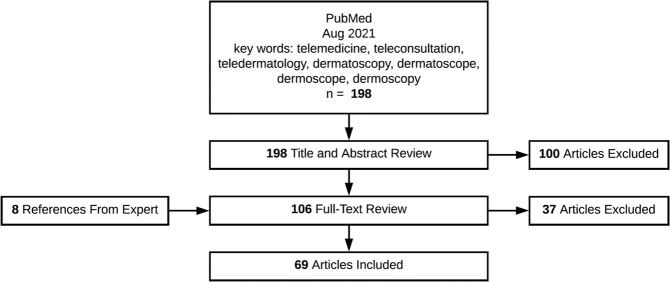
We conducted a PubMed literature search with keywords: teleconsultation, teledermatology, telemedicine, dermatoscope, dermoscope, dermoscopy, and dermatoscopy. After screening abstracts for relevance, those focusing on teledermoscopy background information, indications, types, and recommendations for proper utilization were included, for a total of 69 articles excluding duplicates. Exclusion criteria include articles that were not written in English, those that were abstracts only, and those that focused on topics other than our topic of interest, such as telemedicine, artificial intelligence, machine-learning, reflectance confocal microscopy, and patient-utilized teledermatology applications. See Fig. 3 for literature search, review, and article inclusions. A comprehensive list of equipment for teledermoscopy, including independent devices, those customized for smartphone attachment, and digital camera dermoscopy were adapted from Teledermoscopy for Teledermatology by Singh et al. 2016 along with independent review of manufacturers websites22. For image orientation, guidelines for regional images were summarized from the existing literature on teledermatology and applied for consistent image capture via dermoscopy. Recommendations regarding resolution, scale, measurement, lighting, focus, depth of field, color calibration, and field of view are summarized and adapted from The International Skin Imaging Collaboration (ISIC), which includes 9 image criteria.
Fig. 3. The Decision Tree of literature search.
Literature search, review, and article inclusions.
Final review
The final product was reviewed by dermoscopy and telemedicine experts from the ATA Teledermatology Special Interest Group, including iterative revision to incorporate feedback and commentary.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Clara N. Curiel-Lewandrowski, MD and Delaney B. Stratton, DNP, PhD, University of Arizona/Banner Dermatology Division for providing images used in Supplementary Fig. 1: Example of low-quality versus high-quality image acquisition. These images were developed as part of the International Skin Imaging Collaboration (ISIC) Image Acquisition Guidelines. Informed consent was obtained by the contributors of the image used in Supplemental Fig. 1 (Contributors: Clara N. Curiel-Lewandrowski, MD and Delaney B. Stratton, DNP, PhD, University of Arizona/Banner Dermatology Division).
Author contributions
All authors have contributed significantly to this publication. All authors have made substantial contributions to the conception or design of the work or acquisition, analysis or interpretation of the data, drafting the work or revising it critically for important intellectual content, final approval of the completed version, and accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The first two listed authors contributed equally to the work and are co-first authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Linda Camaj Deda, Rebecca H. Goldberg.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-022-00587-9.
References
- 1.Koelink CJL, Jonkman MF, Van Der Meer K, Van Der Heide WK. Examination of skin lesions for cancer: which clinical decision aids and tools are available in general practice? Eur. J. Dermatol. EJD. 2014;24:297–304. doi: 10.1684/ejd.2014.2275. [DOI] [PubMed] [Google Scholar]
- 2.Giavina Bianchi M, Santos A, Cordioli E. Dermatologists’ perceptions on the utility and limitations of teledermatology after examining 55,000 lesions. J. Telemed. Telecare. 2021;27:166–173. doi: 10.1177/1357633X19864829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrándiz L, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: A randomized teledermoscopy trial. J. Am. Acad. Dermatol. 2017;76:676–682. doi: 10.1016/j.jaad.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Bandic J, Kovacevic S, Karabeg R, Lazarov A, Opric D. Teledermoscopy for skin cancer prevention: a comparative study of clinical and teledermoscopic diagnosis. Acta Inf. Med. AIM J. Soc. Med. Inf. Bosnia Herzeg. Cas. Drustva Za Med. Inf. BiH. 2020;28:37–41. doi: 10.5455/aim.2020.28.37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young AT, et al. The role of technology in melanoma screening and diagnosis. Pigment Cell Melanoma Res. 2021;34:288–300. doi: 10.1111/pcmr.12907. [DOI] [PubMed] [Google Scholar]
- 6.Hussain K, Marghoob AA, Patel NP. Dermoscopy in the COVID-19 era: magnifying the gap for clinicians. Dermatol Pr. Concept. 2021;11:e2021069. doi: 10.5826/dpc.1102a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villani A, Scalvenzi M, Fabbrocini G. Teledermatology: a useful tool to fight COVID-19. J. Dermatol Treat. 2020;31:325. doi: 10.1080/09546634.2020.1750557. [DOI] [PubMed] [Google Scholar]
- 8.Conforti C, et al. Impact of the COVID-19 pandemic on dermatology practice worldwide: results of a survey promoted by the International Dermoscopy Society (IDS) Dermatol Pr. Concept. 2021;11:e2021153. doi: 10.5826/dpc.1101a153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argenziano G, et al. Vascular structures in skin tumors: a dermoscopy study. Arch. Dermatol. 2004;140:1485–1489. doi: 10.1001/archderm.140.12.1485. [DOI] [PubMed] [Google Scholar]
- 10.Braun RP, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch. Dermatol. 2002;138:1556–1560. doi: 10.1001/archderm.138.12.1556. [DOI] [PubMed] [Google Scholar]
- 11.Cuellar F, et al. New dermoscopic pattern in actinic keratosis and related conditions. Arch. Dermatol. 2009;145:732. doi: 10.1001/archdermatol.2009.86. [DOI] [PubMed] [Google Scholar]
- 12.Marghoob AA, Cowell L, Kopf AW, Scope A. Observation of chrysalis structures with polarized dermoscopy. Arch. Dermatol. 2009;145:618. doi: 10.1001/archdermatol.2009.28. [DOI] [PubMed] [Google Scholar]
- 13.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J. Am. Acad. Dermatol. 1987;17:571–583. doi: 10.1016/S0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 14.Scope A, Benvenuto-Andrade C, Agero ALC, Marghoob AA. Nonmelanocytic lesions defying the two-step dermoscopy algorithm. Dermatol Surg. Publ. Am. Soc. Dermatol Surg. Al. 2006;32:1398–1406. doi: 10.1111/j.1524-4725.2006.32312.x. [DOI] [PubMed] [Google Scholar]
- 15.Stricklin SM, Stoecker WV, Oliviero MC, Rabinovitz HS, Mahajan SK. Cloudy and starry milia-like cysts: how well do they distinguish seborrheic keratoses from malignant melanomas? J. Eur. Acad. Dermatol Venereol. JEADV. 2011;25:1222–1224. doi: 10.1111/j.1468-3083.2010.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheraz, A. & Halpern, S. Influence of additional dermoscopy images on teledermatology screening of skin lesions. Br. J. Dermatol. 165, 136 (2011).
- 17.Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol. Clin. 2017;35:559–563. doi: 10.1016/j.det.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Wohltmann, W., Lappan, C. & Henning, S. Teledermoscopy of pigmented lesions: A pilot study: P1702. J. Am. Acad. Dermatol.64, 214–219 (2011).
- 19.Marchetti A, et al. Diagnostic concordance in tertiary (dermatologists-to-experts) teledermoscopy: a final diagnosis-based study on 290 cases. Dermatol. Pr. Concept. 2020;10:e2020071. doi: 10.5826/dpc.1003a71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zalaudek I, et al. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. Melanocytic skin tumors. J. Am. Acad. Dermatol. 2010;63:361–374. doi: 10.1016/j.jaad.2009.11.698. [DOI] [PubMed] [Google Scholar]
- 21.Marwaha SS, et al. Comparative effectiveness study of face-to-face and teledermatology workflows for diagnosing skin cancer. J. Am. Acad. Dermatol. 2019;81:1099–1106. doi: 10.1016/j.jaad.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 22.Singh SR, Meka AP, Nguyen G, Tejasvi T. Teledermoscopy for Teledermatology. Curr. Dermatol. Rep. 2016;5:71–76. doi: 10.1007/s13671-016-0133-x. [DOI] [Google Scholar]
- 23.Tan E, Yung A, Jameson M, Oakley A, Rademaker M. Successful triage of patients referred to a skin lesion clinic using teledermoscopy (IMAGE IT trial) Br. J. Dermatol. 2010;162:803–811. doi: 10.1111/j.1365-2133.2010.09673.x. [DOI] [PubMed] [Google Scholar]
- 24.Piccolo D, et al. Teledermoscopy–results of a multicentre study on 43 pigmented skin lesions. J. Telemed. Telecare. 2000;6:132–137. doi: 10.1258/1357633001935202. [DOI] [PubMed] [Google Scholar]
- 25.Abbott LM, et al. A review of literature supporting the development of practice guidelines for teledermatology in Australia. Australas. J. Dermatol. 2020;61:e174–e183. doi: 10.1111/ajd.13249. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijden JP, Thijssing L, Witkamp L, Spuls PI, de Keizer NF. Accuracy and reliability of teledermatoscopy with images taken by general practitioners during everyday practice. J. Telemed. Telecare. 2013;19:320–325. doi: 10.1177/1357633X13503437. [DOI] [PubMed] [Google Scholar]
- 27.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–165. doi: 10.1016/S1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 28.Piccolo D, et al. Dermoscopic diagnosis by a trained clinician vs. a clinician with minimal dermoscopy training vs. computer-aided diagnosis of 341 pigmented skin lesions: a comparative study. Br. J. Dermatol. 2002;147:481–486. doi: 10.1046/j.1365-2133.2002.04978.x. [DOI] [PubMed] [Google Scholar]
- 29.Janda M, et al. Evaluating healthcare practitioners’ views on store-and-forward teledermoscopy services for the diagnosis of skin cancer. Digit. Health. 2019;5:2055207619828225. doi: 10.1177/2055207619828225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones OT, et al. Dermoscopy use in UK primary care: a survey of GPs with a special interest in dermatology. J. Eur. Acad. Dermatol. Venereol. JEADV. 2019;33:1706–1712. doi: 10.1111/jdv.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies SW, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br. J. Dermatol. 2009;161:1270–1277. doi: 10.1111/j.1365-2133.2009.09374.x. [DOI] [PubMed] [Google Scholar]
- 32.Finnane A, et al. Proposed Technical Guidelines for the Acquisition of Clinical Images of Skin-Related Conditions. JAMA Dermatol. 2017;153:453–457. doi: 10.1001/jamadermatol.2016.6214. [DOI] [PubMed] [Google Scholar]
- 33.Bono A, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br. J. Dermatol. 2006;155:570–573. doi: 10.1111/j.1365-2133.2006.07396.x. [DOI] [PubMed] [Google Scholar]
- 34.Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am. Fam. Physician. 2013;88:441–450. [PubMed] [Google Scholar]
- 35.Koh U, et al. Development of a checklist tool to assess the quality of skin lesion images acquired by consumers using sequential mobile teledermoscopy. Dermatol Basel Switz. 2021;13:1–8. doi: 10.1159/000515158. [DOI] [PubMed] [Google Scholar]
- 36.McKoy K, et al. Practice Guidelines for Teledermatology. Telemed. J. E-Health J. Am. Telemed. Assoc. 2016;22:981–990. doi: 10.1089/tmj.2016.0137. [DOI] [PubMed] [Google Scholar]
- 37.McKoy, K., Nortonm, S. & Lappan, C. Quick Guide to Store-Forward Teledermatology for Referring Providers © American Telemedicine Association (April 2012). https://teledermatology-society.org/wp-content/uploads/2013/08/quick-guide-to-store-forward-and-live-interactive-teledermatology-for-referring-providers.pdf
- 38.Witmer WK, Lebovitz PJ. Clinical photography in the dermatology practice. Semin Cutan. Med. Surg. 2012;31:191–199. doi: 10.1016/j.sder.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Katragadda C, et al. Technique standards for skin lesion imaging: A Delphi consensus statement. JAMA Dermatol. 2017;153:207–213. doi: 10.1001/jamadermatol.2016.3949. [DOI] [PubMed] [Google Scholar]
- 40.Bartley M. Photographic measuring scales. J. Vis. Commun. Med. 2012;35:152–154. doi: 10.3109/17453054.2012.714958. [DOI] [PubMed] [Google Scholar]
- 41.Agero ALC, et al. Conventional and polarized dermoscopy features of dermatofibroma. Arch. Dermatol. 2006;142:1431–1437. doi: 10.1001/archderm.142.11.1431. [DOI] [PubMed] [Google Scholar]
- 42.Wang SQ, et al. Differences in dermoscopic images from nonpolarized dermoscope and polarized dermoscope influence the diagnostic accuracy and confidence level: a pilot study. Dermatol. Surg. Publ. Am. Soc. Dermatol. Surg. Al. 2008;34:1389–1395. doi: 10.1111/j.1524-4725.2008.34293.x. [DOI] [PubMed] [Google Scholar]
- 43.Altamura D, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J. Am. Acad. Dermatol. 2010;62:67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Zalaudek I, et al. Dermoscopy in general dermatology. Dermatol. Basel Switz. 2006;212:7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 45.Pan Y, et al. Polarized and nonpolarized dermoscopy: the explanation for the observed differences. Arch. Dermatol. 2008;144:828–829. doi: 10.1001/archderm.144.6.828. [DOI] [PubMed] [Google Scholar]
- 46.Braun RP, Scope A, Marghoob AA. The “blink sign” in dermoscopy. Arch. Dermatol. 2011;147:520. doi: 10.1001/archdermatol.2011.82. [DOI] [PubMed] [Google Scholar]
- 47.Taheri A, Yentzer BA, Feldman SR. Focusing and depth of field in photography: application in dermatology practice. Ski. Res Technol. J. Int. Soc. Bioeng. Ski. ISBS Int Soc. Digit Imaging Ski. ISDIS Int Soc. Ski. Imaging ISSI. 2013;19:394–397. doi: 10.1111/srt.12058. [DOI] [PubMed] [Google Scholar]
- 48.Quigley EA, Tokay BA, Jewell ST, Marchetti MA, Halpern AC. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883–890. doi: 10.1001/jamadermatol.2015.33. [DOI] [PubMed] [Google Scholar]
- 49.Badano A, et al. Consistency and standardization of color in medical imaging: a consensus report. J. Digit Imaging. 2015;28:41–52. doi: 10.1007/s10278-014-9721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quality Standards for Teledermatology: Using “Store and Forward” Images. Primary Care Commissioning; 2013. Accessed August 24, 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=794.
- 51.Warshaw, E. et al. Teledermatology for Diagnosis and Management of Skin Conditions: A Systematic Review of the Evidence. Department of Veterans Affairs (US); 2010. Accessed October 30, 2021. http://www.ncbi.nlm.nih.gov/books/NBK49157/ [PubMed]
- 52.Maddukuri, S., Patel, J. & Lipoff, J. B. Teledermatology Addressing Disparities in Health Care Access: a Review. Curr. Dermatol. Rep. Published online March 12, 2021:1-8. 10.1007/s13671-021-00329-2 [DOI] [PMC free article] [PubMed]
- 53.Papadimitriou I, et al. Teledermoscopy of common pink, flat and scaly lesions as an adjuvant diagnostic method in everyday clinical practice: so far, so close. J. Eur. Acad. Dermatol. Venereol. JEADV. 2021;35:e507–e509. doi: 10.1111/jdv.17235. [DOI] [PubMed] [Google Scholar]
- 54.Sinz C, et al. Accuracy of dermatoscopy for the diagnosis of nonpigmented cancers of the skin. J. Am. Acad. Dermatol. 2017;77:1100–1109. doi: 10.1016/j.jaad.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Bono A, et al. Micro-melanoma detection. A clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128–131. doi: 10.1177/030089160409000125. [DOI] [PubMed] [Google Scholar]
- 56.Carli P, et al. Effect of lesion size on the diagnostic performance of dermoscopy in melanoma detection. Dermatol. Basel Switz. 2003;206:292–296. doi: 10.1159/000069939. [DOI] [PubMed] [Google Scholar]
- 57.Barcaui CB, Lima PMO. Application of Teledermoscopy in the Diagnosis of Pigmented Lesions. Int J. Telemed. Appl. 2018;2018:1624073. doi: 10.1155/2018/1624073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J. Am. Acad. Dermatol. 2011;64:1068–1073. doi: 10.1016/j.jaad.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 59.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch. Dermatol. 2001;137:1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 60.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008;159:669–676. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 61.Carli P, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J. Am. Acad. Dermatol. 2004;50:683–689. doi: 10.1016/j.jaad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Carli P, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the “dermoscopy era”: a retrospective study 1997–2001. Br. J. Dermatol. 2004;150:687–692. doi: 10.1111/j.0007-0963.2004.05860.x. [DOI] [PubMed] [Google Scholar]
- 63.Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br. J. Dermatol. 2000;143:1016–1020. doi: 10.1046/j.1365-2133.2000.03836.x. [DOI] [PubMed] [Google Scholar]
- 64.Massone C, et al. Melanoma screening with cellular phones. PloS One. 2007;2:e483. doi: 10.1371/journal.pone.0000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroemer S, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele-evaluation using cellular phones. Br. J. Dermatol. 2011;164:973–979. doi: 10.1111/j.1365-2133.2011.10208.x. [DOI] [PubMed] [Google Scholar]
- 66.Piccolo D, et al. Face-to-face diagnosis vs telediagnosis of pigmented skin tumors: a teledermoscopic study. Arch. Dermatol. 1999;135:1467–1471. doi: 10.1001/archderm.135.12.1467. [DOI] [PubMed] [Google Scholar]
- 67.Braun RP, et al. Teledermatoscopy in Switzerland: a preliminary evaluation. J. Am. Acad. Dermatol. 2000;42:770–775. doi: 10.1067/mjd.2000.103977. [DOI] [PubMed] [Google Scholar]
- 68.Moreno-Ramirez D, Ferrandiz L, Galdeano R, Camacho FM. Teledermatoscopy as a triage system for pigmented lesions: a pilot study. Clin. Exp. Dermatol. 2006;31:13–18. doi: 10.1111/j.1365-2230.2005.02000.x. [DOI] [PubMed] [Google Scholar]
- 69.Ferrara G, et al. A pilot study of a combined dermoscopic-pathological approach to the telediagnosis of melanocytic skin neoplasms. J. Telemed. Telecare. 2004;10:34–38. doi: 10.1258/135763304322764176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.