Abstract
OBJECTIVE:
The aim of this study was to investigate whether preterm infants whose families have lower socioeconomic status (SES) or communicate with clinical staff in a language other than English experience differences in the rate, frequency, and duration of Kangaroo Care (KC) in the neonatal intensive care unit (NICU) compared to preterm infants of higher SES or primarily English-speaking families.
METHODS:
Participants were infants born <32 weeks gestational age (GA), N=116. We defined SES by the infants’ health insurance (private/higher vs. public/lower) and Language by the language mothers used to communicate with clinical staff (English vs. Other language). SES or Language groups were compared on: (1) rate of KC infants experienced during hospitalization per visitation days; (2) frequency of KC per visitation days; and, (3) duration of KC events per day.
RESULTS:
Infants in the lower SES and Other language groups experienced KC in reduced amounts, lower frequencies, and shorter durations than infants in either the higher SES or English language groups. SES and Language group differences remained significant after controlling for family visitation and GA at birth. After controlling for SES, language group differences in KC duration remained significant.
CONCLUSION:
Our findings revealed disparities in the rate, frequency, and duration of KC experienced in the NICU as a function of both SES and language. Such disparities reduced infants’ access to this developmental care practice shown to stabilize clinical status and promote neurodevelopment. We recommend that hospital nurseries implement policies that minimize these disparities.
Index terms: Kangaroo Care, disparities, preterm, NICU, infants
INTRODUCTION
Kangaroo Care (KC) is a developmental care practice where infants, clad only in a diaper, are placed against a caregiver’s bare chest and covered with a warm blanket1. It is a core developmental care practice in the neonatal intensive care unit (NICU) and a common avenue for parents and caregivers to participate in hospitalized infant care. This practice has been shown to reduce medical complications of preterm birth, such as hypothermia, sepsis, and rehospitalization2. It has also been shown to promote infant growth, breastfeeding, and mother-infant attachment3. Moreover, KC has been associated with improved neurocognitive developmental outcomes, including better hearing, speech, social, and executive function skills, in preterm infants and children4. All these benefits are crucial for infants born very preterm (<32 weeks gestational age, GA), who are likely to encounter many complications of prematurity that lead to early clinical instability, prolonged hospitalization, and potentially long-term behavioral, cognitive, social-emotional, language, and learning delays5,6. Developmental care practices have been adopted as a part of the standard of medical care in many NICUs7 to reduce preterm-birth-related morbidities, support parent-infant bonding, and to possibly improve long-term developmental outcomes. Despite the many apparent benefits of KC, however, several barriers may reduce opportunities for such practice, including parental factors (e.g., rates of visitation, family comfort with the practice) and health system factors (e.g., unit design, adequate staff support, parent educational programs, access to translators)3,8,9. Health care providers are also susceptible to cultural norms and personal beliefs that affect KC access8,9, including bathing and/or wrapping infants soon after birth rather than doing KC10, not dressing the infant with hat and socks in warm climates11, and general lack of belief in the efficacy of KC or concerns about other medical conditions12.
Studies have shown that child and adult patients with lower socioeconomic status (SES) are consistently less healthy than wealthier and more educated patients13. Differences in health care delivery and medical practices contribute to these disparities14,15. Such findings extend to the NICUs in the United States, where families experience disparities in health care on the basis of socioeconomic and cultural/ethnic factors16. It has been shown that NICUs with higher proportions of patients from lower SES have overall lower quality care as measured by a composite of maternal and infant outcome measures17. Additionally, health insurance (private vs. public), a proxy of SES, relates to prenatal and postnatal health opportunities, negatively impacting the health of those with lower SES18. Moreover, in the United States, non-English-speaking families in the NICU have been shown to be more susceptible to misunderstanding their child’s diagnosis and treatments because clinical staff and families do not share a common language19. To date, there is limited information about whether and how parents’ opportunities to provide KC for their prematurely born infant in the NICU are influenced by their socioeconomic and linguistic background3, despite the beneficial role of this developmental care practice. In the present study, we investigated whether the family’s SES and their preferred language to communicate with clinical staff influenced the rate, frequency, and duration of KC with their preterm infants in the NICU. We hypothesized that infants whose families have lower SES or communicate with clinical staff in a language other than English would receive lower rate, frequency, and duration of KC compared to infants whose families have higher SES or speak English to clinical staff. These findings would have implications for building policies and procedures to increase KC in the NICU for groups at increased risk for adverse health and developmental outcomes.
METHODS
Participants and Settings
Participants were infants born at a GA of less than 32 weeks, who were hospitalized at the Lucile Packard Children’s Hospital (LPCH) in Stanford. From the electronic medical record (EMR), we retrospectively acquired data on these infants’ experience of KC from May 1, 2018, when developmental care practices (including KC) at LPCH started being recorded consistently in the EMR by clinical staff, to March 8th, 2020, when LPCH changed visitation policies due to COVID-19. These infants’ data are part of a broader retrospective study investigating developmental care practices in relation to brain development, and thus we collected the data from the date of birth (DOB) until the infants’ brain imaging session date that is part of routine clinical care at our institution (MRI) that occurs around 36–38 weeks postmenstrual age.
This convenience sample (N=116) was divided into two groups by each of the two key factors: SES and Language. For SES, we used the infant’s health insurance as a proxy for this factor, given our retrospective data acquisition from the EMR and the limited data on sociodemographic variables at our institution. Private insurance was classified as higher SES and public insurance as lower SES (Table 1). For Language, we used a specific field in the EMR that indicated the language that mothers used to communicate with clinical staff, specifically either English or another language (e.g., Spanish, Mandarin, Dari), as a proxy for language primarily used by parents with clinical staff. The Other language group was comprised of the following distribution of languages: 76% Spanish, 12% Mandarin, 3% Dari, 3% Portuguese, 3% Tagalog, and 3% Tamil. For one participant, these data were missing (i.e., the language their mother used to communicate with clinical staff), and thus we used the language reported for the father. At LPCH, translators were available at all times for most common languages either via bedside iPads or in-person interpreters during daytime hours. Of those families who used a language other than English (n=34), 26 families used Spanish, the largest linguistic representation in the study location that is not English.
Table 1.
Description of the participants by Socioeconomic Status group.
| Higher | Lower | X2 or t | p | |
|---|---|---|---|---|
| n (%) or M (SD) | n (%) or M (SD) | |||
| Demographics | ||||
| Total N | 62 | 54 | ||
| Sex: Female | 27 (44%) | 24 (44%) | 0.01a | 0.92 |
| GA at Birth (weeks) | 28.5 (SD 2.5) | 29.2 (SD 2.3) | −1.57b | 0.12 |
| Hospitalization Duration (days) | 62 (SD 32) | 53 (SD 27) | 1.53b | 0.13 |
| Language: English | 55 (89%) | 27 (50%) | 20.87 a | <0.001 |
| Race: White | 13 (21%) | 10 (19%) | 0.11a | 0.74 |
| Clinical Factors | ||||
| Apgar Score 1 Minute | 5 (SD 2) | 6 (SD 2) | −0.56b | 0.58 |
| Apgar Score 5 Minutes | 7 (SD 1) | 8 (SD 2) | −0.76b | 0.45 |
| Intraventricular Hemorrhage Grades 3 & 4 | 1 (2%) | 4 (7%) | 2.35a | 0.12 |
| Bronchopulmonary Dysplasia | 12 (19%) | 9 (17%) | 0.14a | 0.71 |
| Sepsis | 5 (8%) | 2 (4%) | 0.97a | 0.32 |
| Necrotizing Enterocolitis | 5 (8%) | 5 (9%) | 0.05a | 0.82 |
| High Frequency Oscillatory Ventilator | 8 (13%) | 6 (11%) | 0.13a | 0.72 |
| Family Visitation | ||||
| Visitation Days/Hospital Days (%) | 77 (SD 18) | 65 (SD 24) | 2.94 b | <0.001 |
Abbreviations: GA = gestational age; M = mean; SD = standard deviation; bold = significant.
Chi-square test (X2).
Independent samples t-test (t).
The protocols for this study were approved by the Stanford University Institutional Review Board. A waiver of consent was used for data collection, since all the data collected and analyzed for the purposes of this study and the parent study were obtained as part of routine clinical care practices at our institution. These data include MRI scans used to establish participant inclusion in the parent study.
Population description, clinical risk, and family visitation
We extracted the following information about the participants to characterize the sample: sex, GA at birth, days of hospitalization (from DOB to MRI), and race. The distribution of race in our sample, as defined by categories within the EMR, was the following: 64% Other or Unknown, 20% White or Caucasian, 13% Asian, 1% Black or African American, 1% Native Hawaiian or Other Pacific Islander, and 1% Patient Refused. We also extracted information about infants’ medical conditions and clinical risk factors that may have reduced infants’ ability to receive KC (e.g., necrotizing enterocolitis) and medical treatments that, at our center, may have been a barrier to KC per policy in the LPCH NICU (i.e., high frequency oscillatory ventilator). To account for parents’ availability to perform KC, we determined the frequency of family visitation from the EMR. At LPCH, parents were permitted to visit at any time of the day, except during nursing sign out (7:00–7:30 a.m./p.m.). Daily visitation was counted as having occurred if clinical staff charted that any family member engaged in KC with their infant or had visited at bedside. We quantified the frequency of family visitation as the percentage of days that families visited out of the total days an infant was hospitalized (from DOB to MRI).
Kangaroo Care
The NICU at LPCH has standardized unit guidelines on developmental care activities to support parent participation. A nursing-driven bedside tool is used to assess infant readiness for various developmental care practices based on infant maturity and medical stability. Parents are offered some developmental care activity at each visit, including KC, if deemed appropriate, using our LPCH-designed bedside tool. Nurses receive regular education on use of the tool. Parents receive education on the tool and benefits of developmental care activities by developmental care team members assigned to each patient. A translator, when needed, is used to ensure accurate communication of the information to families. KC done by any family member was recorded by clinical staff in the infants’ EMR. We derived the following three metrics from the KC data to assess: (1) KC rate, the total minutes infants experienced KC from DOB to MRI divided by the total days family visited (KC total minutes/family visitation days), since the sum of total minutes of KC is intrinsically biased by the variance in visitation days across infants; (2) KC frequency, the percentage of days that families performed KC out of the total number of days that families visited the hospital (KC days/family visitation days); and, (3) KC duration, the minutes of KC when KC occurred (KC total minutes/KC days).
Data analyses
We performed separate Chi square tests for each categorical variable and independent samples t-tests for each continuous variable to compare groups on demographic, clinical, and visitation variables. For KC rate, frequency, and duration, we performed separate analyses by each group factor: SES or Language. For each of the three metrics, we performed a univariate analysis of variance by SES group or Language group. We also carried out separate univariate analyses of covariance to control for demographic or clinical risk factors that were found to differ between the groups. Threshold for significance was set at p < 0.05. All analyses were conducted using the Statistical Package for Social Sciences 26 (https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-26).
RESULTS
Population description, clinical factors, and family visitation
Table 1 shows demographic, clinical, and family visitation variables of the sample divided by SES group. The groups were balanced in all demographic variables but family language. The lower SES group had a higher proportion of infants whose families used a language other than English to communicate with clinical staff. The groups did not statistically differ on clinical factors. The percentage of days infants in the lower SES group were visited by their families was significantly lower compared to infants in the higher SES group.
Table 2 shows demographic, clinical, and family visitation variables of the sample divided by Language group. The groups were statistically balanced on all demographic variables other than GA at birth and family SES; the Other language group had infants born at an older GA and were predominantly from lower SES families. The groups did not statistically differ on clinical factors. The percentage of days infants in the Other language group were visited by their families was lower, although not statistically significant, relative to infants in the English group.
Table 2.
Description of the participants by Language group.
| English | Other Language | X2 or t | p | |
|---|---|---|---|---|
| n (%) or M (SD) | n (%) or M (SD) | |||
| Demographics | ||||
| Total N | 82 | 34 | ||
| Sex: Female | 34 (41%) | 17 (50%) | 0.71a | 0.40 |
| GA at Birth (weeks) | 28.5 (SD 2.5) | 29.7 (SD 2.0) | −2.63 b | 0.01 |
| Hospitalization Duration (days) | 61 (SD 30) | 52 (SD 31) | 1.40b | 0.16 |
| Socioeconomic Status: Higher | 55 (67%) | 7 (21%) | 20.87 a | <0.001 |
| Race: White | 20 (24%) | 3 (9%) | 3.66a | 0.06 |
| Clinical Factors | ||||
| Apgar Score 1 Minute | 5 (SD 2) | 6 (SD 2) | −1.30b | 0.20 |
| Apgar Score 5 Minutes | 7 (SD 1) | 8 (SD 2) | −1.40b | 0.16 |
| Intraventricular Hemorrhage Grades 3 & 4 | 3 (4%) | 2 (6%) | 0.29a | 0.59 |
| Bronchopulmonary Dysplasia | 16 (19%) | 5 (15%) | 0.37a | 0.54 |
| Sepsis | 4 (5%) | 3 (9%) | 0.67a | 0.42 |
| Necrotizing Enterocolitis | 9 (11%) | 1 (3%) | 1.97a | 0.16 |
| High Frequency Oscillatory Ventilator | 12 (15%) | 2 (6%) | 1.84a | 0.17 |
| Family Visitation | ||||
| Visitation Days/Hospital Days (%) | 74 (SD 21) | 66 (SD 23) | 1.89b | 0.06 |
Abbreviations: GA = gestational age; M = mean; SD = standard deviation; bold = significant.
Chi-square test (X2).
Independent samples t-test (t).
Kangaroo Care
Table 3 compares KC metrics between SES groups. The rate, frequency, and duration of KC was significantly lower for infants in the lower SES group, as compared to the higher SES group (Table 3; Figure 1 A–C). Between-group differences in KC rate, KC frequency, and KC duration remained significant after controlling for either GA at birth or language.
Table 3.
Results of Kangaroo Care by Socioeconomic Status group.
| Higher | Lower | F | p | η p 2 | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | |||||
| Kangaroo Care | ||||||
| Rate (minutes/day)a | 45 (25) | 20 (17) | 37.34 | <0.001 | 0.25 | |
| (controlled for GA at birth) | 36.36 | <0.001 | 0.24 | |||
| (controlled for Language) | 24.08 | <0.001 | 0.18 | |||
| Frequency (%)b | 45 (20) | 23 (16) | 42.68 | <0.001 | 0.27 | |
| (controlled for GA at birth) | 43.44 | <0.001 | 0.28 | |||
| (controlled for Language) | 32.77 | <0.001 | 0.23 | |||
| Duration (minutes)c | 96 (26) | 71 (33) | 21.05 | <0.001 | 0.16 | |
| (controlled for GA at birth) | 18.17 | <0.001 | 0.14 | |||
| (controlled for Language) | 10.73 | <0.001 | 0.09 |
Abbreviations: KC = Kangaroo Care; GA = gestational age; M = mean; SEM = standard deviation; bold = significant.
Rate = KC total minutes/family visitation days
Frequency = KC days/family visitation days
Duration = KC total minutes/KC days
Figure 1. Results of Kangaroo Care by SES group (A-C) or Language group (D-F).
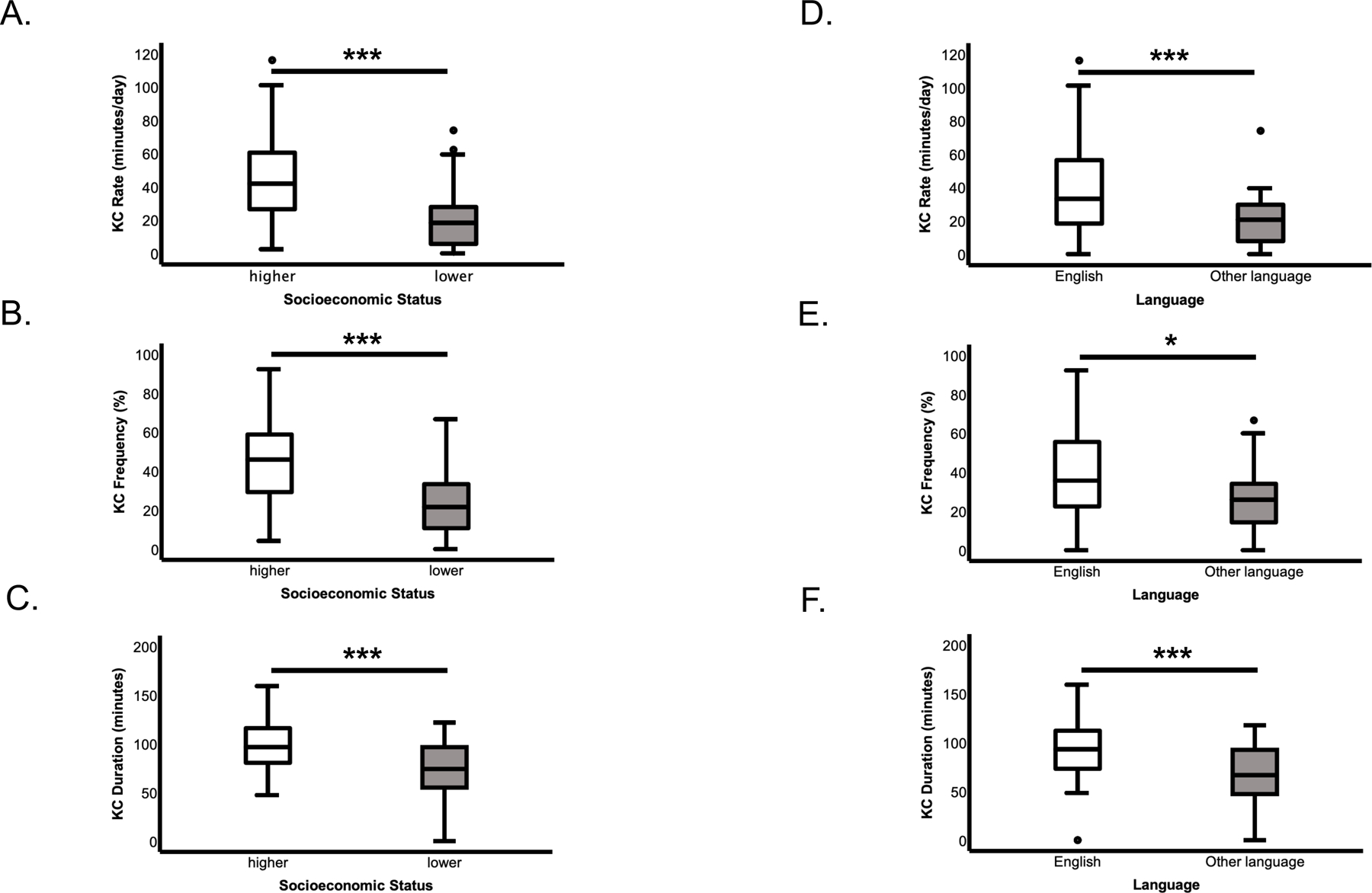
A. Rate of KC during hospitalization out of family visitation days (KC total minutes/family visitation days) by SES. B. Frequency of KC days out of family visitation days (KC days/family visitation days) by SES. C. Duration of KC events during KC days (KC total minutes/KC days) by SES. D. Rate of KC during hospitalization out of family visitation days (KC total minutes/family visitation days) by Language. E. Frequency of KC days out of family visitation days (KC days/family visitation days) by Language. F. Duration of KC events during KC days (KC total minutes/KC days) by Language. Significance: * = p < 0.05; *** = p < 0.001. Box middle line = median; box limits = upper and lower quartiles; whiskers (lines extending from the box) = upper and lower extremes; dots = outliers.
Table 4 compares KC metrics between Language groups. The rate, frequency, and duration of KC was significantly lower for infants in the Other language, as compared to the English group (Table 4; Figure 1 D–F). Between-group differences in KC rate and KC frequency remained significant after controlling for GA at birth, but not when controlling for SES. Group differences in KC duration remained significant after controlling for either GA at birth or SES.
Table 4.
Results of Kangaroo Care by Language group.
| English | Other Language | F | p | η p 2 | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | |||||
| Kangaroo Care | ||||||
| Rate (minutes/day)a | 38 (26) | 21 (16) | 13.44 | <0.001 | 0.11 | |
| (controlled for GA at birth) | 12.79 | 0.001 | 0.10 | |||
| (controlled for SES) | 2.43 | 0.12 | 0.02 | |||
| Frequency (%)b | 38 (22) | 27 (18) | 7.63 | 0.01 | 0.06 | |
| (controlled for GA at birth) | 7.99 | 0.01 | 0.07 | |||
| (controlled for SES) | 0.16 | 0.69 | <0.01 | |||
| Duration (minutes)c | 91 (30) | 67 (32) | 14.83 | <0.001 | 0.12 | |
| (controlled for GA at birth) | 10.35 | <0.001 | 0.08 | |||
| (controlled for SES) | 5.03 | 0.03 | 0.04 |
Abbreviations: KC = Kangaroo Care; GA = gestational age; SES = socioeconomic status; M = mean; SD = standard deviation; SEM = standard error of the mean; bold = significant.
Rate = KC total minutes/family visitation days
Frequency = KC days/family visitation days
Duration = KC total minutes/KC days
DISCUSSION
Consistent with our hypothesis, we found that KC, however measured, rate during hospitalization per visitation, frequency during visitation, and duration per day, was experienced less by infants from lower SES families or whose families spoke a language other than English. These results were significant after controlling for infants’ GA at birth. Disparities in KC found on the basis of SES also remained significant after controlling for language. Disparities found on the basis of language for KC duration, but not rate or frequency, remained significant after controlling for SES.
The present study provides evidence that both SES and the language families use to communicate with clinical staff are factors that contribute to significant disparities in the rate, frequency, and duration of KC experienced by preterm infants in the NICU. Importantly, we show that disparities in KC were not solely explained by how frequently families visited the hospital, given that our metrics accounted for such variability. These findings are generally consistent with previous studies that have examined the contribution of socio-demographic factors to parental involvement in KC activities. For example, a study reported that parental holding increases if mothers are White, married, older, or employed compared to non-White, single, and younger parents20. Another study showed that white mothers are told KC is an activity they could do with their infants 50% more often compared to non-white mothers21. Finally, a survey of parents at multiple European NICUs found longer periods of KC are associated with increased maternal education22. More research is needed to identify specific barriers in subgroups of disadvantaged populations in the NICU on the basis of both SES and language used to communicate with clinical staff. Further studies are required to determine whether these findings are specific to our center or locations where English is the dominant language. Additionally, future studies with larger, more balanced samples by cultural groups could tease apart potential barriers to KC associated with cultural practices/beliefs, such as ethnicity biases, caregiving practices, among other factors.
Our findings reveal that both SES and languages families use to communicate with clinical staff should be individual targets for programmatic interventions to reduce disparities in KC. Recent studies have shown that both disadvantaged individual (e.g., psychological and physical wellness) and systemic (e.g., parental leave policies) factors impede the frequency and duration of KC provided by parents in the NICU9. Barriers to KC may also overlap with those known to impede parental visitation, such as unemployment or low income, lack of support for other children (e.g., childcare), health insurance, marital status, parents’ age, transportation, and work or household responsibilities23. Those barriers that can be addressed at the system-level should include, for example, childcare in the hospital for other children in the family, transportation to/from the home9, and increased training of clinical staff on interpreter use24. Of note, the NICU at LPCH has fairly robust resources compared to many other locations in the United States and yet disparities persist. Until now, most evaluations of disparities in NICU care were done using larger databases or multicenter qualitative studies16,25. While these studies provide certain insights, they can inadvertently lead centers to conclude that the problem exists elsewhere. The single-center construct of our study is limited by sample size but represents a potentially powerful example of critical self-reflection necessary for actionable change through quality improvement initiatives or further interventional research.
It is also possible that the role of SES and language may operate more indirectly to impact care delivery. For example, these factors may impact the quality of interaction between parents and clinical staff. Rates of parent visitation have been shown to be significantly correlated with parental stress and communication with clinical staff26 and to improve when programs are implemented to provide individualized encouragement for maternal visitation27. Targeted parent education about the benefits of KC has been an important part of effective quality improvement efforts to improve rates of KC28–30. Parent education may be hampered by language barriers or ineffective communication due to parent educational level or other social factors. Possible strategies are policies that promote family-integrated approaches in which parents are seen as partners of the clinical staff31 and which expect parents to be present for longer periods and transition to active caregivers, thus potentially removing many barriers to visitation and to KC32. In societies without social supports of extended parental leave and childcare programs, these offered models are likely to have little impact on disparities between groups33. Parent education programs have been, however, effective at increasing KC, even in lower resource countries29,34. Communication that is sensitive to family structures and community norms is vital to effective education programs35. More research is needed to understand the potential impact of family-integrated care programs on mitigating disparities, particularly in countries with fewer social programs to support parents.
Another indirect factor could be ambivalence among NICU clinical staff regarding the importance of family involvement and KC in NICU care, in spite of evidence for its benefits and endorsement by national organizations. In a 2013 survey of NICU parents and nurses, 63% of mothers but only 18% of nurses felt that KC should be offered daily, and 90% of mothers compared to 40% of nurses felt mothers should be partners in care36. While parents may not understand medical barriers to KC, disagreements or communication barriers between family and clinical staff only hamper efforts at family-integrated care. Most national quality metrics used to gauge NICU care do not include direct measures of family-integrated care; best family-integrated care measures are still under discussion37. Clinical staff may interpret quality standards as stressing factors, such as equipment dislodgements like unplanned extubations, as more important than parent engagement in infant care. They may also misinterpret best practice guidelines for KC, which include 60 minutes minimum per session as a reason to limit KC, if parents are unavailable for that period of time. Education of clinical staff and institution of clear guidelines around KC have been shown to be effective in improving rates of KC28–30,38. National quality standards should include measures of family-integrated developmental care, as well as direct measures of disparate care.
Peer support might be an additional support to clinical staff that may help to reduce disparities in KC. Social support is a key component of health care; thus, training families in the NICU to support their peers (e.g., language use/communication, encouragement) could contribute to the family-integrated approach to improve access to this important experience39. It has also been argued that nurses have the ability to ameliorate many of the barriers that parents encounter to participate as partners in their premature infants’ health care, given their important role in the NICU31.
Overall, the current findings emphasize the critical issue of equity. The observed disparities in KC in relation to the family’s socioeconomic and language background represent a challenge to clinical staff in the NICU. Health care professionals must address the need to provide families of lower SES and those who speak a language other than English with the resources and services they need to provide comparable opportunity as enjoyed by infants from wealthier backgrounds and primarily English-speaking families. To achieve equity, NICUs may need to write or modify policies and practices for increasing parents’ visitation, then increasing family education and support when they are at the hospital, and finally addressing medical and nursing practices and education to support families to begin and sustain KC. In addition, a quality improvement approach, now required in many medical settings and in training, may encourage rapid change faster than could be achieved with intervention studies. Quality improvement would allow an iterative process based on intervention and careful measurement, leading to reduced disparities and ultimately improved outcomes for all infants.
A limitation of this study is that data were extracted from EMRs and may thus capture inconsistencies in reporting from clinical staff. Also, the sample was not equally distributed between SES and Language groups and thus an exploration of potential interactions between these two factors was limited. Moreover, our measures of SES and language were limited to subcomponents of these global constructs, given the retrospective nature of our study. Additionally, the potential impact of race on the study findings could not be fully investigated, given that a large proportion of the sample had limited information regarding racial categorization. Finally, this investigation was a single-site study. More studies are needed to further assess the disparities in KC in the NICU, specifically focused on parents-clinical staff partnership quality.
CONCLUSIONS
This investigation presents an in-depth analysis of KC for preterm infants in a NICU in the United States in relation to their family’s SES and language use in the hospital, revealing significant and concerning disparities. Future investigations should include identification of barriers to care at both the local and the system levels. Input from families with lower SES and/or limited English proficiency is key to understanding family experience in the NICU and guide interventions. Quality improvement initiatives that include bundles of interventions need to be studied and scaled to multiple NICUs. We recommend urgency in our completion of these studies to guide rapid modifications of policies that guide and promote this developmental care practice in the NICU. A common goal should be to reduce disparities in KC, a critical early-life experience in this at-risk population.
Acknowledgments:
We want to thank Maya Chan Morales for her assistance. This work was possible also thanks to the Stanford REDCap platform (http://redcap.stanford.edu), which is developed and operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by a) Stanford School of Medicine Research Office and b) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grants KL2 TR001085 and TL1 TR001085.
Also, this research used data or services provided by STARR, “STAnford medicine Research data Repository”, a clinical data warehouse containing live EMR data from Stanford Health Care, the Stanford Children’s Hospital, the University Healthcare Alliance and Packard Children’s Health Alliance clinics, and other auxiliary data from Hospital applications, such as radiology PACS. STARR platform is developed and operated by Stanford Medicine Research IT team and is made possible by Stanford School of Medicine Research Office.
Funding/Support:
This research work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K.E. Travis, PI: 5R00-HD8474904; H.M. Feldman, PI: 2R01-HD069150) and the National Institute of Mental Health Postdoctoral Research Training in Child Psychiatry and Neurodevelopment (A. Reiss, PI: T32-MH019908).
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Campbell-Yeo ML, Disher TC, Benoit BL, et al. Understanding kangaroo care and its benefits to preterm infants. Pediatr Health Med Ther 2015;6:15–32. doi: 10.2147/PHMT.S51869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conde-Agudelo A, Belizán JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011;(3):CD002771. doi: 10.1002/14651858.CD002771.pub2 [DOI] [PubMed] [Google Scholar]
- 3.Mu P-F, Lee M-Y, Chen Y-C, et al. Experiences of parents providing kangaroo care to a premature infant: A qualitative systematic review. Nurs Health Sci Published online August 20, 2019. doi: 10.1111/nhs.12631 [DOI] [PubMed] [Google Scholar]
- 4.Feldman R, Rosenthal Z, Eidelman AI. Maternal-Preterm Skin-to-Skin Contact Enhances Child Physiologic Organization and Cognitive Control Across the First 10 Years of Life. Biol Psychiatry 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 5.Linsell L, Johnson S, Wolke D, et al. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study. Eur Child Adolesc Psychiatry 2019;28(4):531–542. doi: 10.1007/s00787-018-1219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twilhaar ES, Wade RM, de Kieviet JF, et al. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr 2018;172(4):361–367. doi: 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Als H, McAnulty GB. The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) with Kangaroo Mother Care (KMC): Comprehensive Care for Preterm Infants. Curr Womens Health Rev 2011;7(3):288–301. doi: 10.2174/157340411796355216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan G, Bergelson I, Smith ER, et al. Barriers and enablers of kangaroo mother care implementation from a health systems perspective: a systematic review. Health Policy Plan 2017;32(10):1466–1475. doi: 10.1093/heapol/czx098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis TP, Andrews KG, Shenberger E, et al. Caregiving can be costly: A qualitative study of barriers and facilitators to conducting kangaroo mother care in a US tertiary hospital neonatal intensive care unit. BMC Pregnancy Childbirth 2019;19(1):227. doi: 10.1186/s12884-019-2363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charpak N, Ruiz-Peláez JG. Resistance to implementing Kangaroo Mother Care in developing countries, and proposed solutions. Acta Paediatr 2006;95(5):529–534. doi: 10.1111/j.1651-2227.2006.tb02279.x [DOI] [PubMed] [Google Scholar]
- 11.Hill Z, Tawiah-Agyemang C, Manu A, et al. Keeping newborns warm: beliefs, practices and potential for behaviour change in rural Ghana. Trop Med Int Health 2010;15(10):1118–1124. doi: 10.1111/j.1365-3156.2010.02593.x [DOI] [PubMed] [Google Scholar]
- 12.Seidman G, Unnikrishnan S, Kenny E, et al. Barriers and Enablers of Kangaroo Mother Care Practice: A Systematic Review. PLoS ONE 2015;10(5). doi: 10.1371/journal.pone.0125643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic Disparities in Health in the United States: What the Patterns Tell Us. Am J Public Health 2010;100(Suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egede LE. Race, Ethnicity, Culture, and Disparities in Health care. J Gen Intern Med 2006;21(6):667–669. doi: 10.1111/j.1525-1497.2006.0512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasserman J, Palmer RC, Gomez MM, et al. Advancing Health Services Research to Eliminate Health Care Disparities. Am J Public Health 2019;109(S1):S64–S69. doi: 10.2105/AJPH.2018.304922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigurdson K, Mitchell B, Liu J, et al. Racial/Ethnic Disparities in Neonatal Intensive Care: A Systematic Review. Pediatrics 2019;144(2). doi: 10.1542/peds.2018-3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padula AM, Shariff-Marco S, Yang J, et al. Multilevel social factors and NICU quality of care in California. J Perinatol Published online March 10, 2020:1–9. doi: 10.1038/s41372-020-0647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jongh BE, Locke R, Paul DA, et al. The differential effects of maternal age, race/ethnicity and insurance on neonatal intensive care unit admission rates. BMC Pregnancy Childbirth 2012;12(1):97. doi: 10.1186/1471-2393-12-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palau MA, Meier MR, Brinton JT, et al. The impact of parental primary language on communication in the neonatal intensive care unit. J Perinatol 2019;39(2):307–313. doi: 10.1038/s41372-018-0295-4 [DOI] [PubMed] [Google Scholar]
- 20.Pineda R, Bender J, Hall B, et al. Parent Participation in the Neonatal Intensive Care Unit: Predictors and Relationships to Neurobehavior and Developmental Outcomes. Early Hum Dev 2018;117:32–38. doi: 10.1016/j.earlhumdev.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendricks-Munoz K, Mayers R. A Neonatal Nurse Training Program in Kangaroo Mother Care (KMC) Decreases Barriers to KMC Utilization in the NICU. Am J Perinatol 2014;31(11):987–992. doi: 10.1055/s-0034-1371359 [DOI] [PubMed] [Google Scholar]
- 22.Raiskila S, Axelin A, Toome L, et al. Parents’ presence and parent–infant closeness in 11 neonatal intensive care units in six European countries vary between and within the countries. Acta Paediatr Oslo Nor 1992 2017;106(6):878–888. doi: 10.1111/apa.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene MM, Rossman B, Patra K, et al. Maternal psychological distress and visitation to the neonatal intensive care unit. Acta Paediatr Oslo Nor 1992 2015;104(7):e306–313. doi: 10.1111/apa.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores G, Torres S, Holmes LJ, et al. Access to hospital interpreter services for limited English proficient patients in New Jersey: a statewide evaluation. J Health Care Poor Underserved 2008;19(2):391–415. doi: 10.1353/hpu.0.0007 [DOI] [PubMed] [Google Scholar]
- 25.Mujahid MS, Kan P, Leonard SA, et al. Birth hospital and racial and ethnic differences in severe maternal morbidity in the state of California. Am J Obstet Gynecol Published online August 13, 2020. doi: 10.1016/j.ajog.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonya J, Nelin LD. Factors associated with maternal visitation and participation in skin-to-skin care in an all referral level IIIc NICU. Acta Paediatr Oslo Nor 1992 2013;102(2):e53–56. doi: 10.1111/apa.12064 [DOI] [PubMed] [Google Scholar]
- 27.Zeskind PS, Iacino R. Effects of Maternal Visitation to Preterm Infants in the Neonatal Intensive Care Unit. Child Dev 1984;55(5):1887. doi: 10.2307/1129935 [DOI] [PubMed] [Google Scholar]
- 28.Marsh KR, Young HL, Peeples ES. Increasing Skin-to-Skin in a Level IV NICU: A Quality Improvement Project. Neonatal Netw NN 2021;40(2):80–87. doi: 10.1891/0730-0832/11-T-665 [DOI] [PubMed] [Google Scholar]
- 29.Kapoor R, Verma A, Dalal P, et al. Enhancing Kangaroo Mother Care Uptake Through Implementation of an Education Protocol. Indian J Pediatr 2021;88(6):544–549. doi: 10.1007/s12098-020-03537-z [DOI] [PubMed] [Google Scholar]
- 30.Stikes R, Barbier D. Applying the plan-do-study-act model to increase the use of kangaroo care. J Nurs Manag 2013;21(1):70–78. doi: 10.1111/jonm.12021 [DOI] [PubMed] [Google Scholar]
- 31.Brødsgaard A, Pedersen JT, Larsen P, et al. Parents’ and nurses’ experiences of partnership in neonatal intensive care units: A qualitative review and meta-synthesis. J Clin Nurs 2019;28(17–18):3117–3139. doi: 10.1111/jocn.14920 [DOI] [PubMed] [Google Scholar]
- 32.Ortenstrand A, Westrup B, Broström EB, et al. The Stockholm Neonatal Family Centered Care Study: effects on length of stay and infant morbidity. Pediatrics 2010;125(2):e278–285. doi: 10.1542/peds.2009-1511 [DOI] [PubMed] [Google Scholar]
- 33.O’Brien K, Robson K, Bracht M, et al. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health 2018;2(4):245–254. doi: 10.1016/S2352-4642(18)30039-7 [DOI] [PubMed] [Google Scholar]
- 34.Mathias CT, Mianda S, Ginindza TG. Facilitating factors and barriers to accessibility and utilization of kangaroo mother care service among parents of low birth weight infants in Mangochi District, Malawi: a qualitative study. BMC Pediatr 2020;20(1):355. doi: 10.1186/s12887-020-02251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hailegebriel TD, Bergh A-M, Zaka N, et al. Improving the implementation of kangaroo mother care. Bull World Health Organ 2021;99(1):69–71. doi: 10.2471/BLT.20.252361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendricks-Muñoz KD, Li Y, Kim YS, et al. Maternal and Neonatal Nurse Perceived Value of Kangaroo Mother Care and Maternal Care Partnership in the Neonatal Intensive Care Unit. Am J Perinatol 2013;30(10):875–880. doi: 10.1055/s-0033-1333675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klawetter S, Greenfield JC, Speer SR, et al. An integrative review: maternal engagement in the neonatal intensive care unit and health outcomes for U.S.-born preterm infants and their parents. AIMS Public Health 2019;6(2):160–183. doi: 10.3934/publichealth.2019.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenaley KM, Rickolt AL, Vandersteur DA, et al. An intervention to decrease time to parents’ first hold of infants in the Neonatal Intensive Care Unit requiring respiratory support. J Perinatol 2020;40(5):812–819. doi: 10.1038/s41372-019-0569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardal F, Sulman J, Fuller-Thomson E. Support like a walking stick: parent-buddy matching for language and culture in the NICU. Neonatal Netw NN 2011;30(2):89–98. doi: 10.1891/0730-0832.30.2.89 [DOI] [PubMed] [Google Scholar]


