Abstract
High doses of Listeria monocytogenes overcome the ability of a normal mouse to control the infection, due to massive bacterial replication. Treatment with an anti-interleukin 10 (IL-10) receptor monoclonal antibody prevented the fatal course of infection with high doses of bacteria. This work shows that blocking the receptor for IL-10 may have useful therapeutic applications.
Interleukin 10 (IL-10) is a major anti-inflammatory cytokine required for the homeostasis of the host organism subjected to continuous exposure to microorganisms (9). However, IL-10 may lead to ineffective control of microbial infections and, therefore, blocking its activity could prove to be of beneficial value. Listeria monocytogenes is a facultative intracellular bacterium whose proliferation in vivo is controlled by CD8+ and CD4+ cells secreting gamma interferon (IFN-γ) (11). These protective mechanisms can be overcome by increasing the dose of the infecting inoculum, leading to fatal infections. The mechanisms underlying the inability to control the infection with high bacterial numbers have never been analyzed but could be related to the mobilization of an insufficient number of effector cells or cytokines. Alternatively, high bacterial doses may trigger inhibitory mechanisms leading to down-modulation of the protective immune response. Early in infection, macrophages produce a variety of cytokines, including IL-12 and IL-10, in response to bacterial products. The latter cytokine has a suppressive role in the protective immune response in murine listeriosis and is, therefore, a candidate to explain the lethality from infections by high doses of listeriae. Previous studies have indeed shown that antibodies specific for IL-10 can transiently reduce listerial loads in infected mice (12) and that IL-10 gene-deficient mice are more resistant to infection (1). However, lethality from listeriosis in adult mice was not reverted by an IL-10-specific monoclonal antibody (4). The goal of our study was, therefore, to evaluate the effects of a recently produced blocking monoclonal antibody specific for the IL-10 receptor (IL-10R) (8) during listeriosis induced by injecting different doses of L. monocytogenes, namely the antibody's ability to revert the lethality following infection with high doses of bacteria.
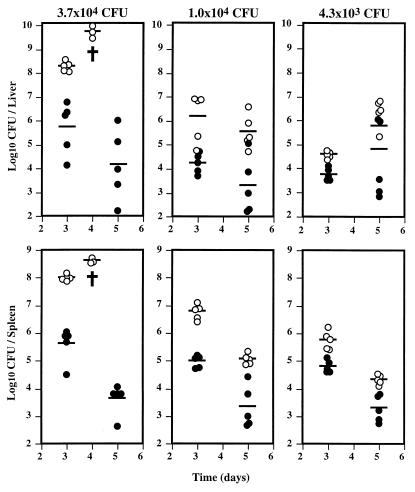
BALB/c mice were injected intravenously with 3.7 × 104, 1.0 × 104, or 4.3 × 103 CFU of L. monocytogenes strain EGD, which had been maintained in frozen stocks prepared from cultures initiated after in vivo passage of the strain. To block the effect of IL-10 during the course of the infection, mice were injected intraperitoneally with an anti-IL-10R monoclonal antibody (produced with the 1B1.2 hybridoma cell line [8] supplied by K. Moore, DNAX, Palo Alto, Calif.) or with rat immunoglobulin as a control (1 mg 24 h before infection and 0.2 mg at days 1 and 3 after infection). The amounts of antibody injected were previously titrated in vivo against the effect on listerial multiplication. Viable bacteria (CFU) were quantified in organ homogenates by plating 10-fold serial dilutions on solid media. The results are shown in Fig. 1. The bacterial loads found in either the livers or spleens of mice treated with anti-IL-10R antibodies were always lower than those in control mice. Mice infected with a lethal inoculum of Listeria and treated with anti-IL-10R not only survived but were also able to control the bacterial multiplication, whereas no mice survived beyond day 4 when only injected with the higher dose of Listeria. Additionally, the bacterial loads in 1B1.2-treated mice were very similar at day 5, irrespective of the initial inoculum dose.
FIG. 1.
Administration of an IL-10R-specific monoclonal antibody protects against the lethal course of infection by high doses of L. monocytogenes. Groups of five BALB/c mice were infected intravenously with the indicated numbers of bacteria and treated with either anti-IL-10R antibodies (closed symbols) or nonimmune rat immunoglobulin (open symbols). Bacterial counts were performed for the livers and spleens of the infected animals at the indicated time points postinfection. Each point represents the log10 CFU value from one animal, and the horizontal lines represent the geometric means. The † represents the death of two animals.
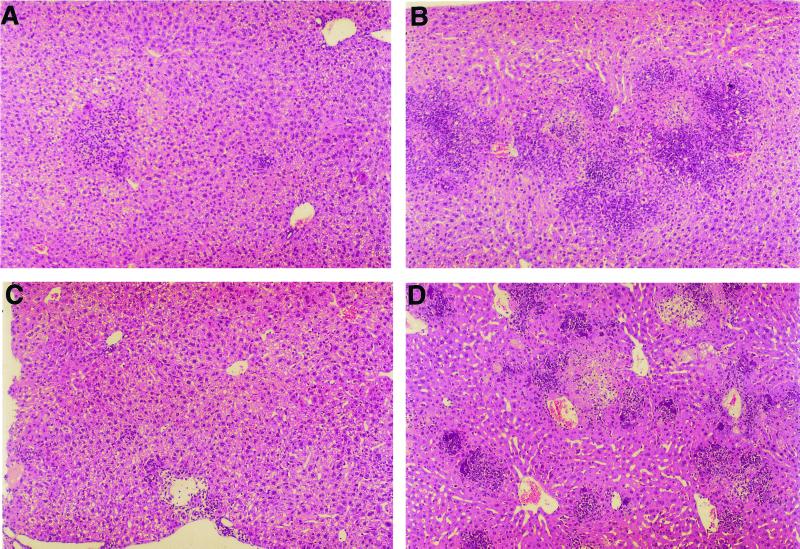
Samples from infected livers were studied by histological analysis (Fig. 2). On day 3, decreases in the number and size of granulomatous lesions were found in the animals treated with anti-IL-10R antibodies (Fig. 2A) compared to controls (Fig. 2B). After 4 days of infection, the control mice showed severe liver injury (Fig. 2D), with a high number of granulomatous lesions and an extensive inflammatory infiltrate, and often with areas of necrosis and microcalcifications. In treated mice, only a small number of inflammatory foci were found in the livers after 5 days of infection (Fig. 2C).
FIG. 2.
Histological analysis of the livers of L. monocytogenes-infected mice (3.7 × 104 CFU/animal). Shown are representative liver sections fixed in 2% paraformaldehyde buffer solution, embedded in paraffin, and stained with hematoxylin and eosin from anti-IL-10R-treated mice on days 3 and 5 (A and C, respectively) postinfection and the respective controls on days 3 and 4 (B and D, respectively).
The mechanisms whereby IL-10 hinders the development of protective immunity in listeriosis are not yet clear. We found that the anti-IL-10R monoclonal antibody induced a slight improvement in the immune response, as measured by increased IFN-γ secretion upon specific stimulation of splenocytes. Mice were sublethally infected, the bacterial loads were determined, and the spleen cells were stimulated in vitro as described elsewhere (10), following stimulation with heat-killed Listeria as antigen (20 × 106 bacilli/ml). The bacterial counts in the spleens at 2, 4, and 7 days postinfection were 5.16 ± 0.44, 4.72 ± 0.39, and 2.51 ± 1.23 log10 CFU, respectively, in the control animals and 4.63 ± 0.34, 4.10 ± 0.23, and 1.59 ± 0.93 log10 CFU, respectively, in the animals that received the anti-IL-10R antibodies. At the same time points, spleen cell supernatants from the control group had 2.9 ± 0.5, 11.7 ± 4.7, and 34.8 ± 12.5 ng of IFN-γ per ml, respectively, whereas those from anti-IL-10R antibody-treated animals had 2.8 ± 0.8, 7.1 ± 3.2, and 59.4 ± 20.5 ng of IFN-γ per ml, respectively. The slight enhancement of the IFN-γ response was apparent late in infection (statistically significant differences were only present at day 7; P < 0.05); this was later than the protection afforded by anti-IL-10R against bacterial multiplication (P < 0.05 at day 2 and P < 0.01 at day 4). Therefore, the deleterious effect of IL-10 may relate to the inhibition of the innate response by IL-10 produced early in the infection by macrophages (2), namely the induction of cytokines or costimulatory molecules that are involved in protective immunity and priming of T cells for IFN-γ production. We could not find any improvement in leukocyte recruitment, namely of neutrophils, or on phagocyte function (e.g., nitric oxide production) after treating listeria-infected mice with the anti-IL-10R antibody (data not shown). However, additional studies should be carried out to understand the role of IL-10 in listeriosis.
The role of IL-10 described here regarding massive infections with L. monocytogenes presumably may be extended to other infections, as was recently done for Klebsiella (13). However, in other models of infection such as Toxoplasma gondii and plasmodium infections, interference with IL-10 signaling led to exacerbated inflammatory responses and, eventually, death of the animals (3, 6). In contrast, IL-10 has counterprotective activities in mycobacterial infections (5, 7). Therefore, the neutralization of the receptor for IL-10 may be a promising approach as an adjunct therapy for severe septicemias and in chronic infections such as mycobacteriosis, namely in human patients, but its use must be avoided in other infections where the anti-inflammatory effects of IL-10 are of importance.
Acknowledgments
We are indebted to J. Pedrosa and C. Oliveira for helpful technical assistance and to K. Moore and DNAX for the gift of the 1B1.2 hybridoma cell line.
REFERENCES
- 1.Dai W, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 2.Flesch I E A, Kaufmann S H E. Role of macrophages and αβ T lymphocytes in early interleukin 10 production during Listeria monocytogenes infection. Int Immunol. 1994;6:463–468. doi: 10.1093/intimm/6.3.463. [DOI] [PubMed] [Google Scholar]
- 3.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFNγ, and TNFα. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 4.Genovese F, Mancuso G, Cuzzola M, Biondo C, Beninati C, Delfino D, Teti G. Role of IL-10 in neonatal mouse listeriosis model. J Immunol. 1999;163:2777–2782. [PubMed] [Google Scholar]
- 5.Jacobs M, Brown N, Aallie N, Gulert R, Ryffel B. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology. 2000;100:494–501. doi: 10.1046/j.1365-2567.2000.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray P J, Young R A. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Farrell A-M, Liu Y, Moore K W, Mui A L-F. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powrie F, Bean A, Moore K W. Interleukin-10. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 143–152. [Google Scholar]
- 10.Silva R A, Pais T F, Appelberg R. Evaluation of interleukin 12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 11.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 12.Wagner R D, Maroushek N M, Brown J F, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M-J, Jeng K-C G, Ping L-I. Exogenous cytokine modulation or neutralization of interleukin-10 enhance survival in lipopolysaccharide-hyporesponsive C3H/HeJ mice with Klebsiella infection. Immunology. 1999;98:90–97. doi: 10.1046/j.1365-2567.1999.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]