Abstract
Stimulation of the medial medullary reticular formation (MMRF) has long been reported to produce generalized inhibition of skeletal muscle activity. However, several studies have reported that in most cases MMRF stimulation produces only increases in muscle tone. In the present investigation we have found that blood pressure is a critical variable, determining whether MMRF stimulation will produce muscle excitation or inhibition. When mean arterial pressure (MAP) was >80 mmHg but <148 mmHg, MMRF stimulation produced muscle atonia. Reductions of blood pressure by pharmacological or mechanical techniques induced a reversal of response to MMRF stimulation; stimulation that produced inhibition in base-line conditions produced excitation after MAP reduction. MAP reductions of as little as 10% could cause the reversal response. In contrast, the EMG reduction to MMRF stimulation was not changed or was augmented when MAP was raised. MMRF induced atonia, and its reversal by blood pressure reduction persisted after bilateral isolation of the carotid sinus combined with vagotomy, and in the 6-hydroxydopamine-treated cat. Spinal transection at the cervicothoracic junction did not block atonia or the reversal response. It is suggested that the reversal is mediated centrally.
Keywords: muscle tone, hypertension, hypotension, chemoreceptor, decerebration, vagotomy, carotid denervation, spinal transection, narcolepsy, cataplexy, rapid-eye-movement sleep
MAGOUN AND RHINES (17) first reported that stimulation of the medial medullary reticular formation (MMRF) produced a nonreciprocal (i.e., occurring simultaneously in muscles that are normally reciprocally activated), generalized inhibition of motor activity. However, other investigators found that many decerebrate preparations do not demonstrate the MMRF-induced motor inhibition (7, 8, 25, 28). Furthermore, MMRF stimulation in the intact animal never produces motor inhibition (28). The physiological mechanisms responsible for this variability of response have not been identified.
In the intact animal, the only similar state of nonreciprocal loss of muscle tone is rapid-eye-movement (REM) sleep (12). In narcoleptics, a similar loss of muscle tone occurs in cataplexy. Cataplectic attacks are triggered by athletic exertion, sexual activity, and other behaviors with strong emotional content (9).
In the present study we have tried to identify physiological variables that might control the response to medullary stimulation in the decerebrate preparation. Such variables might also have a role in the control of muscle tone in the intact animal, particularly in relation to REM sleep and cataplexy. We find that blood pressure has a potent effect on atonia elicited by medullary stimulation. Some of these data have been reported in preliminary form (26).
METHODS
The experiments were performed on 58 decerebrated unanesthetized cats, of either sex, weighing 2.5–4.5 kg. Tracheostomy, ligation of carotid arteries, cannulation of both right femoral artery and vein, and decerebration at the precollicular level were performed under halothane-oxygen anesthesia. Bilateral vagotomy was performed after base-line stimulation studies in nine cats. Bilateral carotid isolation by ligation of the common, external, and internal carotid arteries and the ascending pharyngeal arteries was performed in two vagotomized cats. Bilateral vagotomy combined with spinal cord transection at the cervical thoracic junction was performed in two additional cats. Sympathetic degeneration was produced in one cat by 6-hydroxydopamine (6-OHDA) (10). All animals were allowed to recover from anesthesia for at least 3 h before stimulation experiments were begun. All preparations used in the study had mean arterial pressures (MAP) of >80 mmHg in the undrugged state. Neck, forelimb, and hindlimb muscles were dissected for electromyogram (EMG) recording. Blood pressure was measured using a 4-F balloon-tipped catheter (American Hospital Supply) inserted 20 cm into the femoral artery so that its tip rested in the descending aorta. Core temperature was maintained between 37 and 38°C by a heating pad. Pco2 was measured through a Beckman gas analyzer.
The brain stem was stimulated with a concentric bipolar electrode (Rhodes Medical Instruments) or with a stainless steel monopolar microelectrode with a diameter of 0.25 mm (A & M Systems). The electrode was moved stereotaxically in 0.5-mm steps from H −5.0 to H −10.0 and in l-mm steps from L 0 to L 3 bilaterally and from P 7 to P 14 (1). Stimulation consisted of a 300-ms train of 0.2.ms cathodal rectangular pulses at 20–250 Hz and 10–150 μA.
EMG was recorded with bipolar stainless steel electrodes from occipitoscapularis, splenius, biventer cervicis, and triceps brachii bilaterally and left sartorius and biceps femoris. EMG and blood pressure signals were amplified by a Grass preamplifier (model 78D), displayed on a polygraph and an oscilloscope, and recorded on FM magnetic tape for later analysis.
EMG changes were measured by a stimulus-triggered averaging technique. EMG activities for each muscle were rectified and averaged over a 500-ms period, from 100 ms before the stimulation to 100 ms after it, with a bin width of 100 μs.
Blood pressure.
Blood pressure was manipulated by inflation of a balloon placed in the descending aorta or inferior vena cava (IVC), by bleeding from the femoral vein, or by drug injection. The following drugs were dissolved in Ringer solution and injected intravenously: sodium nitroprusside (SNP, 0.05–0.15 mg/kg), papaverine (PPV, 2.0–3.0 mg/kg), prazosin (PRZ, 10–20 μg/kg), acetylcholine chloride (ACh, 3.0 μg/kg), nitroglycerin (NTG, 20–50 μg/kg), and norepinephrine (NE, 2.0–3.0 μg/kg).
Histology.
The stimulating position was marked by passing 50 μA for 20 s at the end of each tract. The brain stems were removed and stored in potassium ferrocyanide with buffered Formalin solution. Serial 40-μm sections were stained with carbol fuchsin. The effective inhibitory points were verified and reconstructed according to the atlas of Berman (1).
RESULTS
Decreases in MAP reversed or reduced inhibition of EMG activity produced by MMRF stimulation. Increases in MAP either did not change or augmented inhibition of EMG activity produced by MMRF stimulation. MAP changes produced by pharmacological and mechanical means and changes produced by hemorrhage all produced the reversal effect.
Localization of area producing muscle inhibition.
The brain stem area whose stimulation reduced muscle tone ranged from P 7 to 14, L 0 to 3, and H −6 to −9 (Fig. 1). This corresponded to the Magoun and Rhines inhibitory area (17) and included the gigantocellularis, paragigantocellularis dorsalis and ventralis, and raphe obscuris nuclei [nomenclature based on Taber (31)].
FIG. 1.
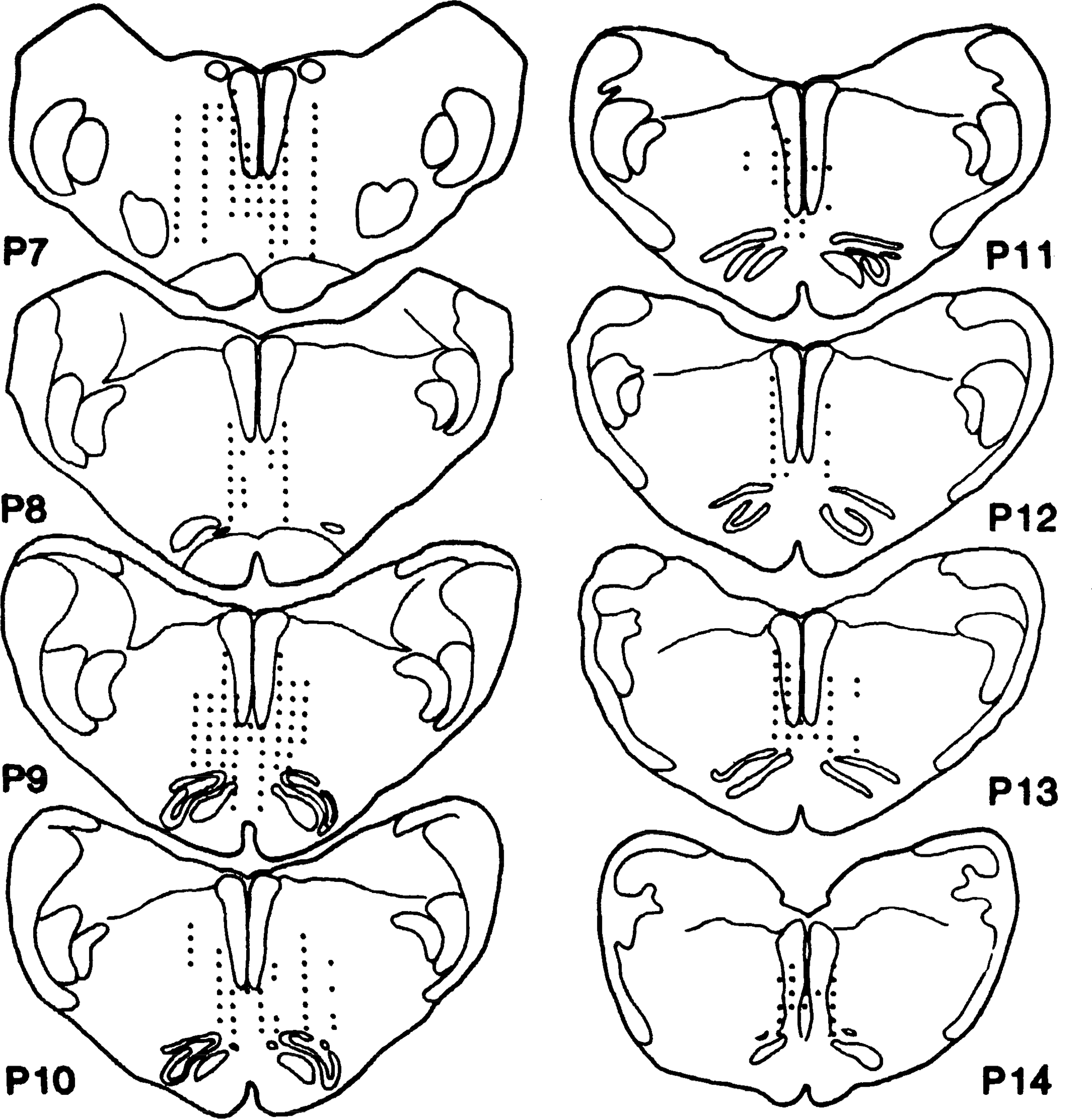
Schematic diagram showing location of medullary inhibitory area in decerebrate cat. Data is summarized from 5 animals. Dots represent points that produced bilateral inhibition when stimulated.
MMRF stimulation effects varied as a function of stimulation site. Stimulation at sites 3 mm from midline produced only contralateral inhibition and ipsilateral facilitation of neck muscles in four of six cats, whereas two of six cats showed bilateral inhibition. Stimulation at sites 2 mm from the midline produced bilateral inhibition in 8 of 14 cats and contralateral inhibition and ipsilateral facilitation in the remaining 6. However, stimulation at sites 1 mm from the midline produced bilateral inhibition in most cats (38 of 43). The most effective area for inducing bilateral inhibition is P 7–10, L 1, and H −7.0 to −9.0 (Fig. 1). This is the area that was stimulated in the studies presented below.
Muscle response to MMRF stimulation.
Figure 2 illustrates the depression of muscle tone produced by MMRF stimulation. The onset of inhibition was defined as the point when EMG fluctuations fell >2 standard deviations below base line. The latency of inhibition in this typical case is 45 ms. Muscle tone recovered to base-line levels 52 ms after the end of the stimulus train. Rebound facilitation was found in most cases (Fig. 2). A comparison of all stimulation sites shows that MMRF inhibition was significantly more pronounced in contralateral than in ipsilateral neck muscles (P < 0.05, sign test; Fig. 3).
Fig. 2.
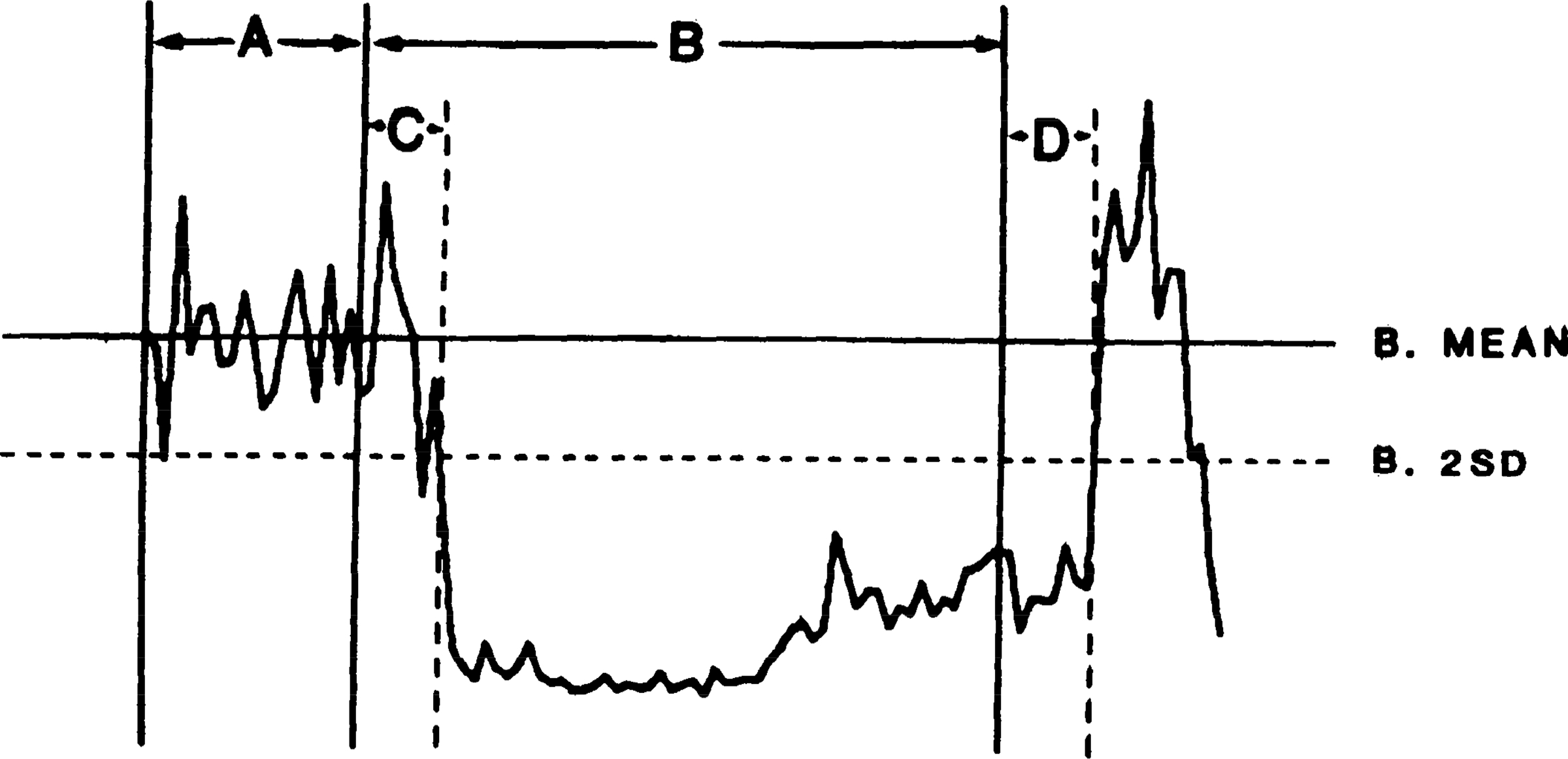
Computer average of electromyogram (EMG) change produced by 20 medullary stimulation trains. Trains were 300 ms in duration with 100-Hz pulse frequency. EMG was recorded from splenius muscle; 100 ms of EMG activity were averaged before stimulation and 100 ms after stimulus termination. Base-line mean and standard deviation were calculated from 100-ms prestimulation base line of muscle activity. A, 100-ms prestimulation base line; B, 300-ms train stimulation; C, latency of inhibition; D, recovery time; B mean, base-line mean EMG activity; B 2SD, 2 times base-line standard deviation.
FIG. 3.
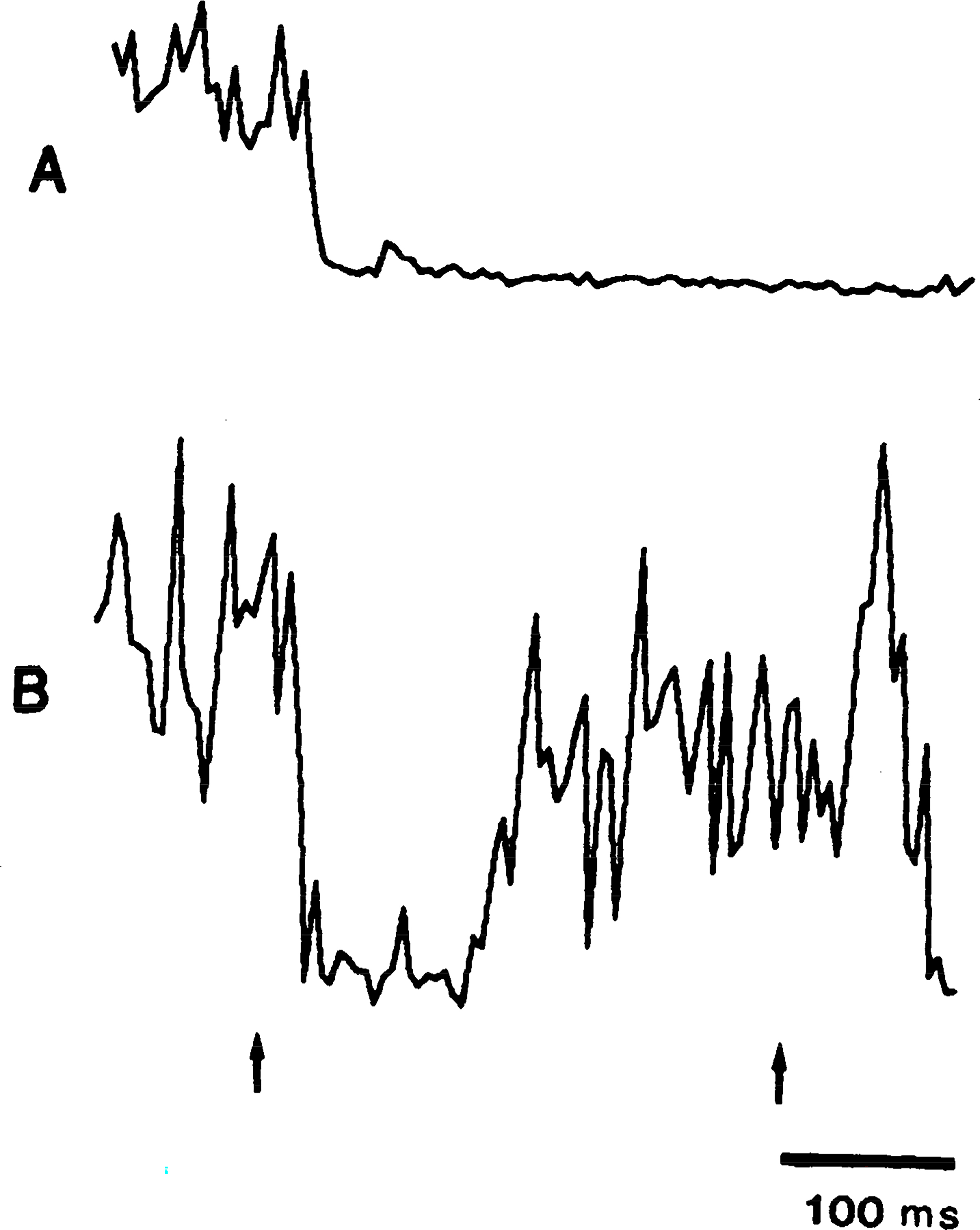
Bilateral inhibition of electromyogram (EMG) to stimulation of medial medullary reticular formation. Train stimulation was delivered to left side of brain stem at P 8, L 1, and H −6. EMG activities were averaged from left and right splenius muscles. EMG reduction was greater in contralateral (A) than in ipsilateral (B) muscle. Left and right arrows indicate start and end of train.
The relationship between the magnitude of inhibition and the stimulus intensity was not linear. In six cats, four neck and two forelimb muscles were examined by varying current with stimulation pulse frequency held constant. When inhibition occurred, it was always visible at lower stimulation intensities than facilitation. For example, in Fig. 4, the threshold for splenius inhibition was 20 μA. More inhibition could be obtained when the stimulus intensity increased, with maximum inhibition visible at 30 μA. At higher intensities, the muscle response to MMRF stimulation was biphasic, with muscle excitation following the initial inhibition. The magnitude of the late facilitation was increased with further increases in the stimulus intensity.
Fig 4.
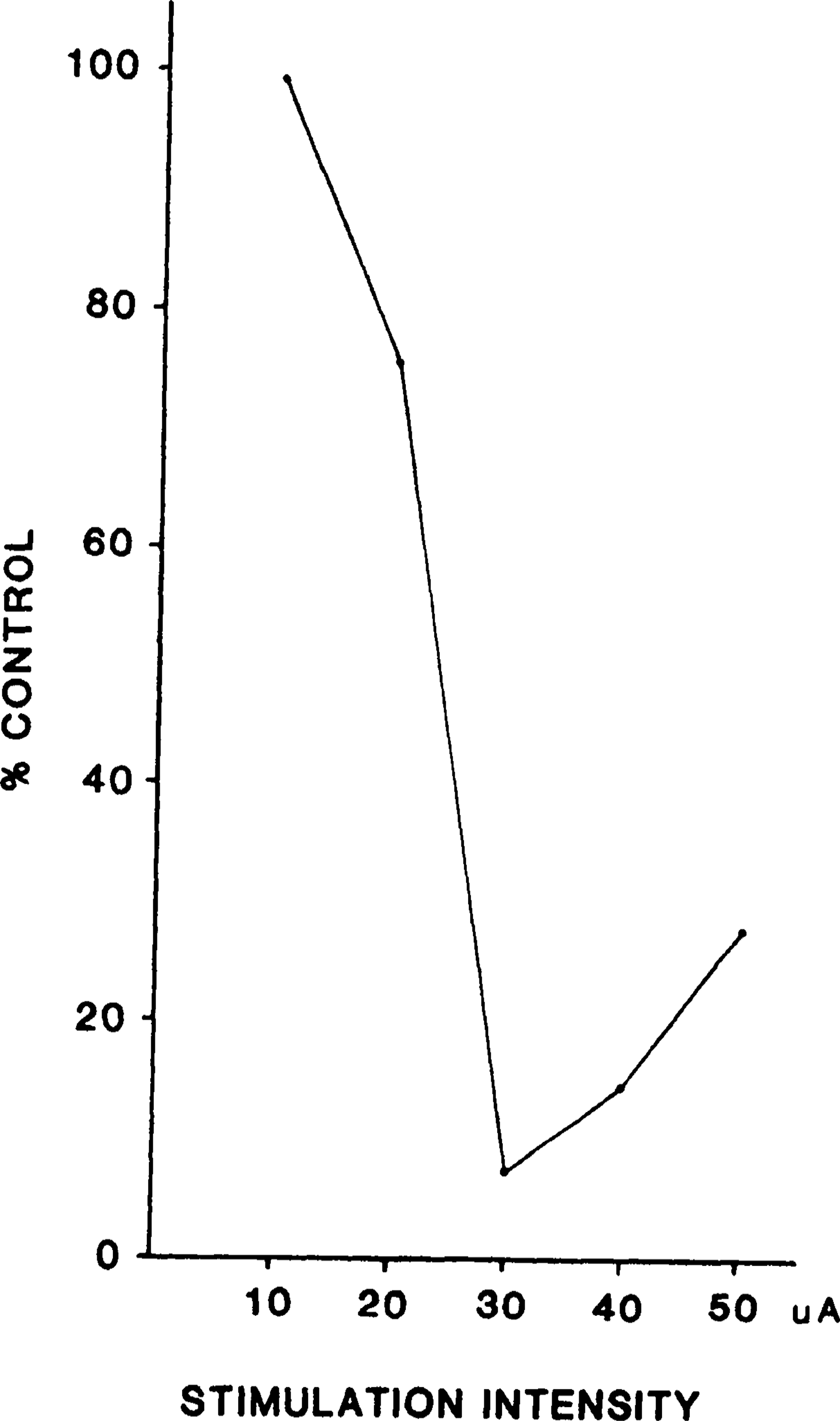
Example of relationship between stimulation intensity and magnitude of muscle (splenius) inhibition. Threshold for inhibition was 20 μA; inhibition was maximal at 30 μA and was diminished at 40 μA.
Blood pressure and muscle tone.
The finding of youmans et al. (36) that changes in blood pressure produce changes in basal muscle tone was confirmed. Increases in blood pressure caused by inflation of a balloon in the descending aorta or by injection of NE produced decreased muscle tone in 15 of 18 cases in 8 cats. This decrease appeared between 4 and 50 s (mean ± SE: 21.2 ± 15.9 s) after the point of maximum blood pressure increase and lasted for 1.5–6.3 min (mean ± SE: 4.0 ± 1.49 min), returning subsequently to base-line levels. Conversely, increased basal muscle tone could be elicited by decreasing blood pressure via inflation of a balloon in the IVC, bleeding from the femoral vein, and injection of hypotensive drugs. This hypertonus occurred between 5 and 42 s after the maximal blood pressure change (mean ± SE: 25.2 ± 12.2 s) and lasted for 1.4–8.1 min (mean ± SE: 4.1 ± 1.9 min).
Youmans and his collaborators (36) reported that the decrease in muscle tone was mediated by the vagus nerve. In contrast with their findings, vagotomy did not abolish the effect on muscle tone and response to MMRF stimulation in the present investigation. This result is in agreement with Trelease et al. (33), who found that vagotomy did not change the diaphragmatic response to blood pressure change.
Effect of blood pressure on EMG response to MMRF stimulation.
Thirty-four cats were studied in this experiment. Trains (300 ms) of 0.2-ms pulses at 100 Hz between 10 and 90 μA (30–50 μA in most experiments) were used to stimulate the MMRF. Blood pressure decreases, induced pharmacologically or mechanically, reduced or reversed the MMRF induced inhibition of muscle tone in 145 of 173 cases and had no effect in 28 cases. Stimulation that had elicited inhibition prior to MAP reduction produced excitation after reduction. This was not merely a change in threshold, since stimulation at lower or higher intensities was also ineffective in eliciting inhibition after blood pressure reduction. In no case was the amount of inhibition elicited by MMRF stimulation increased by blood pressure reduction (P < 0.00001, sign test).
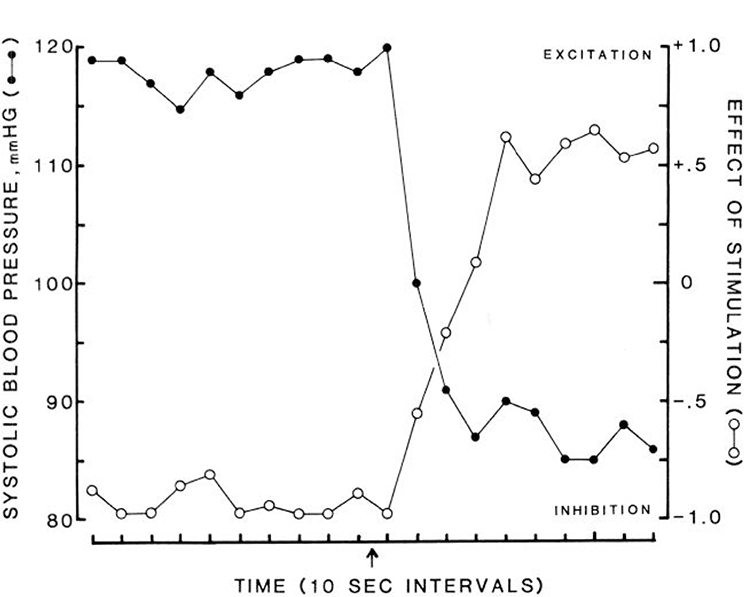
Inflation of a balloon in the IVC, which decreased cardiac return and thereby MAP, reversed the muscle inhibition induced by MMRF stimulation (Fig. 5). The reversal in the response began when the blood pressure had dropped 10% and lasted throughout the period of low blood pressure. After deflation of the balloon, the MAP returned to base-line levels and MMRF inhibition returned. Figure 6 shows the time course of the reversal of inhibition and reduction of blood pressure elicited by SNP. Other hypotensive agents also produced the reversal effect in parallel with their hypotensive effect. Figure 7 shows the reversal effect induced by MAP reduction by ACh.
FIG. 5.
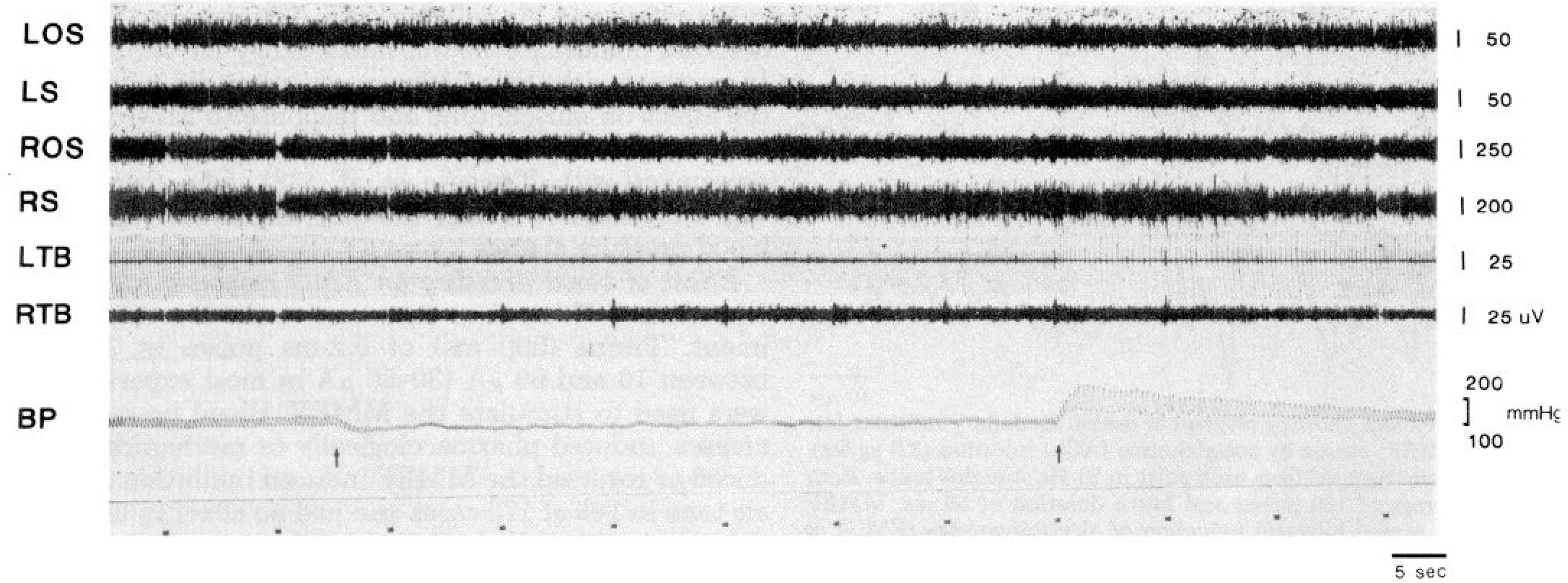
Reversal of medullary atonia by obstruction of inferior vena cava. Inflation of embolectomy balloon at 1st arrow causes a drop in blood pressure and reverses effect of stimulation of medial medullary reticular formation (MMRF). Deflation of balloon at 2nd arrow restores blood pressure to base-line levels and MMRF-induced atonia returns. LOS, left occipitoscapularis; LS, left splenius; ROS, right occipitoscapularis; RS, right splenius; LTB, left triceps brachii; RTB, right triceps brachii; BP, blood pressure. Bottom trace marks stimulation trains.
FIG. 6.
Time course of effect of sodium nitroprusside on medullary inhibition. Sodium nitroprusside injection at arrow. Filled circles, blood pressure; open circles, effect of medullary stimulation on muscle tone. Complete inhibition was scored as −1, excitation as +1. Each data point is average of 24 stimulation sites in 6 cats.
FIG. 7.
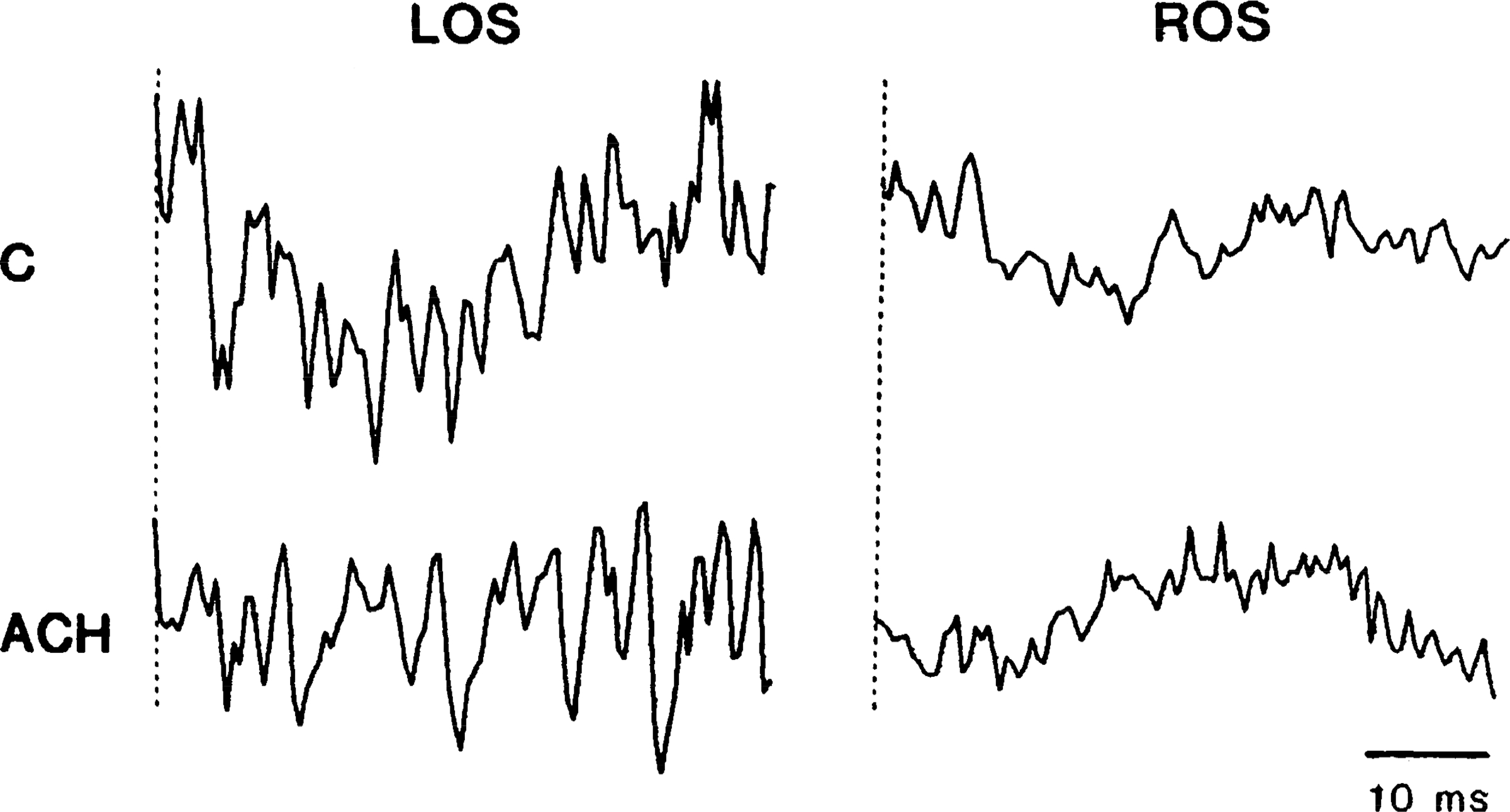
Average showing reversal of medial medullary reticular formation (MMRF) atonia by acetylcholine (ACh) injection (3.0 μg/kg). Averages were triggered from each pulse in 20-Hz stimulus trains. Each trace is average of 100 pulses and has a duration of 50 ms. MMRF stimulation caused bilateral reduction of electromyogram (EMG) in control predrug state (C). Hypotension caused by ACh eliminated (left occipitoscapularis, LOS) or reversed (right occipitoscapularis, ROS) EMG reduction.
Stimulation of a few MMRF sites in four cats produced rapid elevations of MAP during the reversal response. However, stimulation at most MMRF sites effective in producing motor inhibition did not affect blood pressure either before or after the reversal of response by MAP reductions.
Injection of NE shortly after PRZ injection restored blood pressure to base-line levels and immediately restored the inhibitory effect of MMRF stimulation. NE injection by itself either did not alter or increased the amount of atonia induced by stimulation (Fig. 8). A similar effect was produced by blood pressure elevation caused by inflation of a balloon in the descending aorta (Fig. 8).
FIG. 8.

Top: lack of effect of blood pressure (BP) increase on medial medullary reticular formation (MMRF) inhibition. Norepinephrine-induced pressor response seen after second stimulation does not block MMRF-induced atonia. LOS, left occipitoscapularis; LS, left splenius; ROS, right occipitoscapularis; RS, right splenius; LTB, left triceps braii; RTB, right triceps brachii. Middle and bottim: elevated BP, induced by obstruction of the descending aorta does not interfere with MMRF-induced atonia. Note that 2 muscles (LOS, LS) show reversal of atonia response to MMRF stimulation during transient blood pressure reduction immediately after deflation of balloon at 2nd arrow. Calibration, 5 s.
Cats that had a relatively high initial blood pressure level were less sensitive than those with a relatively low initial blood pressure to a given fall in blood pressure (P < 0.0003, Mann-Whitney U test, Table 1). All the animals with an initial blood pressure of <100 mmHg showed low blood pressure induced reversal of the EMG inhibition produced by MMRF stimulation. However, five of nine cats with initial blood pressure >120 mmHg did not show the reversal response after blood pressure reduction. Five cats had major variations in base-line MAP during the experiment. Four showed initially high blood pressure without reversal response and low blood pressure with the reversal response later in the experiment. The fifth cat initially showed low pressure with the reversal response and high pressure without the reversal response later. Even though initial levels of blood pressure were significantly correlated with response to medullary stimulation, all of the variance could not be explained by this variable. Thus some preparations with intermediate initial levels of MAP showed reversal responses at blood pressure levels producing atonia in preparations with lower initial MAP levels. Therefore, although absolute MAP can account for much of the variance in response to MMRF stimulation, the magnitude of change from the initial postdecerebration MAP is also a factor.
TABLE 1.
Relationship between initial MAP and muscle response to MMRF stimulation during reduction of blood pressure
| Initial MAP, mmHg | Muscle Response |
|
|---|---|---|
| Reversal* | No effect† | |
|
| ||
| 80 <90 | 3 | |
| 90 <100 | 6 | |
| 100 <110 | 5 | |
| 110 <120 | 2 | |
| 120 <130 | 3 | 2 |
| 130 | 1 | 3 |
Values represent no. of cats.
More than 50% of mean arterial pressure (MAP) reductions produced reversal of inhibitory response to medial medullary reticular formation (MMRF) stimulation in each of these preparations.
Less than 50% of MAP reductions produced reversal in each of these preparations.
Only a small blood pressure decrease was required to induce reversal of the response to MMRF stimulation. Reversal or reduction in inhibition could be seen when MAP was reduced <10% (˜10 mmHg). However, at a few sites (10 of 83 tested) no reversal could be induced even when MAP was reduced 40% or more (Table 2). These preparations had relatively high base-line MAP.
TABLE 2.
Relationship of %MAP reduction, methodology, and muscle response to MMRF stimulation
| Reversal Response |
No Effect |
Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %Change MAP: | <10 | ≥10 | ≥20 | ≥30 | ≥40 | ≥50 | >60 | Total | % | <10 | ≥10 | ≥20 | ≥30 | ≥40 | Total | % | |
|
| |||||||||||||||||
| Methodology | |||||||||||||||||
| SNP | 1 | 4 | 7 | 11 | 17 | 8 | 48 | 87.3 | 1 | 2 | 4 | 7 | 12.7 | 55 | |||
| ACh | 1 | 1 | 1 | 1 | 4 | 80.0 | 1 | 1 | 20.0 | 5 | |||||||
| PPV | 2 | 4 | 12 | 7 | 4 | 29 | 72.5 | 1 | 7 | 3 | 11 | 27.5 | 40 | ||||
| PRZ | 4 | 3 | 7 | 63.6 | 1 | 1 | 2 | 4 | 36.4 | 11 | |||||||
| NTG | 1 | 3 | 3 | 2 | 1 | 10 | 83.3 | 1 | 1 | 2 | 16.7 | 12 | |||||
| IVC | 5 | 9 | 7 | 3 | 9 | 8 | 41 | 93.2 | 2 | 1 | 3 | 6.8 | 44 | ||||
| Bleeding | 3 | 2 | 1 | 6 | 100.0 | 0.0 | 6 | ||||||||||
| Total | 6 | 16 | 20 | 30 | 33 | 25 | 15 | 145 | 83.8 | 1 | 2 | 5 | 10 | 10 | 28 | 16.2 | 173 |
MAP, mean arterial pressure; MMRF, medial medullary reticular formation; SNP, sodium nitroprusside; ACh, acetylcholine; PPV, papaverine; PRZ, prazosin; NTG, nitroglycerin; IVC, balloon inflated in inferior vena cava.
Inflation of a balloon in the IVC and hemorrhage were the most effective manipulations for inducing the reversal response. The response could be obtained even with MAP reductions of <10% (Table 2). All hypotensive drugs produced the reversal response (Table 2).
Of 54 preparations tested, all but four produced inhibition at one or more medullary sites. Stimulation throughout the brain stem of the remaining four did not produce evidence of nonreciprocal inhibition. In contrast to the “within-animal” situation, preparations without any inhibition had significantly higher MAP (P < 0.0002, t test) than preparations that produced inhibition (which could be reversed by blood pressure reduction). Average MAP in preparations producing inhibition was 118 mmHg (range 80–200). MAP in preparations not producing inhibition was 167 mmHg (range 148–172). Blood pressure reduction and elevation in these latter preparations did not restore medullary inhibition.
Effect of vagotomy, carotid denervation, 6-OHDA treatment, and spinal transection on response to MMRF stimulation.
Bilateral vagotomies were performed in nine cats. Vagotomy did not block the inhibitory response to MMRF stimulation or the reversal of response induced by MAP reduction by SNP (Fig. 9). Furthermore, carotid sinus isolation combined with vagotomy did not prevent either the inhibition evoked by MMRF stimulation or the reversal of inhibition produced by SNP. Transection of the spinal cord at the cervicothoracic junction did not block MMRF inhibition or the reversal response in neck muscles. Bilateral section of the vagus after spinal transection also did not block either MMRF inhibition or the reversal response (Fig. 9). Post-sympathetic nerve degeneration with 6-OHDA by itself and when combined with vagotomy also did not alter MMRF inhibition or the reversal response.
FIG. 9.
Polygraph record showing that bilateral vagotomy (top), spinal transection (middle), and transection combined with vagotomy (bottom), do not block medial medullary reticular formation (MMRF)-induced atonia or its reversal by blood pressure (BP) reduction. LOS, left occipitoscapularis; LS, left splenius; RS, right splenius. A, muscle atonia resulting from MMRF stimulation at beginning of sodium nitroprusside injection period (0.05 mg/kg), prior to blood pressure fall below threshold for the reversal response; B, reversal of MMRF-induced atonia during low blood pressure period; C, MMRF atonia reappeared when blood pressure returned to base-line levels.
DISCUSSION
We have found that blood pressure reduction reversed the EMG inhibition produced by MMRF stimulation. By using both mechanical and a variety of pharmacological methods, we have demonstrated that this effect was not due to the specific pharmacological or physiological effects of any one hypotensive technique. The carotid baroand chemoreceptors and vagus nerve are not required for MMRF induced inhibition or for the reversal of response to MMRF stimulation induced by blood pressure reduction. Neither are afferents entering the spinal cord below the cervical level.
It has been reported that depolarization and hyperactivity of the spinal motoneurons results from spinal ischemia (32). However, this effect was achieved only with blood pressure reductions far greater than those employed in the present study. Although cerebral blood flow is kept within certain limits by autoregulation, changes of flow in intact anesthetized dogs by SNP (3) and in humans by PRZ (21) and PPV (4) have been reported. Hemorrhagic hypotension in intact cats (15) and NTG-induced hypotension in anesthetized dogs (3) have been reported to not produce a significant effect on cerebral blood flow. However, the response of cerebral blood flow to drug-induced and mechanical hypotension may be different in our unanesthetized and open-skull decerebrate cat preparation, since different methods of anesthesia themselves produce different effects on cerebral blood flow (16). Vasoactive drugs are known to alter Pco2 in intracerebral fluid (20). Treatments that elevate MAP produce an increase in central Pco2, presumably by reducing cerebral blood flow, whereas treatments that reduce MAP have the reverse effect. We hypothesize that central chemoreceptors (2), or other central receptors, respond to the Pco2 increase or other changes linked to MAP and excite the medullary cell population responsible for inhibition. Conversely, blood pressure reduction is hypothesized to block the action of medullary neurons responsible for inhibition of muscle tone.
We have found that the initial blood pressure of each animal is an important factor determining whether reduction of blood pressure will produce the reversal response. The four preparations in our series in which medullary stimulation never produced muscle inhibition had significantly higher MAP than the 54 preparations in which inhibition was present. This latter group could be further subdivided into preparations with relatively high MAP (120–200 mmHg) and preparations with moderate MAP (80–120 mmHg). Animals with relatively high MAP produced EMG inhibition that in many cases was not reversed by blood pressure reduction. MMRF stimulation in preparations with moderate MAP produced inhibition that was in all cases reversed to excitation by blood pressure reduction. Preparations with MAP of <80 mmHg were not used in this study. It is interesting to note that in the intact behaving cat MAP ranges between 80 and 95 mmHg (22). Blood pressure is chronically elevated in the decerebrate cat due to the ligation of the carotid arteries and the consequent interruption of blood flow to the carotid bodies. Thus the reversal response after blood pressure reduction occurs when blood pressure approaches the normal range. The inhibitory response to MMRF stimulation that has long been observed in decerebrate animals may be related to their elevated MAP. In accordance with the hypothesis discussed previously, MAP reduction may act through a reduction in central Pco2. It has been shown that very high blood pressure levels are associated with a “break-through of autoregulation” in which cerebral blood flow increases with increasing perfusion pressure (29). This may be responsible for the paradoxical loss of atonia response that we find in preparations with very high MAP. In these preparations, the high cerebral blood flow levels would be associated with low Pco2, just as they are in preparations with moderate MAP in which blood pressure was decreased. It is also possible that other mechanisms are responsible for this relatively rare effect. Nevertheless, it is clear that an MAP between 80 and 148 mmHg is optimal for producing medullary inhibition. Small MAP reductions or very high initial MAP are incompatible with medullary inhibition.
Many investigators (5, 6, 11, 13, 14, 18, 19) have reported that stimulation of the MMRF produces bilateral inhibition of muscle activity. However, the nonreciprocal, global inhibitory effect of MMRF stimulation described by Magoun and Rhines (17) cannot be obtained in all decerebrated animals (25, 27). As a result, there has been continuing uncertainty over whether the MMRF is truly an inhibitory area, and if so, what experimental conditions were responsible for causing some preparations to produce MMRF inhibition when stimulated, whereas other preparations produced only excitation. We have previously reported that one variable affecting the likelihood of eliciting generalized inhibition after MMRF stimulation is transection level. Although generalized inhibition is relatively common after transections at the precollicular level, MMRF stimulation of preparations with transections through the pons usually produce only reciprocal effects (25). In the present study we identify blood pressure as another potent variable affecting the motor changes produced by MMRF stimulation. Since blood pressure is under the control of pontine mechanisms (30, 34, 35), it is likely that these two effects are related. Together, the blood pressure and transection level effects may be responsible for much of the variability that has been reported in different laboratories in the likelihood of producing atonia by MMRF stimulation.
Cataplexy, a condition associated with narcolepsy, appears to involve an increase in the activity of the medullary inhibitory mechanism. In cataplexy, a sudden emotional stimulus leads to an inhibition of skeletal muscle tone and reflex activity similar to that seen in REM sleep (9). In normal individuals, a similar stimulus usually produces increased levels of muscle tone. Because cataplexy is thought to imolve an abnormal activation of the same medullary region manipulated in the present study, we studied the effect of blood pressure manipulations on its occurrence. We found that NE injection produced an increase in the occurrence of cataplexy, time locked to the blood pressure rise (23). We also found that a rapid increase in heart rate consistently precedes the onset of spontaneous cataplexy (24). Therefore the cardiovascular changes identified in the present study, possibly linked to changes in central Pco2, may also underlie the triggering of cataplexy.
Acknowledgments
This study was supported by the Medical Research Service of the Veterans Administration, National Institute of Neurological and Communicative Disorders and Stroke Grant NS-1640 and National Science Foundation Grant BNS 84-19782.
REFERENCES
- 1.Bergman AL The Brain Stem of the Cat. Madison: Univ. of Wisconsin Press, 1968. [Google Scholar]
- 2.Caker L, and Terzioglu M. Localization of CO2 sensitive units in the rostral medullary chemosensitive area of the cat. In: Central Neurone Environment, edited by Schlaefke ME, Koepchen HP, and See WR. Berlin: Springer-Verlag, 1983, p. 52–69. [Google Scholar]
- 3.Colley PS, and Sivrajan M. Regional blood flow in dogs during halothane anesthesia and controlled hypotension produced by nitroprusside or nitroglycerin. Anesth. Analg. Cleveland 63: 503–510, 1984. [PubMed] [Google Scholar]
- 4.Conway RS, and Weiss HR. Effect of papaverine on regional cerebral blood flow and small vessel blood content. Eur. J. Pharmacol 68: 17–24, 1980. [DOI] [PubMed] [Google Scholar]
- 5.Engberg I, Lundberg A, and Ryall RW. Reticulospinal inhibition of transmission in reflex pathways. J. Physiol. Lond 194: 201–223, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gassel MM, Marchiafava PL, and Pompeiano O. Tonic and phasic inhibition of spinal reflexes during deep, desynchronized sleep in unrestrained cats. Ach. Ital. Biol 102: 471–499, 1964. [PubMed] [Google Scholar]
- 7.Gernandt BE, and Thulin CA. Reciprocal effects upon spinal motoneurons from stimulation of bulbar reticular formation. J. Neurophysiol 18: 113–129, 1955. [DOI] [PubMed] [Google Scholar]
- 8.Giaquinto S, Pompeiano O, and Somogyi I. Descending inhibitory influences on spinal reflexes during natural sleep. Arch. Ital. Biol 102: 282–307, 1964. [PubMed] [Google Scholar]
- 9.Guilleminault C Cataplexy. In: Nurcolepsy, edited by Guilleminault C, Dement WC, and Passouant P. New York: Spectrum, 1976, p. 125–143. [Google Scholar]
- 10.Haeusler G, Haefely W, and Thoenen H. Chemical sympathectomy of the cat with 6-hydroxydopamine. J. Phurmacol. Exp. Ther 170: 50–61, 1969. [PubMed] [Google Scholar]
- 11.Jankowska E, Lund S, Lundberg A, and Pompeiano O. Inhibitory effects evoked through ventral reticulospinal pathways. Arch. Ital. Biol 106: 124–140, 1968. [PubMed] [Google Scholar]
- 12.Jouvet M Recherches sur les structures nerveuses et les mechanismes responsables des différentes phases du sommeil physiologique. Arch. Ital. Biol 100: 125–206, 1962. [PubMed] [Google Scholar]
- 13.Lunas R, and Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons. J. Neurophysiol 27: 579–591, 1964. [DOI] [PubMed] [Google Scholar]
- 14.Llinas R, and Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons. J. Neurophysiol 28: 413–422, 1965. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie ET, Farrafx JK, Fitch W, Graham DI, Gregory PC, and Harper AM. Effects of hemorrhagic hypotension on the cerebral circulation. I. Cerebral blood flow and pial arteriolar caliber. Stroke 10: 711–718, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Maeuwa T, Mcdowall DG, and Okuda Y. Brain-surface oxygen tension and cerebral cortical blood flow during hemorrhagic and drug-induced hypotension in the cat. Anesthesiology 51: 313–320, 1979. [DOI] [PubMed] [Google Scholar]
- 17.Magoun HW, and Rhines R. An inhibitory mechanism in the bulbar reticular formation. J. Neurophysiol 9: 165–171, 1946. [DOI] [PubMed] [Google Scholar]
- 18.Peterson BW, Pitts NG, and Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp. Brain Res 36: l–20,1979. [DOI] [PubMed] [Google Scholar]
- 19.Peterson BW, Pitts NG, Fukushima K, and Mackel R. Reticulospinal excitation and inhibition of neck motoneurons. Exp. Brain Res 32: 471–489,1978. [DOI] [PubMed] [Google Scholar]
- 20.Pinard E, and Seylaz J. Intracerebral gas partial pressure changes under vasoactive drugs: a mass spectrometry study. pfluegers Arch. 375: 25–30, 1978. [DOI] [PubMed] [Google Scholar]
- 21.Rutland MD, Lee TY, Nimmon CC, Granowska M, and Britton KE. Measurement of the effects of a single dose of prazosin on the cerebral blood flow in hypertensive patients. Postgrad. Med. J 56:818–822,1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiromani P, Siegel JM, Tomaszewski KS, and Mcginty DJ. Alterations in blood pressure and REM sleep after pontine carbachol microinfusion. Exp. Neural 91: 285–292, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel JM Blood pressure, blood flow and cataplexy (Abstract). Proceedings of the 5th Internatiunal Congress of Sleep Research. In press, 1987. [Google Scholar]
- 24.Siegel JM, Fahringer H, Tomaszewski KS, Kaitan K, Kilduff T, and Dement WC. Heart rate and blood pressure changes associated with cataplexy in canine narcolepsy. Sleep Basel 9: 216–221,1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel JM, Nienhuis R, and Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 268: 344–348,1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel JM, Wilson WJ, and Tomaszewski KS. Effect of blood pressure changes on atonia produced by stimulation of the medial medulla (Abstract). Sleep Res. 13: 39, 1984. [Google Scholar]
- 27.Spracue JM, and Chambers WW. Regulation of posture in intact and decerebrate cat. I. Cerebellum, reticular formation, vestibular nuclei. J. Neurophysiol, 16: 451–463, 1953. [DOI] [PubMed] [Google Scholar]
- 28.Sprague JM, and Chambers WW. Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat. Am. J. Physiol 176: 52–64, 1954. [DOI] [PubMed] [Google Scholar]
- 29.Strandgaard S, Mackenzie ET, Jones JV, and Harper MA. Studies on the cerebral circulation of the baboon in acutely induced hypertension. Stroke 7: 287–290, 1976. [DOI] [PubMed] [Google Scholar]
- 30.Svensson TH, and Thoren P. Brain noradrenergic neurons in the locus coeruleus: inhibition by blood volume load through vagal afferents. Brain Res. 172: 174–178, 1979. [DOI] [PubMed] [Google Scholar]
- 31.Taber E The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of the cat. J. Comp. Neurol 116: 27–70, 1971. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y Electrophysiological studies of spinal activity by occlusion of aorta. Nagoya J. Med. Sci 31: 155–170, 1968. [PubMed] [Google Scholar]
- 33.Trelease RB, Sieck GC, Marks JD, and M Harper R. Respiratory inhibition induced by transient hypertension during sleep in unrestrained cats. Exp. Neurol 90: 173–186, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Ward DG, and Gunn CG. Locus coeruleus complex: elicitation of a pressor response and a brain stem region nm for its occurrence. Brain Res 107: 401–406, 1976. [DOI] [PubMed] [Google Scholar]
- 35.Ward DG, and Gunn CG. Locus coeruleus complex: differential modulation of depressor mechanisms. Brain Res 107: 407–411, 1976. [DOI] [PubMed] [Google Scholar]
- 36.Youmans WB, Murphy QR, Turner JK, Davis LD, Briggs DI, and Hoye AS. Activity of abdominal muscles elicited from the circulatory system. Am. J. Phys. Med 42: l–70, 1963. [PubMed] [Google Scholar]