Abstract
In many dorsolateral pontine neurons, auditory stimulation produces an initial excitation followed by a sustained inhibition. We now report that rapid eye movement (REM) sleep deprivation, for periods of from 22–48 h, reduced this auditory evoked inhibition of unit discharge. Inhibition returned to baseline levels after recovery REM sleep. Prior work indicates that the auditory evoked inhibition seen in noradrenergic cells in this region is partially mediated by norepinephrine. We hypothesize that the reduction in inhibition that we see is a consequence of either downregulation/desensitization of norepinephrine receptors or reduced norepinephrine release resulting from REM sleep deprivation.
Keywords: Rapid eye movement sleep, Pons, Audition, Norepinephrine
More than 35 years after its discovery, the function(s) of rapid eye movement (REM) sleep remain unclear. We have hypothesized that an important function of REM sleep is to upregulate, or interrupt the downregulation of norepinephrine (NE) receptors occurring in waking20. This hypothesis is based on the following findings. Noradrenergic neurons of the locus coeruleus discharge continuously during both active and quiet waking states. The only time at which such activity is substantially reduced is in sleep, with a virtually complete cessation of activity occurring during REM sleep4,9,10,11,16. The continuous application of NE agonists has been shown to produce a downregulation/desensitization of NE receptors20. We have therefore hypothesized that the interruption of NE release in REM sleep is a mechanism for preventing this desensitization. This hypothesis predicts that synaptic responses utilizing NE will be attenuated by REM sleep deprivation.
Sensory stimulation has been shown to produce a phasic excitatory response followed by a long duration suppression of activity in dorsolateral pontine cells recorded in the cat and rat5,10,11,14,15. Two mechanisms have been found to be involved in the stimulation-evoked suppression of activity. One is a norepinephrine mediated collateral inhibition from locus coeruleus cells1. The second is a calcium-mediated potassium conductance, not utilizing NE receptors3. Recent work has determined that both calcium and NE-mediated mechanisms contribute, in approximately equal amounts, to the inhibition following sensory stimulation7.
Therefore, our hypothesis would predict that loss of REM sleep, and consequent downregulation of NE receptors, will produce a reduction in the NE-mediated inhibition resulting from sensory stimulation. In the present study, we recorded from single units in the dorsolateral pons of the freely moving cat and determined their response to auditory stimulation. We then deprived the animal of REM sleep, while permitting non-REM sleep, for 22–68 h, and again observed the response of the same unit to auditory stimulation. We found that REM sleep deprivation reduced the stimulation evoked inhibition and that this inhibition returned after recovery sleep. To our knowledge, this is the first demonstration of an effect of sleep deprivation on neuronal responsiveness at the single unit level.
Three mongrel female cats served as subjects. They were habituated to the chamber and recording platforms and then implanted with electrodes for recording unit activity in the dorsolateral pons, as previously described13,19.
We were able to acquire extremely long duration single unit recordings by using flexible formvar insulated 32 µm microwires protruding 7 mm from the head-mounted microdrives, and by moving the drives at a very slow rate while searching for active units. Drives were advanced no more than 100 µm/day and were not moved at all for the duration of the experiment after single units were isolated. Once a unit with signal to noise ratio > 3/1 was discriminated, recording was performed for at least one hour to confirm the stability of the waveform. The cat was then transferred to a 30 × 35 cm control platform 27 cm above the surface of a 5 cm deep pool of water. The tank was placed in a well ventilated 55 × 58 × 85 cm recording chamber, with a 15 × 40 cm glass window.
The unit signal, electroencephalogram (EEG), electromyogram (EMG), electro-oculogram (EOG), and lateral geniculate nucleus (LGN) ponto-geniculo-occipital (PGO) spikes, were recorded. The cats were fed once a day (between 12.00 and 13.00 h) throughout the study. After baseline recording on the 30 × 35 cm control platform was completed, the platform was replaced by a smaller 12 cm × 12 cm platform13,17,23 to deprive the animal of REM sleep while permitting non-REM sleep. The shallow pool of water serves to prevent the animal from sleeping on the chamber floor. Cats walk and play in the water. However, they climb back to the platform to sleep. While they can have slow wave sleep on the platform, the muscle relaxation that accompanies REM sleep causes them to extend their body over the edge of the platform and arouse. This is analogous to the case of a human sleeping in a chair without adequate head support. We have never seen a cat fall off the platform in waking or sleep. The large platform was returned to the chamber for recovery sleep13.
Five ms, 3 kHz, 100–105 dB SPL tones were presented in blocks of 50, given in a randomized sequence at intervals of 15–30 s. Peristimulus interval histograms were calculated. Histograms were collected during quiet wakefulness22 in pre-deprivation, deprivation and recovery periods. Units which were lost prior to 22 h of deprivation were not used in our analyses. All other units with auditory response were studied. Three of the cells (U19 [Unit 19 in our series],U21,U25) were first discriminated at the start of the deprivation procedure, and therefore did not have pre-deprivation data, but did have recovery data.
After the end of deprivation, and once the neuronal response to auditory stimulation had returned to the control level for at least 12–24 h, the effect of the α2 adrenergic agonist clonidine on auditory response was studied. Baseline histograms were collected, then clonidine (25 µg/kg) was injected i.p. and auditory response retested 30 min later.
The baseline prestimulus mean spikes per bin was calculated for each histogram for the 160 ms preceding the stimulus. The period of discharge suppression (inhibition) was defined as follows: the bins after the stimulus having the minimum spike count were identified. Adjacent bins before and after the midpoint of these bins were included up to the point where the number of spikes per bin was 50% of the mean firing rate of the pre-stimulus period. The period thus defined for the first recorded baseline histogram was then employed in the analysis of subsequent histograms from that unit. For all the statistical analyses, values from at least 5 histograms in each condition were available.
Six cells (U19, U21, U22, U25, U27, U35), localized to the dorsolateral pontine reticular formation (Fig. 1), were each continuously recorded during a mean of 33 h of REM sleep deprivation and 53.7 h of recovery and pre-deprivation sleep. In baseline recordings, the discharge rates in these cells were 18.4 ± 15.7 in active waking, 8.7 ± 8.7 in quiet waking, 8.8 ± 9.3 in slow wave sleep, and 22.3 ± 22.2 in REM sleep, thus it is unlikely that these cells were noradrenergic2,4,6,8,9,11.
Fig. 1.
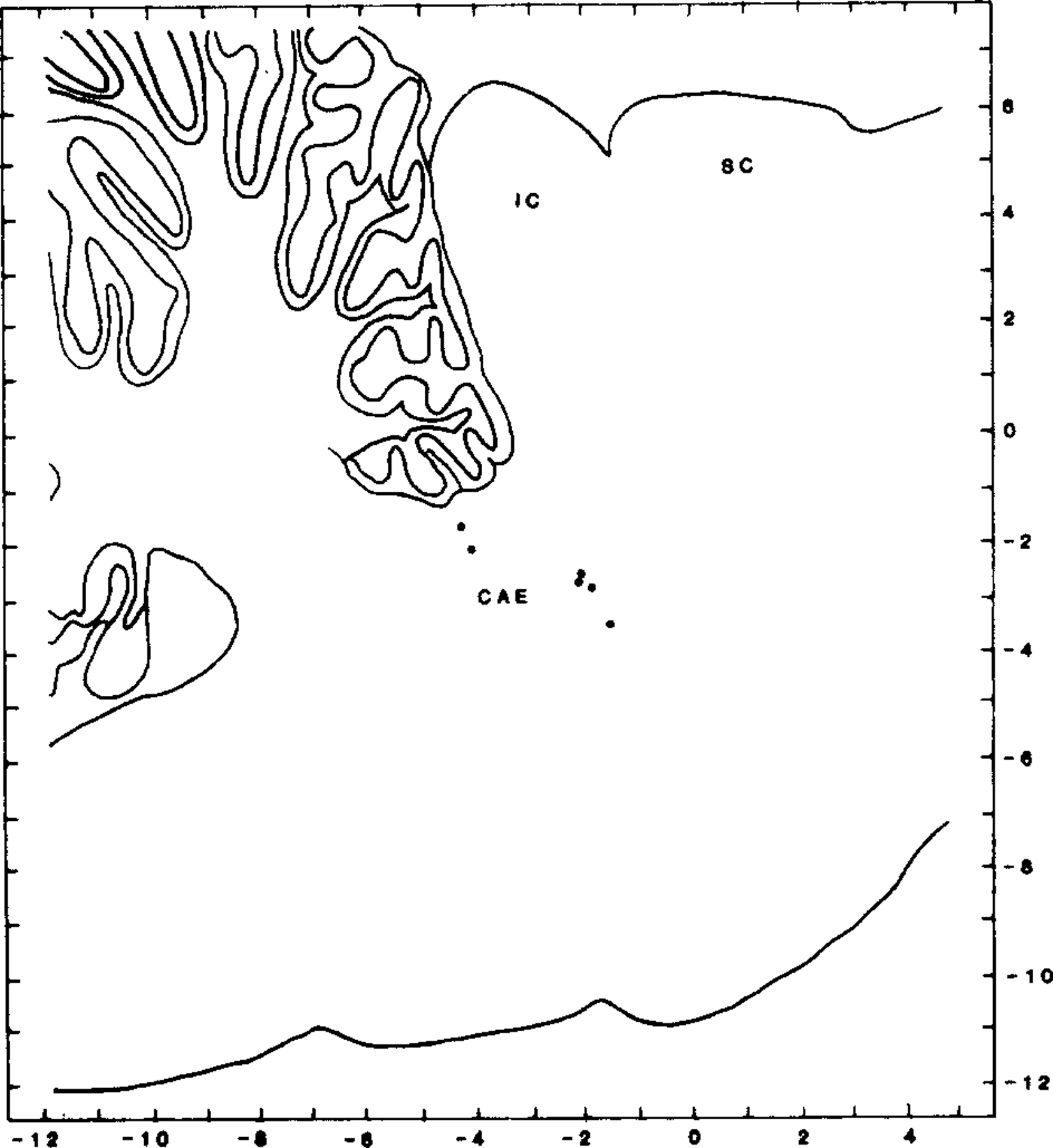
Distribution of recorded cells within the dorsolateral pons. Cells are plotted on sagittal section, 2.9 mm lateral to midline. Laterality of recorded cells ranged between 2 and 3.5 mm from midline. CAE indicates position of locus coeruleus; IC, inferior colliculus; SC, superior colliculus.
Restriction to the small platform eliminated REM sleep and reduced stage 2 non-REM sleep. The REM sleep loss was largely made up during the first 14 h of the recovery period13.
Stimulation produced an inhibition of unit discharge with a latency of 37 ± 17.2 ms after auditory stimulus onset and a duration of 54 ± 40.4 ms. This inhibition (Fig. 2) was preceded by an increase in discharge rate with a mean latency of 12.4 ± 3.4 ms and 20.6 ± 8.6 ms duration in 4 of the cells. The other two cells showed inhibition of background discharge without a preceding facilitation. Auditory response was tested at the end of deprivation and at the end of the recovery period in all 6 cells, and also prior to deprivation in 3 of these cells. The inhibition was significantly smaller after REM sleep deprivation than after recovery sleep (P < 0.001, paired t5 = 6.57; Fig. 2, Fig. 3). The pre-deprivation and recovery data were not significantly different (Table I).
Fig. 2.

Oscilloscope tracing of 5 superimposed sweeps showing auditory evoked excitation followed by inhibition. REM sleep deprivation produces a reduction in inhibition, which is restored with recovery sleep. Stimulus artifact indicates onset of auditory stimulus.
Fig. 3.
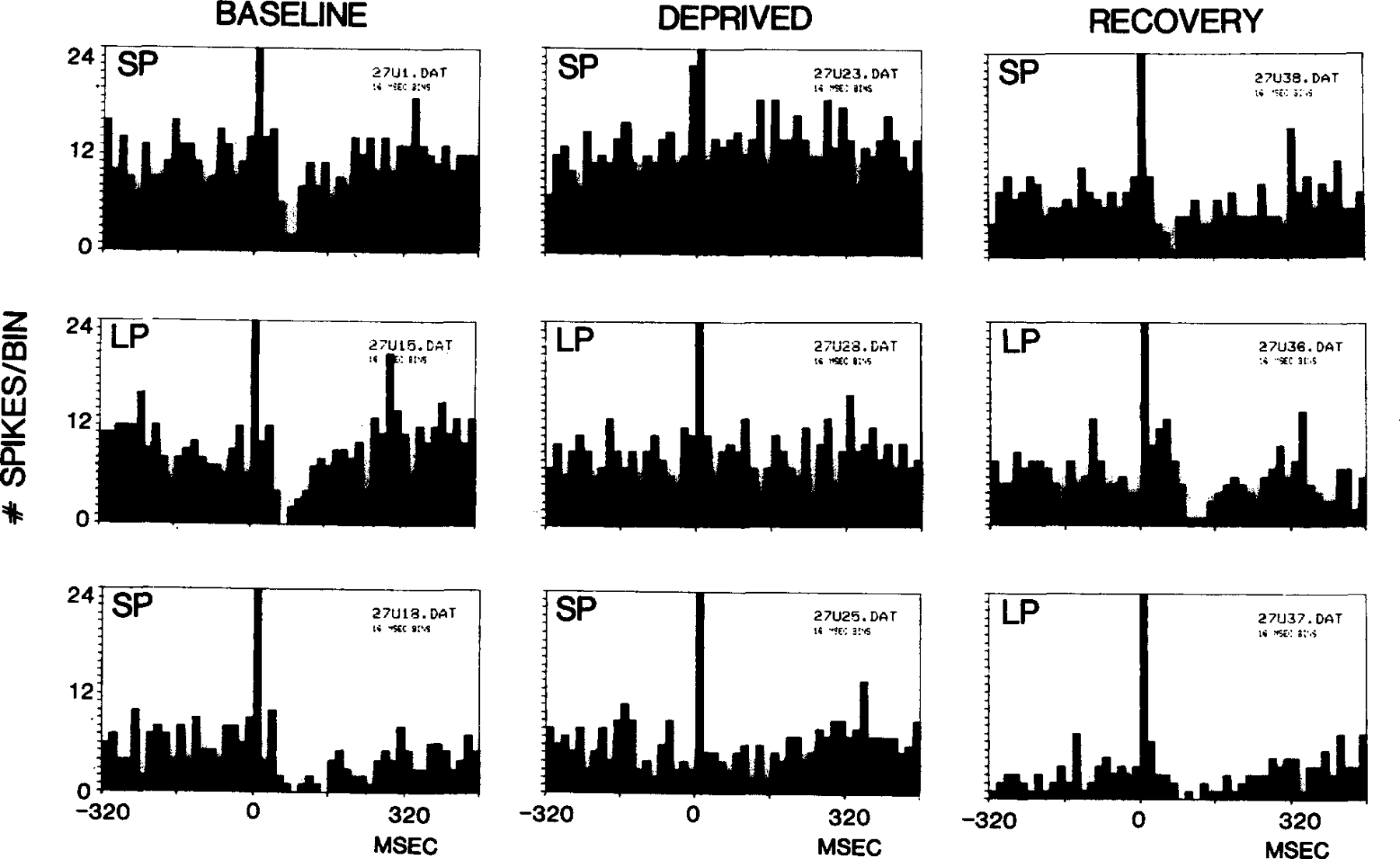
Poststimulus interval histograms of unit discharge to auditory stimulation with deprivation and recovery. Figure illustrates the reduction of inhibition in U27 with REM sleep deprivation, and the persistence of this effect with variations in firing rate and with both small platform (SP) and large platform (LP) testing. In the top 3 panels, the unit was tested on the small platform prior to REM sleep deprivation, after REM sleep deprivation and after recovery. In the middle series, all tests were done on the large platform. In the bottom series the first two tests were performed on the small platform and the third on the large platform.
TABLE I. Changes in inhibition with REM sleep deprivation.
Ratio of prestimulus spikes per bin to spikes per bin during period of inhibition. Inhibition ratio was lower after deprivation than in baseline and recovery conditions. Duration of baseline, deprivation, and recovery conditions is given in parentheses.
| Unit number | Baseline | Deprivation | Recovery |
|---|---|---|---|
| 19 | 1.6 (22 h) | 2.4 (50 h) | |
| 21 | 1.9 (22 h) | 3.8 (50 h) | |
| 22 | 3.2 (20 h) | 1.5 (24 h) | 2.4 (22 h) |
| 25 | 1.4 (23 h) | 3.0 (22 h) | |
| 27 | 2.3 (3 h) | 1.3 (40 h) | 2.4 (30 h) |
| 35 | 1.7 (24 h) | 1.1 (68h) | 2.0 (101 h) |
| Mean | 2.4 (15.7 h) | 1.5 (33 h) | 2.7 (45.8 h) |
The change in inhibition was not due simply to restriction to the small platform during testing. The effect was present in cells recorded from cats moved to either large or small platforms for either the baseline or deprivation auditory stimulation tests, after deprivation on the small platform (Fig. 3). The effect could also not be attributed to small changes in EEG state during the test procedure. As illustrated in Fig. 4, the reduction of inhibition with deprivation could be seen if auditory stimulation was delivered during epochs with EEG synchrony or on a desynchronized background.
Fig. 4.
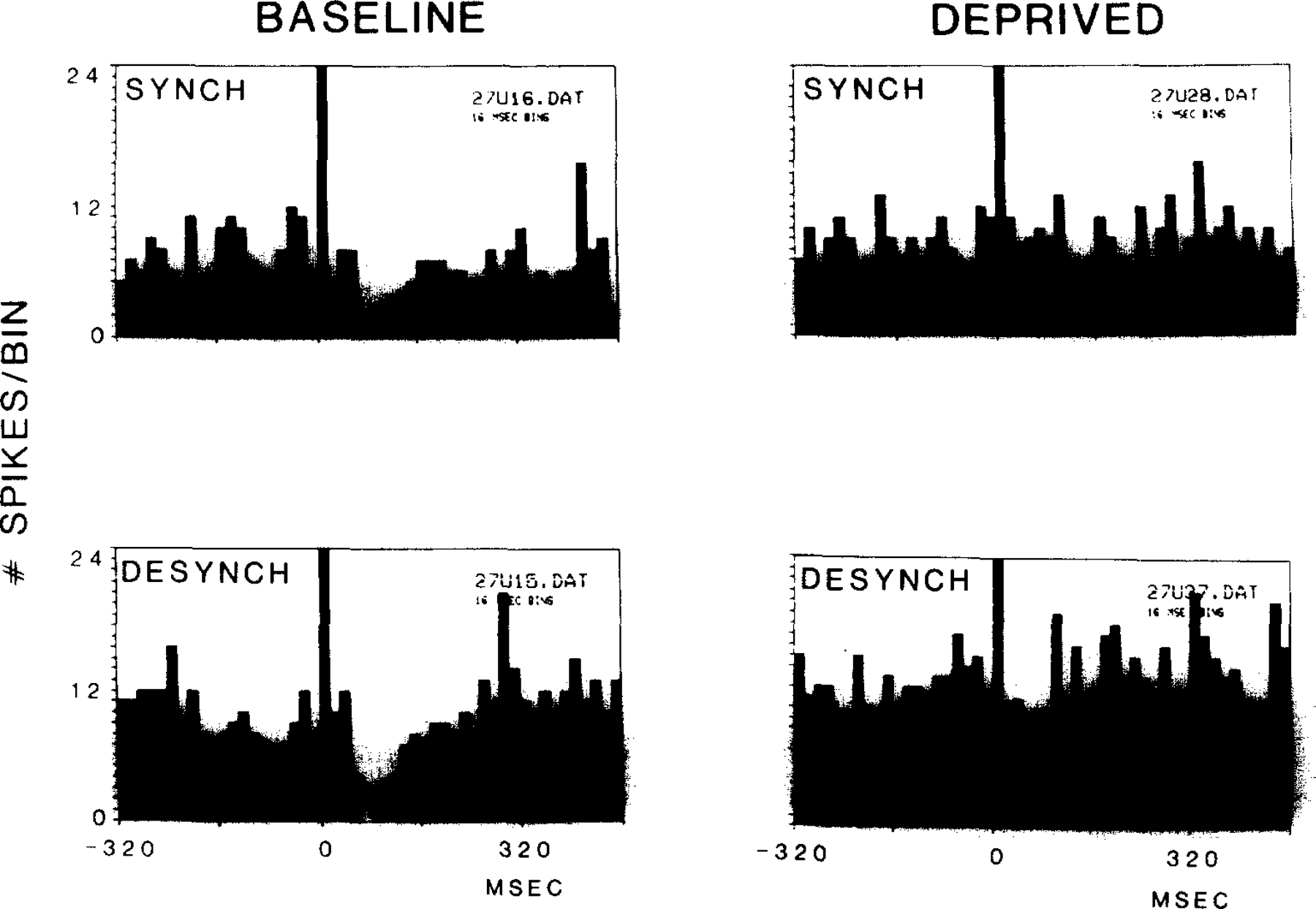
Independence of auditory evoked inhibition from variations in the EEG. Inhibition was present prior to REM sleep deprivation in both drowsy (synchronized) and desynchronized states22 prior to deprivation, but was absent in both conditions after deprivation.
Since clonidine at doses of 2–4 µg/kg is known to dramatically reduce discharge in locus coeruleus neurons16, we hypothesized that clonidine administration would reduce auditory evoked inhibition, as did REM sleep deprivation. Three cells with auditory induced inhibition were tested after clonidine administration. In each case, clonidine reduced the auditory induced inhibition (P < 0.001, paired t2 = 8.12; Fig. 5).
Fig. 5.
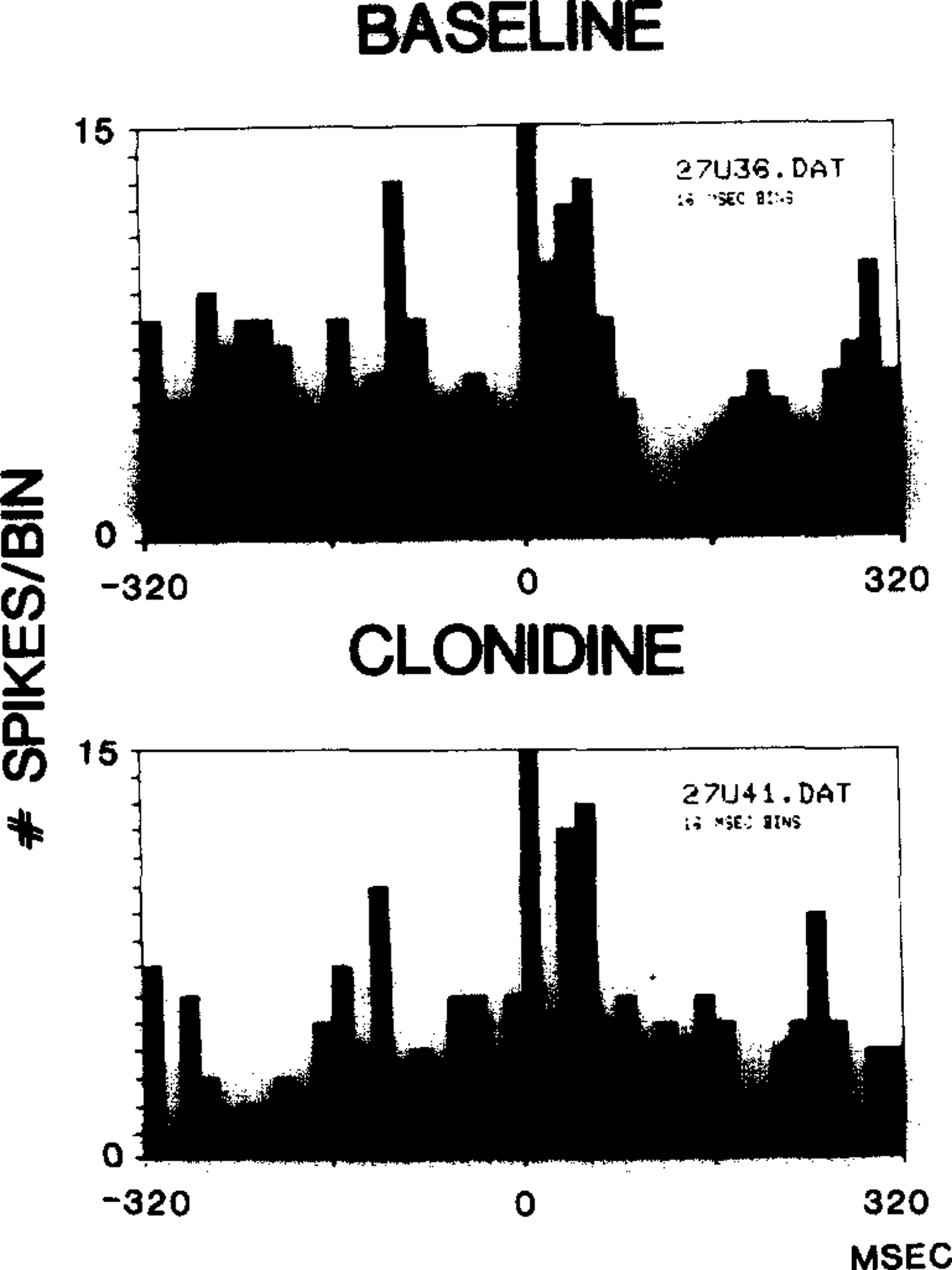
Clonidine reduced auditory evoked inhibition of unit discharge.
REM sleep deprivation decreased or abolished the auditory induced inhibition of unit discharge. Recovery REM sleep restored this inhibition to baseline levels. Since these cells were not of the ‘REM sleep-off’ type, it is unlikely that they were noradrenergic2,4,6,8,9,11. They were, however, intermixed with REM sleep-off cells that we were studying at the same time13. The recorded cells may be cholinergic or may have any of a number of other transmitters that are concentrated in this region12,18,21,24. We emphasize that all dorsolateral pontine cells we encountered that showed the auditory induced inhibition, had a reduction of this inhibition with REM sleep deprivation. We hypothesize that the auditory evoked inhibition seen in these cells is mediated by collaterals of neighboring NE cells, as is the case with auditory evoked inhibition in norepinephrine-containing cells. Our evidence that clonidine reduces the auditory stimulus induced inhibition seen in these cells is consistent with the hypothesis that this inhibition is mediated, at least in part, by norepinephrine7.
The reduction of inhibition that we have seen demonstrates an effect of REM sleep deprivation on sensory response at the single unit level. We have previously reported a reduction in discharge of NE containing REM sleep-off cells with REM sleep deprivation13. These findings are both consistent with our hypothesis that REM sleep serves to maintain the sensitivity of NE receptors. Thus, we interpret the reduction in the waking discharge of NE-containing cells with REM sleep deprivation as a protective response, that evolved to slow the progressive reduction of NE receptor sensitivity resulting from sleep loss. In this context, the reduction of auditory-induced inhibition that we see in the present study can be seen to be a result of a reduced NE release. Alternatively, the reduced inhibition could be a result of a reduction of NE receptor sensitivity caused by the REM sleep deprivation. Further work would be necessary to distinguish between these alternatives, which are not mutually exclusive. It is also possible that other transmitters are involved in the loss of the auditory evoked inhibition of unit activity that we have observed after REM sleep deprivation.
Acknowledgments
Supported by the Medical Research Service of the Veterans Administration, and PHS Grants NS14610, HL41370 and MH43811
References
- 1.Aghajanian GK, Cedarbaum JM and Wang RY, Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons, Brain Research, 136 (1977) 570–577. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG and Sinnamon HM, The locus coeruleus: neurobiology of a central noradrenergic nucleus, Prog. Neurobiol, 9 (1977) 147–196. [DOI] [PubMed] [Google Scholar]
- 3.Andrade R and Aghajanian GK, Locus coeruleus activity in vitro: intrinsic regulation by a calcium-dependent potassium conductance but not α2-adrenoceptors, J. Neurosci, 4 (1984) 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aston-Jones G and Bloom FE, Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle, J. Neurosci, 8 (1981) 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aston-Jones G and Bloom EE., Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli, J. Neurosci, 1 (1981) 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Mansari M, Sakai K and Jouvet M, Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats, Exp. Brain Res, 76 (1989) 519–529. [DOI] [PubMed] [Google Scholar]
- 7.Ennis M and Aston-Jones G, Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons, Brain Research, 374 (1986) 299–305. [DOI] [PubMed] [Google Scholar]
- 8.Foote SL, Bloom FE and Aston-Jones G, Nucleus locus ceruleus: new evidence of anatomical and physiological specificity, Physiol. Rev, 63 (1983) 844–914. [DOI] [PubMed] [Google Scholar]
- 9.Hobson JA, McCarley RW and Nelson JP, Location and spike-train characteristics of cells in anterodorsal pons having selective decreases in firing rate during desynchronized sleep, J. Neurophysiol, 50 (1983) 770–783. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs BL, Brain monoaminergic unit activity in behaving animals. In Epstein AN and Morrison AR (Eds.), Progress in Psychobiology and Physiological Psychology, Academic Press, New York, 1987, pp. 171–206. [Google Scholar]
- 11.Jacobs BL, Single unit activity of locus coeruleus neurons in behaving animals, Prog. Neurobiol, 27 (1986) 183–194. [DOI] [PubMed] [Google Scholar]
- 12.Jones BE and Beaudet A, Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study, J. Comp. Neurol, 261 (1987) 15–32. [DOI] [PubMed] [Google Scholar]
- 13.Mallick BN, Siegel JM and Fahringer H, Changes in pontine unit activity with REM sleep deprivation, Brain Research, 515 (1989) 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen K, Morilak DA and Jacobs BL, Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli, Brain Research, 371 (1986) 324–334. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen K, Strecker R and Jacobs B, Single unit response of noradrenergic, serotonergic and dopaminergic neurons in freely moving cats to simple sensory stimuli, Brain Research, 369 (1986) 336–340. [DOI] [PubMed] [Google Scholar]
- 16.Reiner PB, Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats, Brain Research, 378 (1986) 86–96. [DOI] [PubMed] [Google Scholar]
- 17.Satinoff E, Drucker-Colin RR and Hemandez-Peon R, Paleocortical excitability and sensory filtering during REM sleep deprivation, Physiol. Behav, 7 (1971) 103–106. [DOI] [PubMed] [Google Scholar]
- 18.Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV and Gillin JC, Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation, Sleep, 11 (1988) 1–16. [DOI] [PubMed] [Google Scholar]
- 19.Siegel JM, McGinty DJ and Breedlove SM, Sleep and waking activity of pontine gigantocellular field neurons, Exp. Neurol, 56 (1977) 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel JM and Rogawski MA, A function for REM sleep: regulation of noradrenergic receptor sensitivity, Brain Research Rev, 13 (1988) 213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutin EL and Jacobowitz DM, Immunocytochemical localization of peptides and other neurochemicals in the rat laterodorsal tegmental nucleus and adjacent area, J. Comp. Neurol, 270 (1988) 243–270. [DOI] [PubMed] [Google Scholar]
- 22.Ursin R and Sternum MB, A manual for standardized scoring of sleep and waking states in the adult cat, Brain Information Service/Brain Research Institute, University of California, Los Angeles, 1981. [Google Scholar]
- 23.Vimont-Vicary P, Jouvet-Mounier D and Delorme F, Effets et comportementaux des privations de sommeil paradoxal chez le chat, Electroencephalogr. Clin. Neurophysiol, 20 (1966) 439–449. [DOI] [PubMed] [Google Scholar]
- 24.Vincent SR and Reiner PB, The immunohistochemical localization of choline acetyltransferase in the cat brain, Brain Res. Bull, 18 (1987) 371–415. [DOI] [PubMed] [Google Scholar]


