Abstract
Introduction
Durvalumab 10 mg/kg every 2 weeks for 1 year after chemoradiation has improved overall survival (OS) in unresectable stage III NSCLC. Subsequently, a 20 mg/kg 4-weekly regimen was approved. The study goal was to compare the efficacy and toxicity of the two regimens.
Methods
All patients with NSCLC treated with curative-intent chemoradiation followed by durvalumab from March 1, 2018 to December 31, 2020 at BC Cancer, British Columbia, Canada were included in this retrospective review. Durvalumab dosing schedule, toxicity, progression, and OS were collected. Comparisons between treatment groups were made using chi-square and independent t tests. Kaplan-Meier curves and log-rank test were used to analyze OS.
Results
A total of 152 patients were included in the 2-weekly group and 53 patients in the 4-weekly group. The median follow-up was 19.7 months and 12.0 months, respectively. The median OS was not reached, but 12-month survival rates were 88.4% versus 85.2% (p = 0.55). Toxicity profiles were similar in terms of sites and severity.
Conclusions
There was no significant difference in efficacy or toxicity between the 2-weekly and 4-weekly durvalumab in this cohort of patients with advanced NSCLC previously treated with curative-intent chemoradiation.
Keywords: Advanced non–small cell lung cancer, Durvalumab, Adjuvant, Immune checkpoint inhibitors, Immune-related toxicity
Introduction
Stage III NSCLC is a heterogenous group characterized by locally invasive tumors, multiple tumor nodules in the same lobe, with or without mediastinal adenopathy.1 The potential for cure depends on the feasibility of surgical resection and the ability to encompass disease within a radiation field. Despite curative-intent treatment, 5-year survival remains poor, from 36% in stage IIIA to 13% in stage IIIC.2 Recently, the PACIFIC (A Global Study to Assess the Effects of MEDI4736 Following Concurrent Chemoradiation in Patients With Stage III Unresectable Non-Small Cell Lung Cancer) trial reported that adjuvant durvalumab 10 mg/kg given every 2 weeks for 1 year after chemoradiation was associated with improved overall survival (OS) compared with placebo (5-year OS 42.9% versus 33.4%, hazard ratio [HR] 0.72).3,4
Durvalumab was initially approved for stage III NSCLC with the 2-weekly dosing in February 2018. The European Medicines Agency and the Food and Drug Administration labels were amended in January and February 2021, respectively, to allow the 4-weekly dosing schedule at 1500-mg flat dose.5,6 In Canada, weight-based dosing was permitted on the basis of a pharmacokinetics metric model built with individual patient data (n = 1409) from two large trials in NSCLC and other solid tumors.7 Results revealed that a 1500-mg 4-weekly dosing led to similar median steady-state exposure, variability, and incidence of extreme concentration values compared with weight-based or fixed 2-weekly regimens. Moreover, this dosing schedule was successfully used in the CASPIAN (Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer: a randomised, controlled, open-label, phase 3 trial) trial, reporting improved OS with the addition of durvalumab to platinum-etoposide in the treatment of extensive-stage SCLC.8
Whereas the pharmacokinetics data suggest that 2-weekly and 4-weekly administration intervals are equivalent, the clinical impact is unknown. This study aimed to compare the two dosing schedules in terms of efficacy and toxicity in patients with advanced, unresectable NSCLC.
Materials and methods
BC Cancer is a provincial cancer care program that serves a population of 5.1 million residents in British Columbia (BC). BC Cancer is a single-payer health care system, and as a result, has completed records on the billing and prescribing of all cancer therapies in BC. A retrospective chart review of all patients with NSCLC treated with curative-intent chemoradiation between March 1, 2018 and December 31, 2020 was conducted. All patients who received at least one dose of durvalumab were included. Data on demographics, diagnosis, durvalumab dosing schedule, treatment, progression, survival, and toxicity were collected. Patients were divided into two groups, 2-weekly and 4-weekly, according to the dosing schedule that was used for most (>50%) of the treatment. The administration schedule was at the treating physician’s discretion, and consent for the treatment plan and schedule was obtained per institutional practice. Crossover was defined as switching from one administration schedule to the other at some point during durvalumab treatment. Crossover rates were collected for both treatment groups; however, crossover patients were not analyzed as a separate group. Dosing was weight-based for both regimens. The PACIFIC patient support program for durvalumab was offered by AstraZeneca Canada with the 2-weekly dosing from May 2018 to December 2019. The 2-weekly dosing was launched in February 2020 and the 4-weekly in April 2020 at BC Cancer.
The primary outcome was OS, defined as the date of the first durvalumab treatment to the date of death. Secondary outcomes were real-world progression-free survival (PFS), progression pattern, reasons for stopping treatment, and adverse events. Real-world PFS was the time between the date of the first durvalumab treatment and progression identified on imaging, performed at the discretion of the attending physician. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.09 and classified by organ system. Clinically relevant retoxicity was defined as toxicity that caused a missed dose, treatment cessation, or hospital admission. Pulmonary toxicity was divided into three categories: immune-mediated, radiation-mediated, and mixed (unclear between the first two mechanisms).
Comparisons were made using the chi-square test for categorical variables and independent t tests for continuous variables. Kaplan-Meier curves and log-rank test were used to analyze OS. A multivariable survival model was built with the demographic, diagnostic, chemotherapy-related, and radiation-related variables that were significantly associated with survival in univariate analyses. The data cutoff date was July 23, 2021. For all the analyses, the statistical significance threshold was p value less than 0.05.
This study received approval from the local institutional research ethics board (University of British Columbia—BC Cancer Research Ethics Board; H19-02361), and approval was given for a waiver of consent to extract and analyze the archival data from the database.
Results
Between March 1, 2018 and December 31, 2020, a total of 453 patients with NSCLC were treated with chemoradiotherapy at BC Cancer. Of those, 205 patients who had at least one dose of durvalumab were identified.
A total of 152 patients belonged to the 2-weekly group and 53 to the 4-weekly group. Patient characteristics were well-balanced between groups (Table 1). Crossover between the two regimens was less frequent in the 2-weekly (7.2%) compared with the 4-weekly group (52.8%) (p < 0.001). Programmed death-ligand 1 tumor proportion score was unknown for 48.8% of the study population and was at least 50% for 27.0% and 24.5%, respectively. EGFR mutation and ALK and ROS-1 fusion status were unknown. In the 2-weekly versus 4-weekly group, 90.7% and 94.3% had two cycles of chemotherapy, and 95.4% versus 96.2% had a minimum of 60 Gy. The median time between radiation completion and durvalumab start was 40 and 43 days, respectively. The durvalumab median (range) cumulative dose was 180 (10–270) mg/kg in the 2-weekly group and 180 (20–270) mg/kg in the 4-weekly group (p = 0.91).
Table 1.
Patients Characteristics
| Patient Characteristics and Treatment | 2-Weekly (n = 152) | 4-Weekly (n = 53) | p Value |
|---|---|---|---|
| Age, y | 66 ± 8 | 68 ± 7 | 0.051 |
| Sex | 0.34 | ||
| Male | 89 (58.6) | 27 (50.9) | |
| Female | 63 (41.4) | 26 (49.1) | |
| Smoking history | 0.91 | ||
| Current | 61 (40.1) | 23 (43.4) | |
| Past | 77 (50.7%) | 25 (47.2) | |
| Never | 14 (9.2) | 5 (9.4) | |
| Living area | 0.27 | ||
| Urban | 117 (77.5) | 44 (84.6) | |
| Rural | 34 (22.5) | 8 (15.4) | |
| ECOG PS for durvalumab | 0.13 | ||
| 0 | 26 (17.1) | 9 (17.0) | |
| 1 | 97 (63.8) | 31 (58.5) | |
| 2 | 19 (12.5) | 4 (7.5) | |
| Unknown | 10 (6.6) | 9 (17.0) | |
| Histologic type | 0.90 | ||
| Squamous | 52 (34.2) | 17 (32.1) | |
| Nonsquamous | 91 (59.9) | 32 (60.4) | |
| Other | 9 (5.9) | 4 (7.5) | |
| Stage | 0.051 | ||
| IIBa | 0 (0.0) | 3 (5.7) | |
| IIIA | 84 (55.3) | 27 (50.9) | |
| IIIB | 57 (37.5) | 19 (35.8) | |
| IIIC | 10 (6.6) | 3 (5.7) | |
| IVAb | 1 (0.7) | 1 (1.9) | |
| PD-L1 TPS | 0.88 | ||
| <1% | 20 (13.2) | 6 (11.3) | |
| 1%–49% | 17 (11.2) | 8 (15.1) | |
| ≥50% | 41 (27.0) | 13 (24.5) | |
| Unknown | 74 (48.7) | 26 (49.1) | |
| Chemotherapy | |||
| Platinum type | 0.20 | ||
| Cisplatin | 64 (42.1) | 17 (32.1) | |
| Carboplatin | 88 (57.9) | 36 (67.9) | |
| ≥2 cycles | 136 (90.7) | 50 (94.3) | 0.41 |
| Radiation | |||
| Dose, Gy | 60 ± 2 | 60 ± 2 | 0.91 |
| Dose ≥60 Gy | 145 (95.4) | 50 (96.2) | 0.82 |
| Radiation completion to durvalumab start, d | 40 (13–128) | 43 (13–92) | 0.64 |
| ≤42 d | 86 (56.6) | 26 (49.1) | 0.34 |
| Durvalumab median number of cycles | |||
| 2-weekly | 17 (1–26) | 2 (0–12) | < 0.001 |
| 4-weekly | 0 (0–6) | 7 (1–13) | < 0.001 |
| Durvalumab median cumulative dose, mg/kg | 180 (10–270) | 180 (20–270) | 0.91 |
| Durvalumab regimen crossover | 11 (7.2) | 28 (52.8) | < 0.0001 |
| Durvalumab treatment durationc, mo | 9.1 (0.0–15.0) | 8.8 (0.0–14.8) | 0.26 |
| Durvalumab | 0.068 | ||
| Ongoing | 5 (3.3) | 7 (13.2) | |
| Completed | 62 (40.8) | 18 (34.0) | |
| Stopped | 82 (53.9) | 27 (50.9) | |
| Unknown | 3 (2.0) | 1 (1.9) | |
| Reason for stopping durvalumab | 0.68 | ||
| Progression | 35 (42.7) | 9 (33.3) | |
| Toxicity | 33 (40.2) | 13 (48.1) | |
| Other | 14 (17.1) | 5 (18.5) | |
| Disease Progression | 73 (48.0) | 18 (34.0) | 0.076 |
| Locoregional | 25 (16.4) | 9 (17.0) | 0.22 |
| Metastatic | 48 (31.6) | 9 (17.0) |
Note: Data are presented as mean (±SD), median (range), and n (%).
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; PS, performance status; TPS, tumor proportion score.
Unresectable or incomplete resection.
Single, resected extrathoracic metastasis (n = 1) and N3 upstaged to M1a.
In patients who have completed or stopped treatment (n = 189).
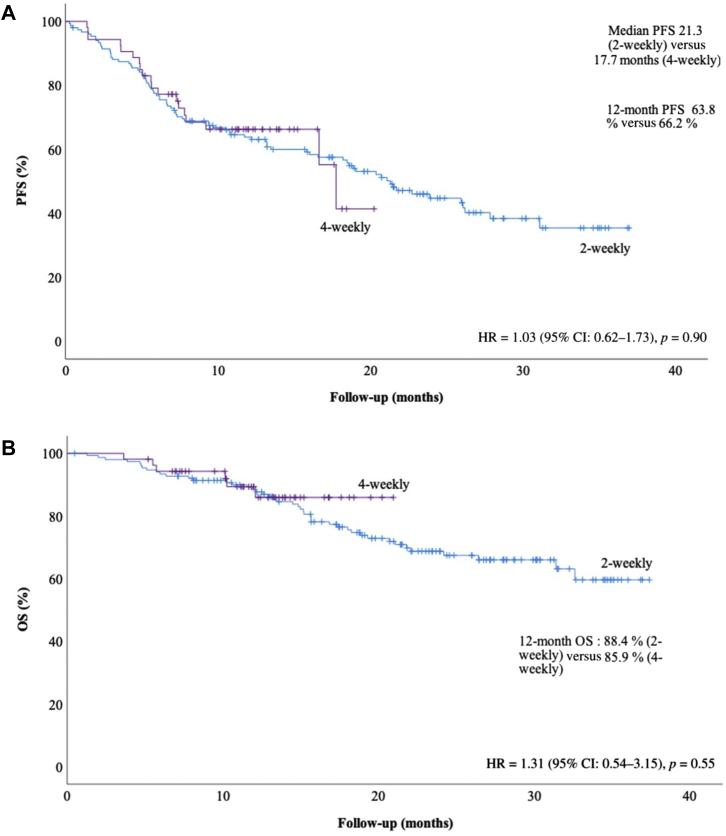
At data cutoff on July 23, 2021, after a median follow-up of 19.7 months (2-weekly) and 12.0 months (4-weekly), 50 patients had died. The median OS was not reached in either group, the HR for death was 1.31 (95% confidence interval [CI]: 0.54–3.15, p = 0.550) (Fig. 1). The 12-month survival rates were similar (88.4% versus 85.9%). We performed univariate analyses with all demographic, diagnostic, chemotherapy-related and radiation-related variables from Table 1 and included significant variables (age, male sex and cisplatin-based chemotherapy) in a multivariable survival model. Adjusted for age (HR = 1.02 [95% CI: 0.98–1.06], p = 0.41), male sex (HR = 1.92 [95% CI: 1.03–3.58], p = 0.04), and cisplatin-based chemotherapy (HR = 0.38 [95% CI: 0.18–0.80], p = 0.01), the durvalumab HR for death was 1.49 ([95% CI: 0.62–3.60], p = 0.38). Median real-world PFS was not different between groups, 21.3 versus 17.7 months, (HR = 1.03 [95% CI: 0.62–1.73], p = 0.90), with 12-month PFS rates of 63.8% versus 66.2%. Progression occurred in 48.0% versus 34.0% of patients (Table 1).
Figure 1.
(A) Real-world PFS and (B) OS according to durvalumab dosing schedule. Data cutoff was July 23, 2021. Median follow-up was 19.7 months (2-weekly group) versus 12.0 months (4-weekly group). PFS was defined as the time from durvalumab start to progression or death. OS was defined as time from durvalumab start to death. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
The median durvalumab treatment duration was 9.1 versus 8.8 months. The toxicity profiles of the two regimens were similar (Table 2). All-grade adverse events occurred in 58.7% versus 55.6% of patients in the 2-weekly and 4-weekly groups, respectively, for 12.7% versus 11.6% of grade 3 or worse events. Clinically relevant toxicity was observed in 34.0% versus 38.5%. Lung and gastrointestinal adverse effects were the most common for grade 3 or higher toxicity in both groups. One case of mixed radiation and immune-related pneumonitis led to death in the 2-weekly group. There were no toxicity-related deaths in the 4-weekly group.
Table 2.
Immune-Related Adverse Events
| Patient Characteristics and Treatment | 2-Weekly (n = 150) |
4-Weekly (n = 52) |
||
|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Any | 88 (58.7) | 19 (12.7) | 29 (55.8) | 6 (11.5) |
| Skin | 18 (12.0) | 0 | 7 (13.5) | 0 |
| Endocrine | 28 (18.7) | 2 (1.3) | 7 (13.5) | 0 |
| Lung | 15 (10.0) | 5 (3.3) | 6 (11.5) | 3 (5.8) |
| Lung, mixeda | 13 (8.7) | 6 (4.0) | 2 (3.8) | 0 |
| Gastrointestinal | 22 (14.7) | 6 (4.0) | 5 (9.6) | 2 (3.8) |
| Rheumatologic | 17 (11.3) | 1 (0.7) | 7 (13.5) | 0 |
| Otherb | 7 (4.7) | 1 (0.7) | 5 (9.6) | 1 (2.9) |
| Durvalumab treatment held at least once (p = 0.75) | 39 (25.7) | 12 (22.6) | ||
| Durvalumab treatment stopped because of toxicity (p = 0.68) | 33 (22.0) | 13 (25) | ||
| Clinically relevant toxicityc (p = 0.56) | 51 (34.0) | 20 (38.5) | ||
Note: Data are presented as n (%).
Unclear or mixed cause between radiation and durvalumab.
Cardiovascular, neurologic, hematologic.
Defined as toxicity that caused a missed dose, treatment cessation, or hospital admission.
Discussion
The 4-weekly dosing schedule for stage III NSCLC durvalumab consolidation after chemoradiotherapy is accepted in standard practice on the basis of pharmacokinetic data. Our real-world study compared the 2-weekly and 4-weekly dosing intervals for consolidative durvalumab and identified no differences in OS and toxicity. This confirms the pharmacokinetic analysis that suggests both regimens are equally effective and safe.
The findings of the present study reveal that the 12-month survival rates were similar. The HR for death was 1.31 favoring the 4-weekly dosing; however, this is likely attributable to differences in duration of follow-up. In the multivariate analysis incorporating other variables that significantly impacted survival on univariate analyses, the association between durvalumab schedule and OS remained not significant. Median real-world PFS in the 2-weekly and the 4-weekly groups was similar. The median real-world PFS in both groups was longer and had a higher proportion of metastatic recurrences than the PACIFIC trial3,4 because of the lack of standardized imaging follow-up in this observational study.
The immune-related toxicity rates with the 2-weekly and 4-weekly dosing were similar. With other immunotherapy agents, there has been an association between grade 3 or higher toxicity and dosing.10 Our data do not raise a concerning signal with the higher 4-weekly dosing. The most common grade greater than 3 toxicity was pneumonitis—unsurprising in the light of recent chemoradiotherapy.
The strengths of our study include the real-world population of patients receiving combined modality chemoradiotherapy followed by durvalumab and the completeness of follow-up owing to the provincial oversight for cancer treatment. The lack of standardized imaging follow-up and reporting of adverse events is a limitation inherent to the retrospective design. The small number of patients and the shorter follow-up in the 4-weekly group may also have impacted the rates of immune-related adverse events and the OS analysis. Finally, the high crossover rate in the 4-weekly group might have impacted the treatment group's effect on survival.
Consolidative durvalumab is now the standard of care in patients with advanced NSCLC treated with chemoradiation. This retrospective study did not find statistically significant differences in efficacy or adverse events between the 2-weekly and 4-weekly administration schedules. This study is providing clinical evidence to reinforce the conclusions of previous pharmacokinetics analyses supporting both administration intervals. Of course, other factors such as logistics and patient preference need to be considered in clinical decision-making.
CRediT Authorship Contribution Statement
Marie-Hélène Denault: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing - original draft, Writing - review & editing.
Shelley Kuang: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Aria Shokoohi, Bonnie Leung, Mitchell Liu, Eric Berthelet, Janessa Laskin, Sophie Sun, Tina Zhang, Barbara Melosky: Conceptualization, Investigation, Methodology, Resources, Writing - review & editing.
Cheryl Ho: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing.
Footnotes
Disclosure: Dr. Laskin reported receiving honoraria from Roche, Pfizer, Jazz, Takeda, and Eli Lilly; and research funding to institution from Roche and AstraZeneca. Dr. Sun has had an advisory/consulting role for AstraZeneca, Bristol-Myers Squibb, Merck, Novartis, Pfizer, and Takeda. Dr. Melosky has been on advisory boards for and has received honoraria from AstraZeneca, Merck, Bristol-Myers Squibb, Pfizer, Roche, Jazz, Boehringer Ingelheim, Janssen Johnson & Johnson, Amgen, Bayer, and Lilly. Dr. Ho has received funding from the BC Cancer Foundation for this article; received research grants paid to institution from AstraZeneca, EMD Serono, and Roche; and honoraria from AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, EMD Serono, Merck, Novartis, Pfizer, Roche, and Takeda. The remaining authors declare no conflict of interest.
The results of this study have been the object of a poster presentation at the 2021 Virtual World Conference on Lung Cancer.
Cite this article as: Denault MH, Kuang S, Shokoohi A, et al. Comparison of 2-weekly versus 4-weekly durvalumab consolidation for advanced NSCLC treated with chemoradiotherapy: a brief report. JTO Clin Res Rep. 2022;3:100316.
References
- 1.Canadian Cancer Society Canadian Cancer Statistics 2021. https://cancer.ca/en/research/cancer-statistics/canadian-cancer-statistics Accessed December 1, 2021.
- 2.Goldstraw P., Chansky K., Crowley J., et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 4.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: an update from the PACIFIC trial. J Clin Oncol. In press.
- 5.European Medical Association IMFINZI (durvalumab): Annex 1: summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf
- 6.Food and Drug Association IMFINZI (durvalumab) injection, for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761069s029lbl.pdf Accessed December 1, 2021.
- 7.Canadian Agency for Drugs and Technologies in Health Dosing and timing of immuno-oncology drugs. https://cadth.ca/sites/default/files/ou-tr/ho0008-dosing-timing-immuno-oncology-drugs.pdf Accessed December 1, 2021.
- 8.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed December 1, 2021.
- 10.Hellmann M.D., Rizvi N.A., Goldman J.W., et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]