Abstract
Eleven of 40 decerebrated cats were found to exhibit periods of spontaneous or sensory induced myoclonus and locomotion beginning 24 h after decerebration. Histological analysis showed that the cats generating myoclonus had hemorrhagic lesions in the retrorubral nucleus (RRN) and ventral mesopontine junction (vMPJ). However, animals with intact RRN and vMPJ never showed myoclonus. To verify that the lesions were responsible for myoclonus, 6 additional cats received N-methyl-D-aspartate (NMDA, 0.5 M/0.5 μl). injections in the areas of RRN and vMPJ to produce bilateral lesions. Coordinated rhythmic leg movement (locomotion) or myoclonic twitches developed in all of these cats beginning 3 hours after NMDA injection. These NMDA lesion-induced movements appeared either spontaneously (5 out of 6 cats) or after sensory stimulation (1 cat). Four cats received saline control injections in the RRN and vMPJ and did not have spontaneous, or sensory stimulation-induced, myoclonic twitches during the 48 h observation period. These results indicate that the RRN and vMPJ have a suppressive effect on myoclonic twitches and rhythmic leg movement. Dysfunction of these regions could release motor activity into sleep and waking states.
Keywords: NMDA, Retrorubral nucleus, Atonia, Twitch, REM behavior disorder, Narcolepsy, Periodic leg movement
1. Introduction
“Myoclonus” is a sudden, brief and involuntary muscular contraction. It can occur unilaterally or bilaterally and may be symmetrical or asymmetrical. Myoclonus is observed in the head, neck, trunk, and limbs. Myoclonic jerks can occur while falling asleep (nocturnal myoclonus) and in patients with cofactor deficiency (biotin, pyridoxine), metal dysmetabolism (copper, lead, magnesium), central nervous system lesion, intoxications, and metabolic abnormalities [6,21,29,33].
The mechanism of myoclonus is unclear. Neural substrates related to myoclonus may include spinal cord, brainstem, and cortex [22,36]. However, myoclonus has been observed in patients with no known cause and no other neurologic abnormality, although rhythmic myoclonus is usually associated with some pathology of the brainstem or spinal cord [7]. Swanson et al. [34] and Denny-Brown [10] hypothesized that myoclonus is due to the release of the brainstem from forebrain inhibition. We have found that electrical stimulation of the ventral part of the mesopontine junction (vMPJ). produces muscle tone suppression during stimulation and locomotion during the interstimulus interval with repetitive stimulation [18]. In the course of our studies we noted that damage to this region appeared to be associated with a dramatic increase in the incidence of spontaneous myoclonus or stepping-like activity. Therefore, we undertook a systematic study of the effect of damage to this area on myoclonus and spontaneous movement.
2. Materials and methods
2.1. Surgical preparations
Fifty-one cats, 18 males and 33 females, weighing 2.5–4.5 kg were used. Animals were decerebrated at the precollicular-postmammillary level. Tracheostomy, ligation of both carotid arteries, cannulation of right femoral artery and vein, and decerebration were performed under halothane-oxygen anesthesia as described previously [17]. Halothane anesthesia was discontinued after removal of the forebrain. The tentorium was removed to expose the brainstem and cerebellum for electrical stimulation and microinjection. Muscles in the neck both sides of occipitoscapularis, splenius, and biventer cervicis, both forelimbs (triceps brachii), and left hindlimb (gastrocnemius.) were dissected and implanted with stainless steel bipolar electrodes for electromyograph (EMG). recording. Blood pressure was measured from the right femoral artery through a Statham transducer and data were collected only from animals with mean arterial pressure above 80 mm Hg. Rectal temperature was maintained at 38 ± 0.5°C through a heating pad regulated by a thermoregulator. EMG activity and blood pressure were recorded by a polygraph (Grass, Model 78D).
2.2. Stimulation and lesion
The animals’ heads were fixed in the stereotaxic frame and stabilized on the recording table for the entire period of the experiment to prevent labyrinthine modulation of posture [28]. The limbs and torso rested in a prone position on the stereotaxic table, located 15 cm below the earbars. Ten hours after decerebration, electrical stimulation (500 ms trains with 100 Hz, 0.2 ms, and 20–70 μA rectangular cathodal pulses.) was applied through a stainless steel monopolar microelectrode (A-M Systems, Model 5710 to). identify the areas of RRN and vMPJ [18]. These areas were located 2.5–0.5 mm anterior, 2.5–3.5 mm from the midline, and dorso-ventral at −5.0, according to Berman’s atlas [5]. In our previous study [18] we showed that electrical stimulation in these areas produced global muscle tone suppression during stimulation, while stepping-like activity occurred in between stimulations. After the area was located, 0.5 μl of 0.5 M NMDA was microinjected into the vMPJ, bilaterally through a 1 μl Hamilton micro-syringe (25 sG, Model 7001) in 7 cats. The injection rate was 0.1 μ1/min and the needle was retained in position for an additional 15 min. Four cats received 0.5 μl of Ringer’s saline in the areas of RRN and vMPJ, bilaterally. Neither NMDA lesion nor saline injection was made in the other 40 cats.
2.3. Data collection
The occurrence of muscle twitches was recorded by polygraph. However, visual observation was also used to asses the extent of muscle jerks in facial, trunk and right hindlimb musculature, in which EMG electrodes were not implanted.
2.4. Histology
Sodium pentobarbital 35 mg/kg (i.p.) was administered to the animal before thoracotomy to suppress reflexes, and then the cats were perfused intra-cardially with saline followed by buffered formalin solution. The brainstem was removed and sectioned in the coronal plane at 60 μm. Staining with neutral red was used to identify the lesion area.
3. Results
3.1. Motor phenomena
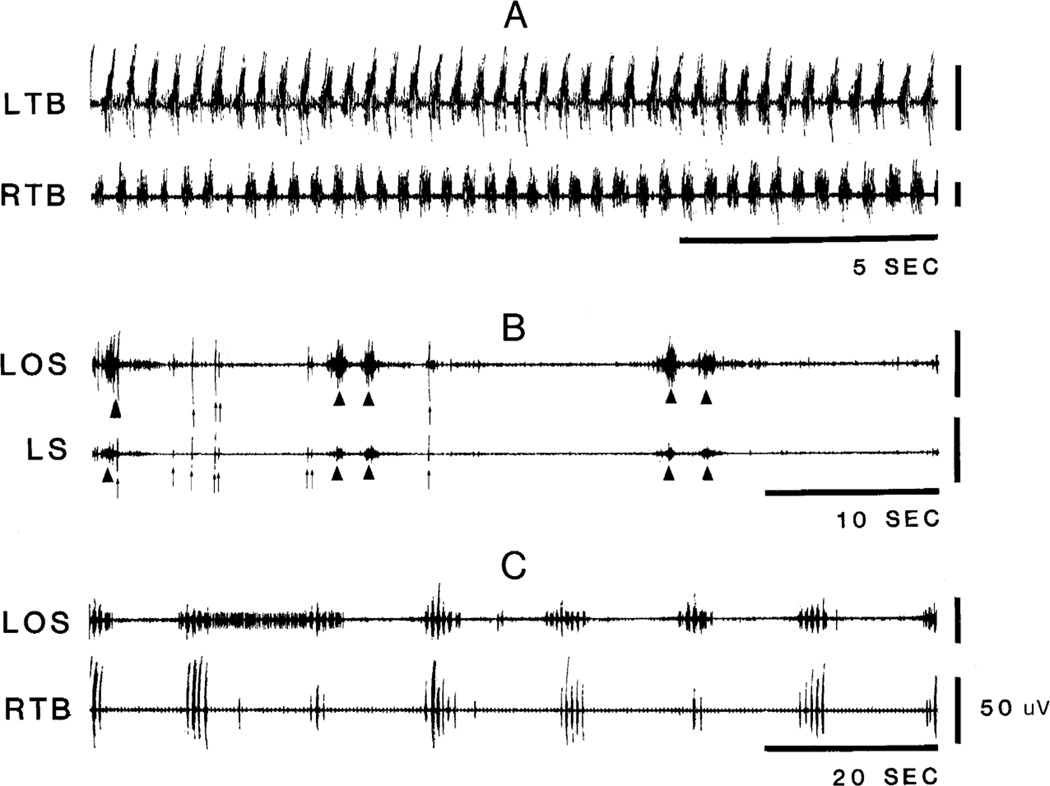
Motor phenomena seen in the decerebrate cats after lesion could be separated into two catagories, coordinated stepping-like behavior and rhythmic or non-rhythmic contractions of single muscles or muscle groups. Coordinated stepping-like activities (Fig. 1A) occurred in both forelimbs or in all four limbs. Rhythmic or non-rhythmic contractions could be subdivided into short muscle twitches of “myoclonus” and longer “tonic” contractions (Fig. 1B). Muscle contractions could occur repetitively in a. short “episode” (Fig. 1C).
Fig. 1.
Example of muscle hyperactivity in the decerebrate cat. Recordings were taken from 3 animals. A: stepping-like activity. B: a brief period of muscle twitches arrow and a longer tonic muscle contraction arrowhead. C: “episode” of muscle twitches consisted of several muscle contractions at short intervals. LOS, left occipitoscapularis; LS, left splenius; LTB, left triceps brachii; RTB, right triceps brachii
Myoclonus could be seen uni- or bilaterally in forelimbs and hindlimbs, uni- or bilaterally in the shoulder musculature or uni- or bilaterally in neck muscles. The most common location for myoclonus was in the neck muscles and the shoulder musculature, which occurred in 100% and 88% of the myoclonic episodes, respectively (Table 1). Tonic contractions were most commonly seen in the forelimbs. However, tonic contractions were also seen in the facial and trunk musculature (Table 1).
Table 1.
The occurrence of muscle contractions and myoclonic twitches in 17 animals that had ventral mesopontine junction lesion
| n | % | |
|---|---|---|
|
| ||
| Neck muscles | 17 | 100 |
| Shoulder | 15 | 88 |
| Forelimb | ||
| Proximal end | 14 | 82 |
| Distal end | 5 | 29 |
| Unilateral forelimb | 10 | 59 |
| Bilateral forelimb | 4 | 24 |
| Hindlimb | ||
| Unilateral hindlimb | 6 | 35 |
| Bilateral hindlimb | 2 | 12 |
| Facial muscles | 11 | 65 |
| Trunk | 4 | 24 |
3.2. Spontaneous motor phenomena
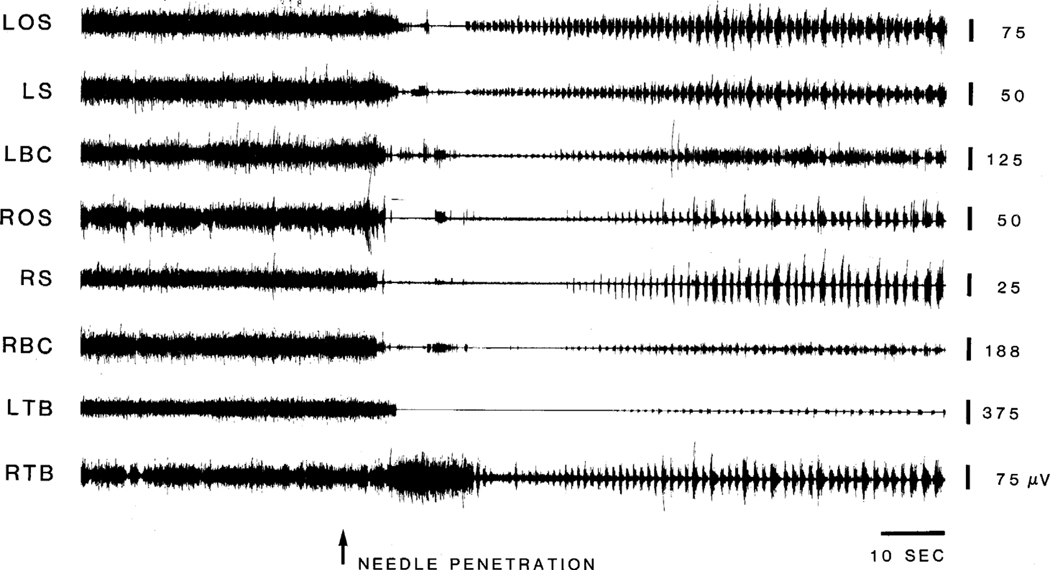
On the second day after decerebration, 11 out of the 40 cats which did not receive any NMDA injections generated either spontaneous or mechanical/sensory stimulation-induced movement or myoclonic twitches. Of these 11 decerebrated animals, 8 exhibited spontaneous episodes of locomotion, 3 had auditory finger snap, opening of sliding door to the recording room, tactile (light touching of the. neck and back). and mechanical (electrode and needle penetration into the nucleus magnocellularis) stimulation-induced locomotion and/or myoclonic jerks, which persisted for 40 s to 10 min per stimulation. As shown in Fig. 2, insertion of a needle into the nucleus magnocellularis induced a decrease in muscle tone followed by locomotion. In all of these animals, it was found that the vMPJ and RRN were damaged bilaterally by blood penetration (Fig. 3C).In contrast, decerebrated animals with intact ventral. mesopontine junctions never generated spontaneous locomotion or myoclonus (n = 29).
Fig. 2.
Mechanical stimulation in the brainstem-induced locomotion. Needle penetration arrow into the nucleus magnocellularis of the medulla on day 2 post-decerebration produced decreases in muscle tone and followed by locomotion. LBC and RBC, left and right biventer cervicis.
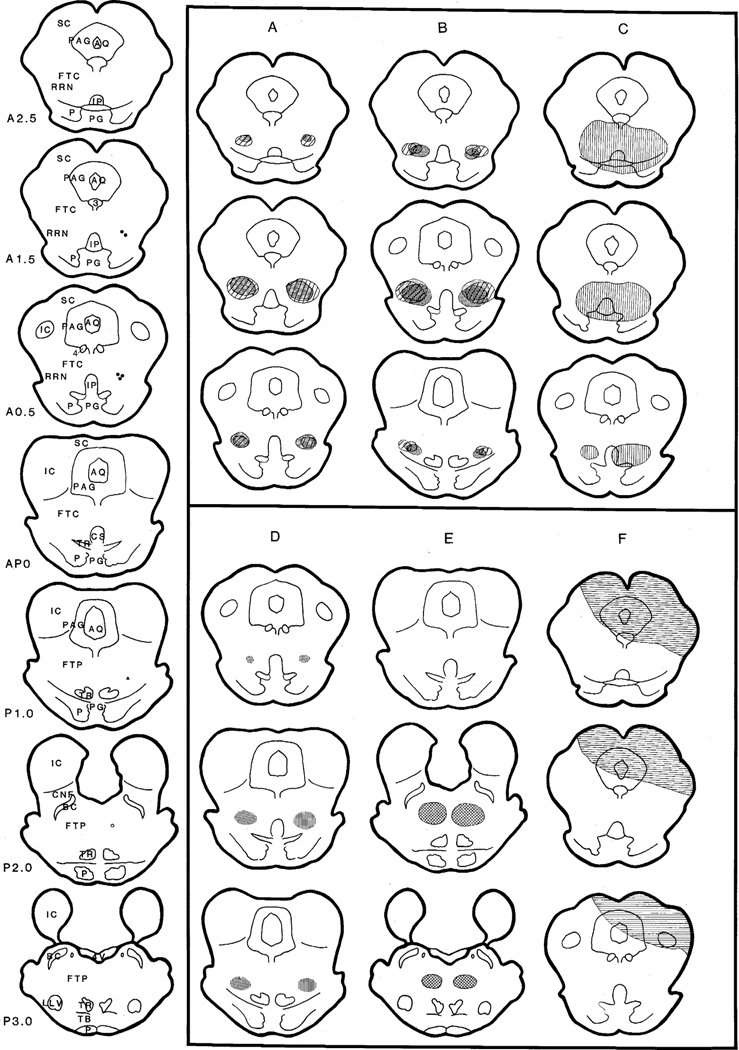
Fig. 3.
Histological mapping showing lesions induced by either NMDA injection or blood penetration. Serial coronal sections shown on the left side of the figure represent the location of the center of the NMDA injection in each animal. Filled circles represent the cats that received an NMDA injection centered in the rostral ventral mesopontine junction and exhibited spontaneous movement. Filled triangle represents the cat that received NMDA injections in the middle portion of the vMPJ and produced sensory induced movement. However, no movement was found in the cat which received an NMDA injection in the mid-pons (open circle). A, B, D and E: shaded areas represent regions of neuronal degeneration induced by NMDA injections. A was taken from two cats that received NMDA injection at the A1.5 level; B was taken from 3 cats that received NMDA injections at the level of A0.5; D was taken from one cat which received NMDA injections at the P1.0 level; E was taken from one cat which received NMDA injections at the P2.0 level. C and F represent areas damaged by penetration of blood. Cats produced spontaneous movement when damaged areas included the vMPJ (C), while no movement was found when the damaged area was located in the dorsal part of the brainstem (F). AQ, aqueduct; BC, brachium conjunctivum; CNF, nucleus cuneiformis; CS, superior central nucleus; FTC, mesencephalic reticular formation; FTP, paralemniscal tegmental field; IC, inferior colliculus; IP, interpeduncle nucleus; LLV, ventral nucleus of the lateral lemniscus; P, pyramidal tract; PAG, periaqueductal gray; PG, pontine gray; RRN, retrorubral nucleus; TB, trapezoid body; TR, tegmental reticular nucleus; SC, superior colliculus; 3, oculomotor nucleus; 4, trochlear nucleus.
Hemorrhagic lesions in the dorsal part of the pons and/or dorsal midbrain were found in 8 of 40 cats without NMDA injection. Areas of neuronal degeneration included the superior colliculus, periaqueductal gray, dorsal mesencephalic reticular formation, and inferior colliculus (Fig. 3F).These cats never generated locomotion or myoclonus. over the 5 day survival period.
3.3. Time course of motor activities induced by NMDA lesion
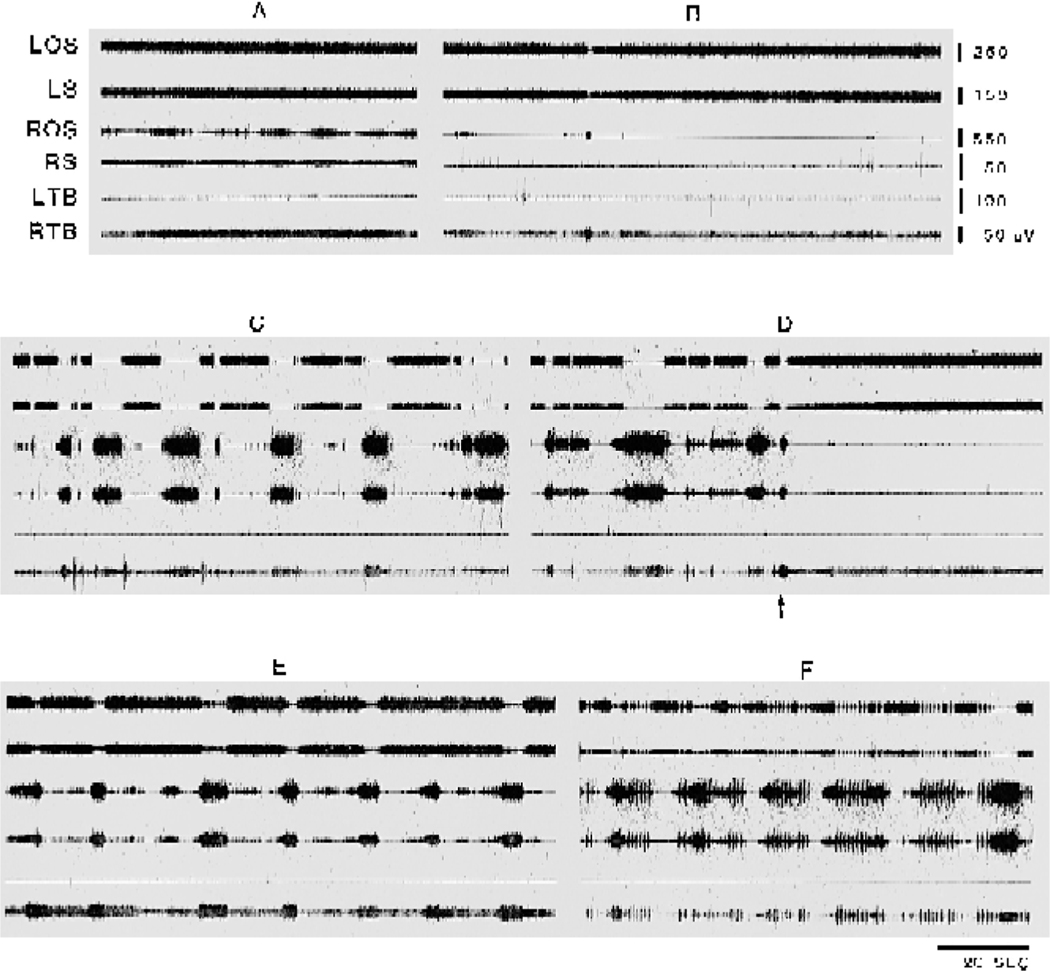
A high concentration of NMDA (0.5 M) injected into the rostral vMPJ produced a tonic suppression of tone in neck muscles contralateral to the injection site and both sides of forelimb muscles, 5 min (5.2 ± 0.38 min) after the. start of the injection (5 cases, Fig. 4), while caudal vMPJ injection (2 cases) did not change muscle tone. Starting 6 min (6.7 ± 0.92 min). after injection onset, contralateral forelimb extension accompanied by ipsilateral forelimb flexion (Fig. 4C). was visually and polygraphicallly observed in all cases studied and lasted for 70 min (65–80 min).Needle penetration into the contralateral rostral vMPJ. blocked forelimb extension and flexion (Fig. 4D) in 5 of 7 cases. If this introduced needle was then used to inject NMDA, the forelimb extension and flexion were reinstated (Fig. 4E) at a 5 min (5.6 ± 0.87 min) latency. The frequency of these muscle contraction episodes reached a maximum 40 min after the second injection into vMPJ with the episodes recurring with a mean frequency of 5.2/min (5.2 ± 0.21/min; based on 5 cases). Ninety minutes after injection onset, a mixture of tonic contraction episodes and myoclonus was seen in the neck and forelimb muscles (Fig. 4F). By 2 h after injection, this pattern was replaced by a regular coordinated stepping-like activity in 4 of 7 cases. Spontaneous myoclonic twitching was found in 1 of the 7 cats. Histology showed that lesions in these 5 cats were localized to the vMPJ including the caudal portion of the ventral mesencephalic reticular formation, retrorubral nucleus, and ventral mesopontine junction (Fig. 3 and Fig. 5).
Fig. 4.
NMDA effect on muscle activity. One half microliter of 0.5 M NMDA was injected into the ventral part of the mesopontine junction vMPJ. A: baseline level of muscle activity. B: muscle tone slightly decreased 2 min after the end of NMDA injection into the left side of vMPJ. C: combination of short, brief muscle twitches with prolonged muscle contraction appeared 15 min after NMDA injection into the left side of RRN and vMPJ. D: injection needle inserted into the right side (arrowhead) of the vMPJ induced a cessation of muscle hyperactivity. E: hyperactivity reappeared 7 min after the second NMDA injection into right side of the vMPJ. F: a combination of myoclonus with prolonged muscle contraction reappeared 12 min after the second NMDA injection. ROS, right occipitoscapularis; RS, right splenius.
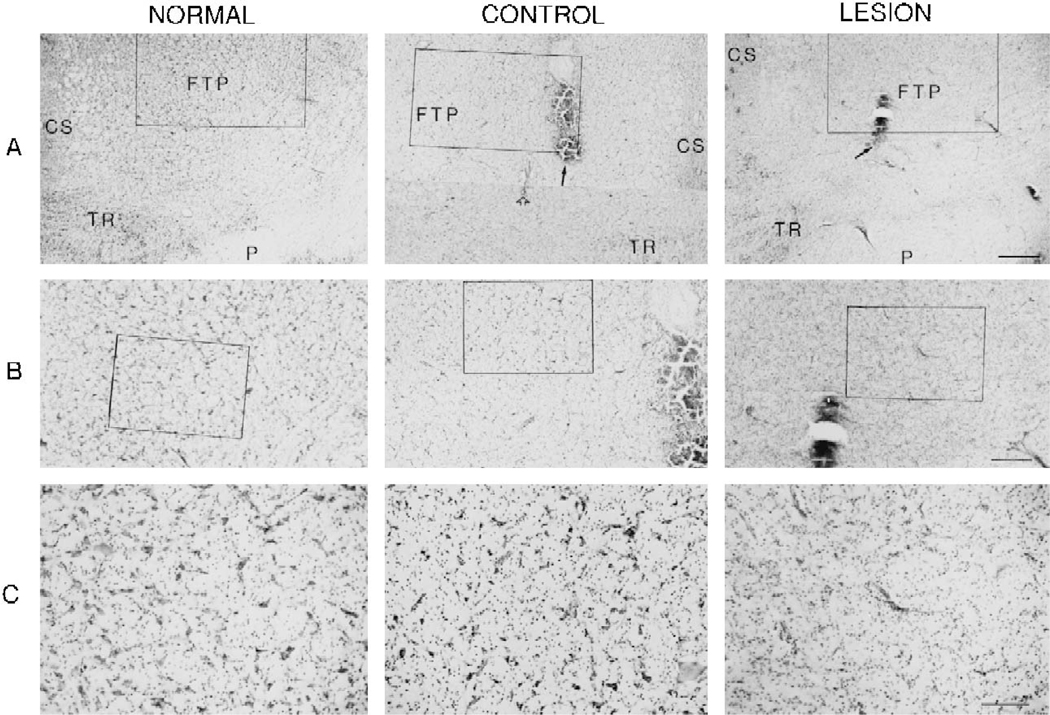
Fig. 5.
Microphotographs showing neuronal degeneration induced by NMDA injection. The normal and control sections of the microphotograph were taken from the unoperated and saline injection cats, respectively. Solid and open arrows represent NMDA injection center and electrical stimulation site, respectively. A: low magnification microphotograph taken from the ventral portion of the mesopontine junction shows lesion area. B: a higher magnification of the rectangular areas of A. C: higher magnification of rectangular areas of B. No neurons were seen in the lesioned animal. CS, superior central nucleus; FTP, paralemniscal tegmental field; P, pyramidal tract; TR, tegmental reticular nucleus. Calibration: A = 500 μm; B = 250 μm; C = 100 μm.
Of the other 2 NMDA injected cats, one developed muscle twitches in response to auditory stimulation and gentle touching the skin. This cat had a small lesion including vMPJ and adjacent regions of the paralemniscal tegmental field (Fig. 3D). Neither muscle twitches nor locomotion were found in another cat in which the lesioned area was located in the medial pons (Fig. 3E). Saline injection in the vMPJ (n = 4) did not generate motor activity.
3.4. Histology
A high concentration (0.5 M) of NMDA produced neuronal degeneration during the 5 day duration of the experiment (Fig. 5). The lesion size produced by 0.5 μl of 0.5 M NDMA was approximately 2.5 mm in diameter. The areas of neuronal degeneration which produced locomotion or muscle jerks were localized to the ventral part of the paralemniscal tegmental field, retrorubral nucleus, and caudo-ventral mesencephalic reticular formation (Fig. 3A, B, D). However, cats which had lesions localized in the. medial paralemniscal tegmental field never generated muscle hyperactivity (Fig. 3E).
4. Discussion
In the present study, we found that lesion of the RRN and vMPJ produced myoclonus and spontaneous locomotion in the decerebrate cat. Cats transected at the precollicular-postmammillary level with an intact brainstem or with dorsal brainstem lesions never generated this pattern of motor hyperactivity over the 5 day period of the experiment. However, animals prepared in the same way developed myoclonus and spontaneous locomotion if their ventral mesopontine junction was damaged by either chemical or hemorrhagic lesion. In these animals, myoclonus and locomotion could be elicited by sensory input, such as touching, tapping, and auditory stimulation.
In most cases, we saw a decrease in muscle tone immediately before the occurrence of locomotion and/or muscle jerks. Mori et al. [26], as well as our previous study [18], had found that an optimal level of muscle tone was necessary to generate locomotion. Clinically, muscle atonia occurring immediately before leg jerks has been reported in patients with periodic leg movement [22].
As early as 1922, Bazett and Penfield [2] found that cats transected at the level of mid-pons ventrally had spontaneous movement 2–4 days after decerebration. Similar results were also found in dogs by Keller [16] who reported that spontaneous running behavior developed in animals transected at the level of the rostral one-third of the pons. Cats transected at the mid-collicular level were reported to have rhythmic muscle jerks [8,32]. Cats generating myoclonus and locomotion in the present study had a common lesion in the area of the ventral mesopontine junction. In contrast, our precollicular-postmammillary decerebrate cats with either intact ventral mesopontine reticular formation or damage at the dorsal mesopontine junction never generated spontaneous or stimulus-induced movements over as long as 5 survival days. These results indicate that an intact vMPJ is critical to the control of muscle activity.
Clinically, the brainstem has been implicated in myoclonus. All posthypoxic or post-methyl bromide intoxication-induced myoclonic patients reported by Hauw et al.[14] had a pathological change in the mesencephalic reticular formation. Pathophysiological changes in the pontine reticular formation were also found in myoclonic patients with magnetic resonce imaging (MRI) [13] or CT scanning [27]
The retrorubral nucleus, A8 group of monoaminergic neurons [9], and ventral mesopontine junction contain dopaminergic [15,35] and glutamatergic neurons [19]. Ret-rograde transport of HRP combined with immunohisto-chemical and fluorescence studies have shown that glutamatergic neurons project to the pontine reticular formation [19] and nucleus magnocellularis of the medulla [20], while dopaminergic neurons project to the caudate-putamen [11] Clinically L-DOPA, which is a dopaminergic agonist, has been found to be the most potent pharmacological treatment for periodic leg movement PLM in sleep [3,23,24], suggesting that a dopaminergic mechanism is involved in PLM [25]. The present finding that lesion in the areas of RRN and vMPJ induces motor hyperactivity leads us to suggest that these areas participate in the control of periodic motor activity.
Our previous study [18] found that repetitive stimulation of the ventral part of the mesopontine junction produced muscle tone suppression. However, with repetitive stimulations, “spontaneous” locomotion appeared between stimulations when basal muscle tone was low. A similar phenomenon is also found in normal REM sleep in which phasic muscle twitches are superimposed on muscle atonia. Clinically, narcoleptic patients have a higher level of phasic muscle activities during REM sleep [12,31] and increased body movement in sleep [1,4], as well as increased occurrence of nocturnal myoclonus (periodic leg movement). and REM sleep behavior disorder. In REM sleep behavior disorder, abnormal motor activity and “dream re-enactment” occur during sleep. Muscle twitches persist in either slow wave sleep or REM sleep in affected patients when the basal muscle tone is low. Neuropathological studies have been negative in most of these cases. However, dysfunction of the pontomesencephalic region was found in some REM sleep behavior disorder patients [30]. Our present finding, that lesion of the RRN and vMPJ produces motor hyperactivity, suggests that degeneration or hypoactivity of this region may contribute to some movement disorders in waking and sleep.
Acknowledgements
This study was supported by the Medical Research Service of the VA and HL41370.
References
- [1].Aldrich MS, Narcolepsy N. Engl. J. Med, 323 (1990) 389–394. [DOI] [PubMed] [Google Scholar]
- [2].Bazett HC and Penfield WG, A study of the Sherrington decerebrate animal in the chronic as well as the acute condition, Brain, 45 (1922) 185–265.. [Google Scholar]
- [3].Becker PM, Jamieson AO and Brown WD, Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: response and complications of extended treatment in 49 cases, Sleep, 16 (1993) 713–716. [DOI] [PubMed] [Google Scholar]
- [4].Bédard M-A, Montplaisir J, Godbout R. and Lapierre O, Nocturnal γ-hydroxybutyrate. Effect on periodic leg movements and sleep organization of narcoleptic patients, Clin. Neuropharmacol, 12 (1989) 29–36. [PubMed] [Google Scholar]
- [5].Berman AL, The Brain Stem of the Cat, University of Wisconsin Press, Madison, WI, 1968. [Google Scholar]
- [6].Bressman S. and Fahn S, Essential myoclonus. In Fahn S, Marsden CD and Van Woret MH (Eds.), Myoclonus. Advances in Neurology, Vol. 43, Raven Press, New York, 1986, pp. 287–294. [PubMed] [Google Scholar]
- [7].Bressman S, Fahn S, Eisenberg M, Brin M. and Maltese W, Biotin-responsive encephalopathy with myoclonus, ataxia, and seizures. In Fahn S, Marsden CD and Van Woret MH (Eds.), Myoclonus. Adances in Neurology, Vol. 43, Raven Press, New York, 1986, pp. 119–125. [PubMed] [Google Scholar]
- [8].Burke W. and Ramzan I, Myoclonus in the decerebrate cat produced by gallamine, Brain Res., 580 (1992) 189–196. [DOI] [PubMed] [Google Scholar]
- [9].Dahlström A. and Fuxe K, Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons, Acta Physiol. Scand, 62, Suppl. 232 (1964) 1–55. [PubMed] [Google Scholar]
- [10].Denny-Brown D, Quelques aspects physioloques des myoclonies. In Bonduelle M. and Gastaut H. (Eds.), Les Myoclonies, Masson et Cie, Paris, 1968, pp. 121–129. [Google Scholar]
- [11].Fallon JH and Moore RY, Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum, J. Comp. Neurol, 180 (1978). 545–580. [DOI] [PubMed] [Google Scholar]
- [12].Geisler P, Meier-Ewert K. and Matsubayshi K, Rapid eye movements, muscle twitches and sawtooth waves in the sleep of narcoleptic patients and controls, Electroencephalogr. Clin. Neurophysiol, 67 (1987) 499–507 [DOI] [PubMed] [Google Scholar]
- [13].Hattori T, Hirayama K, Imai T, Yamada T. and Kojima S, Pontine lesion in opsoclonus-myoclonus syndrome shown by MRI, J. Neurol. Neurosurg. Psychiatry, 51 (1988) 1572–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hauw JJ, Escourolle R, Baulac M, Morel-Maroger A, Goulon M. and Castaigne P, Postmortem studies on posthypoxic and post-methyl bromide intoxication: case reports. In Fahn S, Marsden CD and Van Woret MH (Eds.), Myoclonus. Advances in Neurology, Vol. 43, Raven Press, New York, 1986, pp. 201–214. [PubMed] [Google Scholar]
- [15].Jones BE and Beaudet A, Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study, J. Comp. Neurol, 261 (1987) 15–32. [DOI] [PubMed] [Google Scholar]
- [16].Keller AD, Generalized atonia and profound dysreflexia following transection of the brain stem through the cephalic pons, J. Neurophysiol, 8 (1945) 275–288. [Google Scholar]
- [17].Lai YY and Siegel JM, Medullary regions mediated atonia, J. Neurosci, 8 (1988) 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai YY and Siegel JM, Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation, J. Neurosci, 10 (1990) 2727–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lai YY, Clements JR and Siegel JM, Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry, J. Comp. Neurol, 336 (1993) 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai YY, Clements JR and Siegel JM, Afferents to the nucleus magnocellularis of the medulla, Soc. Neurosci. Abstr, 19 (1993). 1437. [Google Scholar]
- [21].Lapresle J, Palatal myoclonus. In Fahn S, Marsden CD and Van Woret MH (Eds.), Myoclonus. Advances in Neurology, Vol. 43, Raven Press, New York, 1986, pp. 265–273. [PubMed] [Google Scholar]
- [22].Marsden CD, Hallett M. and Fahn S, The nosology and pathophysiology of myoclonus. In Marsden CD and Fahn SF (Eds.), Movement Disorders, Raven Press, New York, 1982, pp. 196–248. [Google Scholar]
- [23].Montplaisir J. and Godbout R, Restless legs syndrome and periodic movements during sleep. In Kryger MH, Roth T. and Dement WC (Eds.), Principles and Practice of Sleep Medicine, Saunders, Philadelphia, PA, 1989, pp. 402–409. [Google Scholar]
- [24].Montplaisir J, Godbout R, Poirier G. and Bedard MA, Restless legs syndrome and periodic movements in sleep: physiolopathology and treatment with L-DOPA, Clin. Neuropharmacol, 9 (1986) 456–463. [DOI] [PubMed] [Google Scholar]
- [25].Montplaisir J, Lorrain D. and Godbout R, Restless legs syndrome and periodic leg movements in sleep: the primary role of dopaminergic mechanism, Eur. Neurol, 31 (1991) 41–43. [DOI] [PubMed] [Google Scholar]
- [26].Mori S, Ohta Y, Sakamoto T. and Nonaka S, Excitability level-setting mechanisms in the pons: their behavioral support in decerebrate, reflex standing and freely moving, intact cats, Brain Dev., 8 (1986) 408–415. [DOI] [PubMed] [Google Scholar]
- [27].Palmer JB, Tippett DC and Wolf JS, Synchronous positive and negative myoclonus due to pontine hemorrhage, Muscle Nerve, 13 (1991) 124–132. [DOI] [PubMed] [Google Scholar]
- [28].Pompeiano O, A comparison of the response characteristics of vestibulospinal and medullary reticulospinal neurons to labyrinth and neck inputs. In Barnes CD (Ed.), Brainstem Control of Spinal Cord Function, Academic Press, New York, 1984, pp. 87–140. [Google Scholar]
- [29].Pranzatelli MR, The neurobiology of the opsoclonus-myoclonus syndrome, Clin. Neuropharmacol, 15 (1992) 186–228. [DOI] [PubMed] [Google Scholar]
- [30].Schenck CH and Mahowald MW, Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clon-. azepam efficacy in 89.5% of 57 treated patients, Cleve. Clin. J. Med, 57, Suppl. (1990) S9–S23. [Google Scholar]
- [31].Schenck CH and Mahowald MW, Motor dyscontrol in narcolepsy: rapid-eye-movement REM sleep without atonia and (REM). sleep behavior disorder, Ann. Neurol, 32 (1992) 3–10. [DOI] [PubMed] [Google Scholar]
- [32].Sears TA, Seers C. and Burke W, Synchronization of neural activity in the cat following decerebration, Neurosci. Lett, Suppl. 27 (1987) S121. [Google Scholar]
- [33].Snodgrass SR, Myoclonus: analysis of monoamine, GABA, and other systems, FASEB J., 4 (1990) 2775–2788. [DOI] [PubMed] [Google Scholar]
- [34].Swanson PD, Luttrell CN and Magladery JW, Myoclonus – a report of 67 cases and a review of the literature, Medicine (Baltimore), 41 (1962) 339–356. [DOI] [PubMed] [Google Scholar]
- [35].Wiklund L, Leger L. and Persson M, Monoamine cell distribution in the cat brain stem. A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups, J. Comp. Neurol, 203 (1981) 613–647. [DOI] [PubMed] [Google Scholar]
- [36].Zuckerman EG and Glaser GH, Urea-induced myoclonic seizures, Arch. Neurol, 27 (1972) 14–28. [DOI] [PubMed] [Google Scholar]