Abstract
Previous studies in our laboratory have found that muscle atonia could be triggered by two distinct areas of the medial medulla, a caudal region, corresponding to the nucleus paramedianus (NPM) and a rostral region, corresponding to the nucleus magnocellularis (NMC). The former region is responsive to acetylcholine (ACh) and the latter region is responsive to glutamate. In this study we have measured the endogenous ACh release across the sleep–wake cycle in these two areas with the microdialysis technique in unanesthetized, freely moving cats. We found that ACh release in NPM was state-dependent and was about 30% higher P < 0.001) during rapid eye movement (REM) sleep than during slow-wave sleep and wakefulness. However, ACh release in NMC was not selectively elevated in REM sleep. The enhancement of ACh release in NPM during REM sleep supports our hypothesis that ACh release onto cholinoceptive neurons in this area mediates the muscle atonia of REM sleep.
Keywords: REM sleep, Atonia, Muscle activity, Acetylcholine, Nucleus paramedianus, Nucleus magnocellularis, In vivo microdialysis, Narcolepsy, Cataplexy
Muscle atonia, the tonic feature characteristic of rapid eye movement (REM) sleep5,17, can be induced by two supraspinal structures, the pontine reticular formation and the medial medu1la14. A loss of muscle tone can be evoked by injection of the cholinergic agonist carbachol into the pontine inhibitory area (PIA), which corresponds to dorsolateral regions of the nucleus reticularis pontis oralis17. Recent studies show that there are many small-sized cholinoceptive neurons and cholinergic terminals in PIA4,7. A specific increase of ACh release is also found in this region prior to and during REM sleep6. The second area in which stimulation can elicit atonia is the medial medulla3,12. Our previous studies in decerebrate animals have shown that glutamate stimulation but not ACh stimulation of the rostral portion of the medial medulla, corresponding to the nucleus magnocellularis (NMC), will trigger atonia8. In contrast, cholinergic stimulation but not glutamatergic stimulation is effective in the caudal portions of this area, corresponding to the nucleus paramedianus (NPM)8. However, there are cholinoceptive neurons both in NMC and NPM1,15. If cholinoceptive neurons in NPM are responsible for muscle atonia, there should be a specific increase of ACh release in this area during REM sleep. In the present study, we attempted to clarify the relationship between local ACh release in NPM and NMC and sleep stages in cats using the microdialysis technique.
Four adult cats (two males and two females) weighing 3.0–4.5 kg were anesthetized with sodium pentobarbital (Nembutal, 35 mg/kg i.p.) and chronically implanted with standard sleep electrodes for recording cortical electroencephalograms (EEG), ponto-geniculo-occipita1 (PGO) waves, eye-movement potentials (EOG) and nuchal electromyograms (EMG). Guide cannulae (21G) for microdialysis probes were also stereotaxically implanted above the target regions, NMC (A –9.5, L 1.0 D –7.5 to –9.5) and NPM (A –13.5, L 1.0, D –7.0 to –9.0) (Fig. 1). After the experiment, the positions of the probes were histologically verified.
Fig. 1.

A diagram demonstrating the positions of microdialysis probes. The tip (semipermeable membrane) of the probe is located in NPM (A –13.5, L 1.0, D –7.0 to –9.0) and in NMC (A –9.5, L 1.0, D –7.5 to –9.5). The shaded portions indicate semipermeable membrane. NPM, nucleus paramedianus of medulla; NGC, nucleus gigantocellularis; IO, inferior olive; 6, nucleus abducens; 7G, genu of the facial nerve.
Following recovery from surgery, at least 12 h prior to the collection of dialysate, a microdialysis probe was inserted, through the guide cannulae into NMC or NPM. Brain microdialysis and sleep monitoring were simultaneously performed between 08.00 h and 17.00 h in the freely moving, unanesthetized cat. A coaxial type of microdialysis probe, made of polyamido-coated quartz glass, was covered by a semipermeable membrane at its tip (O.D.: 220 µm; length: 2 mm; 90% cut off, 50 kDA; supplied by EICOM, Kyoto. Ringer solution containing 0.1 mM physostigmine, a specific acetylcholinesterase inhibitor, was perfused at the flow rate of 2 µl/min. Dialysate was collected for 5 min during a specific sleep–waking state and injected into the mobile phase. The mobile phase was 100 mM phosphate buffer (pH 8.5), containing 0.6 mM tetramethylammonium and 0.55 mM 1-decanesulfonic acid (NAKARAI, sodium salt). ACh level in the perfusate was determined by a high-performance liquid chromatography (HPLC) and electrochemical detection (ECD) system (EICOM). The coefficient of variation in the determination of 1 pmol of acetylcholine and choline by this HPLC-ECD system was 1.4% and 2.6%, respectively. ACh peak height on the ECD recorder was linearly correlated with the amount of ACh applied to the HPLC-ECD system within the range between 0.06 and 5.0 pmol.
Twelve dialysate samples were obtained during each stage of wakefulness (W), slow–wave sleep (SWS) and REM sleep from both NMC and NPM. A two-way analysis of variance (sleep–wake stage by region) with replicated measures (r = 12) and paired t-tests revealed that the amount of ACh release was significantly (P < 0.001) different among W, SWS and REM and also significantly (P < 0.001) different between NMC and NPM. The relationship of ACh release to sleep–wake stages was different in NMC and NPM (interaction, P < 0.05). In NPM (Fig. 2A), the mean (± S.E.) amount of ACh relaease during REM sleep (31.66 ± 1.75 fmol/min perfusion) was 31% higher than that during SWS (24.22 ± 1.25 fmol) and 35% higher than that during W (23.45 ± 1.64 fmol). These differences were both significant (P < 0.001). In NMC (Fig. 2B), by contrast, the mean amount of ACh release was not significantly different between REM (55.78 ±2.87 fmol) and W (54.83 ±3.67 fmol). ACh release in NMC during REM or W was significantly higher P < 0.01) than that during SWS (49.67 ± 3.13 fmol).
Fig. 2.
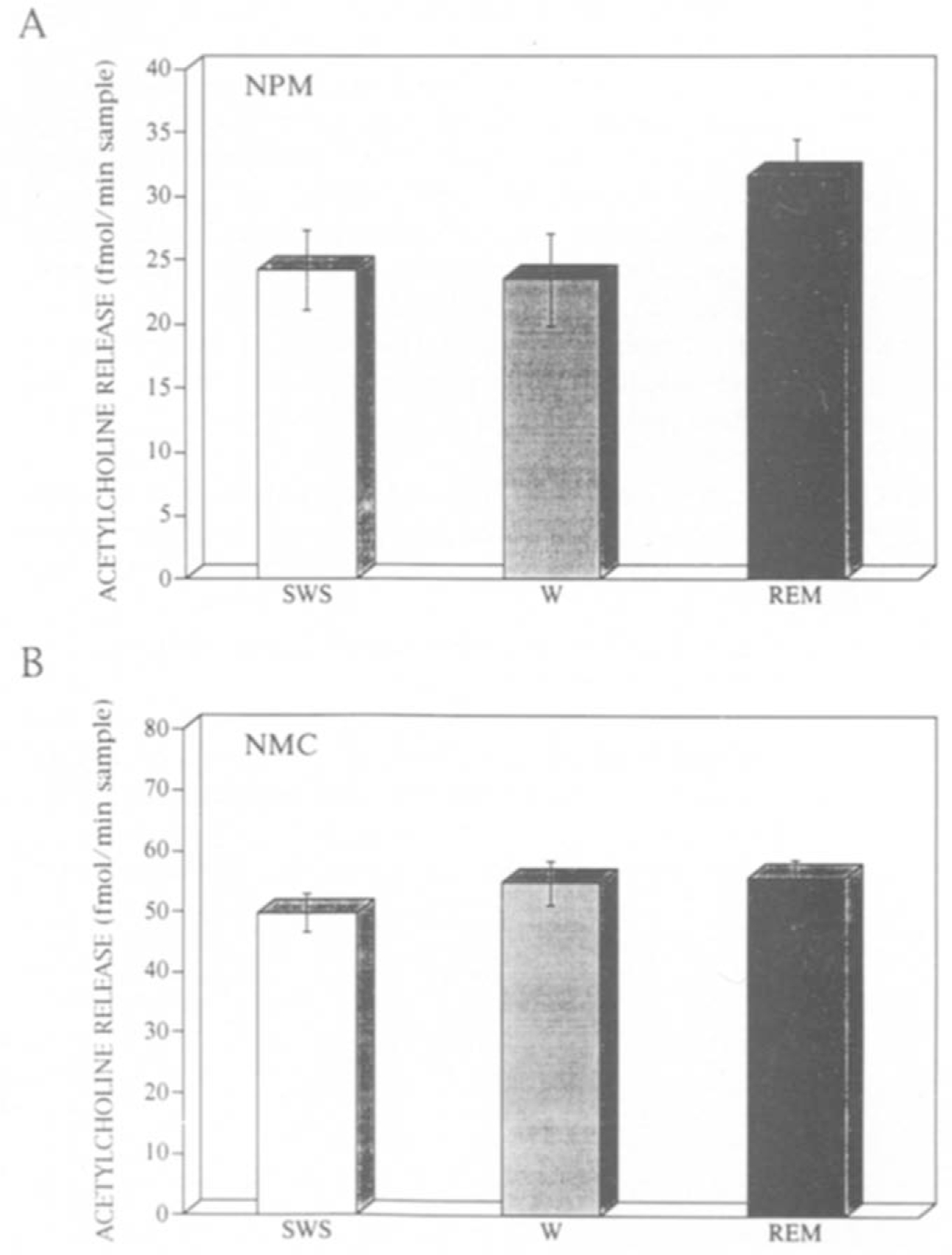
Mean and standard error of acetylcholine (ACh) release during SWS, REM sleep and W in A, the nucleus paramedianus of medulla (NPM) and B, nucleus magnocellularis (NMC). Ordinate, the amount of ACh release (fmol per 1 min sampling time).
These results indicate that the pattern of ACh release is different in NMC and NPM. ACh release is significantly higher during REM than during SWS and W in NPM, but not in NMC. It is noteworthy that this increased release is observed only in the region where ACh microinjection induces muscle atonia.
The pattern of ACh release in NPM and NMC can be compared with that in PIA. The ACh release in PIA during REM sleep (51.67 ± 1.92 fmol/min dialysate, 15 cases) is markedly higher than that during SWS (27.72 ± 1.75 fmol) and W (32.23 ± 1.35 fmol). This pattern is similar to that in NPM but not to that in NMC. Furthermore, the microinjection of ACh and/or ACh analogs into NPM induces muscle atonia8. These findings are consistent with the hypothesis that cholinoceptive neurons in NPM are responsible for muscle atonia, but, that cholinoceptive neurons in NMC are not.
In contrast to NPM, ACh is not selectively released in NMC in REM sleep. The level of ACh release in NMC during REM sleep is almost the same as that during W and both W and REM sleep rates are higher than that during SWS. There are a considerable number of putative cholinergic neurons firing highly both in REM and W2,13,18. The elevated ACh release increase which we see both in REM sleep and waking may be related to the activities of this neuronal type. The average basal ACh release in NMC is higher that that in NPM. This suggests a difference in the tonic level of cholinergic neuron terminal activation, but it is difficult to assess the significance of differences in the absolute levels of ACh in the dialysate10. The tonic release of ACh in NMC may be related to cardiovascular control, since microinjections of cholinergic agonists in this area have a potent effect on blood pressure and heart rate9.
ACh release in PIA and NPM is high during REM sleep and relatively low both during SWS and W. ACh release in these two regions is likely to be related to the activities of ACh neurons active during REM sleep, but relatively inactive in W. Cholinoceptive regions in PIA and NPM receive projections from dorsolateral pontine cholinergic neurons11,16.
We hypothesize that muscle atonia generated by cholinergic neurons is mediated by two distinct pathways. One pathway is via glutamatergic and cholinoceptive neurons in pons projecting to glutamate sensitive, non-cholinergic neurons in NMC. Thes NMC neurons then project to the spinal cord to either activate interneurons responsible for muscle atonia, or directly hyperpolarize motoneurons. The other pathway is via cholinergic neurons in the pons projecting to cholinoceptive neurons in NPM which in turn project to the spinal cord. The increase of ACh release in NPM during REM sleep that we find in the present study supports the existence of a REM-sleep-active cholinergic pathway to NPM.
Acknowledgments
This work was supported by the Medical Research Service of the VA, USPHS Grant HL 41370 and Uehara Memorial foundation.
References
- 1.Duggan AW, Headley PM and Lodge D, Acetylcholine-sensitive cells in the caudal medulla of the cat: distribution, pharmacology and effects of pentobarbitone, Br. J. Pharmacol, 54 (1974) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Mansari M, Sakai K and Jouvet M, Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep–waking cycle in freely moving cats, Exp. Brain Res, 76 (1989) 519–529. [DOI] [PubMed] [Google Scholar]
- 3.Jankowska E, Lund S, Lundberg A and Pompeiano O, Inhibitory effects evoked through ventral reticulospinal pathways, Arch. Ital. Biol, 106 (1968) 124–140. [PubMed] [Google Scholar]
- 4.Jones BE and Beaudet A, Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study, j. Comp. Neurol, 261 (1987) 15–32. [DOI] [PubMed] [Google Scholar]
- 5.Jouvet M, Recherches sur les structures nerveuses et les mechanismes responsables des differentes phases du sommeil physiologique, Arch Ital. Biol, 100 (1962) 125–206. [PubMed] [Google Scholar]
- 6.Kodama T, Takahashi Y and Honda Y, Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem, Neurosci. Lett, 114 (1990) 277–282. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H, McGeer PL, Peng JH and McGeer EG, The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat, J. Comp. Neurol, 200 (1981) 151–291. [DOI] [PubMed] [Google Scholar]
- 8.Lai YY and Siegel JM, Medullary regions mediating atonia, J. Neurosci, 8 (1988) 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai YY and Siegel JM, Cardiovascular and muscle tone changes produced by microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medulla, Brain Res, 514 (1990) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonnroth P, Jansson P-A and Smith U, A microdialysis method allowing characterization of intercellular water space in humans, Am. J. Physiol, 253 (1987) E228–E231. [DOI] [PubMed] [Google Scholar]
- 11.Luppi PH, Sakai K, Fort P, Salvert D and Jouvet M, The nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat nucleus reticularis magnocellularis: a double-labeling study with cholera toxin as a retrograde tracer, J. Comp. Neurol, 277 (1988) 1–20. [DOI] [PubMed] [Google Scholar]
- 12.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, j. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Anatomical and physiological basis of paradoxical sleep. In McGinty DJ, Drucker-Colin R, Morrison A and Parmeggiani L (Eds.), Brain Mechanisms of Sleep, Raven Press, New York, (1985), pp. 111–137. [Google Scholar]
- 14.Sakai K, Sastre JP, Kanamori N and Jouvet M, State-specific neurons in the ponto-medullary reticular formations with special reference to the postural atonia during paradoxical sleep in cat, Ajmone Marsan C and Pompeiano O (Eds.), Brain Mechanisms of Perceptual Awareness and Purposeful Behavior, Raven Press, New York, 1981, pp. 405–429. [Google Scholar]
- 15.Salmoiraghi GC and Steiner FA, Acetylcholine sensitivity of cat’s medullary neurons, J. Neurophysiol, 26 (1963) 581–597. [DOI] [PubMed] [Google Scholar]
- 16.Shiromani PJ, Lai YY and Siegel JM, Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat, Brain Res, 517 (1990) 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel JM, Mechanisms of sleep control, J. Clin. Neurophysiol, 7 (1990) 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steriade M, Pare D, Oakson G and Curro Dossi R, Different cellular types in mesopontine cholinergic nuclei related to ponto-genicu1o-occipita1 waves,j. Neurosci, 10 (1990) 2560–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]


