Abstract
Abstract
The present research sought to experiment the effect of additives such as dry gluten powder (DGP), fungal α-amylase (FAA), sodium stearoyl-2-lactylate (SSL) and their combination (GAS) on the rheological and nutritional profile of bread. The incorporation of varied percentage (0–20%) of stenostylis stenocarpa flour (ssF) into WF(Wheat Flour) was optimized at 15%. The results showed that the incorporation of 15% ssF decreased the farinograph dough stability and extensograph resistance to extension. There was protein matrix disruption in 15% ssF dough contrary to 15% ssF + GAS dough as shown in scanning electron micrograph (SEM). However, the addition of GAS to 15% ssF produced bread with an improved quality score that competed favourably with control bread. The organoleptic evaluation revealed an overall quality score of 52.5, 40 and 49% for control, 15% ssF and 15% ssF + GAS bread respectively. Furthermore, 15% ssF + GAS revealed a higher value of ash, protein, dietary fibre, total phenol contents and IC50 of DPPH radical scavenging activity in comparison to control bread. Hence, the present study showed that partial replacement of WF with 15% ssF + GAS has a positive effect on the overall sensorial acceptability and nutritional value of bread.
Graphical abstract
Keywords: Sphenostylis stenocarpa, Wheat flour, Rheology, Farinograph, Extensograph, Additives
Introduction
The problem of food insecurity, scarcity and malnutrition continues to torment the developing world due to the greenhouse effect (Asogwa and Onweluzo 2010). Urbanisation on the other end of the divide has redesigned the consumption of convenient foods which are nutritionally inadequate and expensive. One of the ways to preclude this is to upgrade dietary diversity with the use of indigenous crops with high nutritive potentials to improve baked products that are commonly consumed by the populace to overcome these challenges (Shelef et al. 2017).
Sphenostylis stenocarpa also known as African yam bean is one of the under-utilised grain legume of tropical origin with high nutritional content. It is a plant that exceptionally adapts to lowland tropical conditions. It is widely cultivated majorly in the southern part of Nigeria (Malumba et al. 2017). Earlier studies had revealed its nutritional characteristics with potentials that can broaden the food base for human consumption. Sphenostylis stenocarpa in comparison to cowpea and pigeon pea is rich in protein and starch (Agunbiade and Longe 1999). Its protein content was analyzed to be comparable to that of Bambara groundnut, pigeon pea, lima bean, groundnuts and cowpea but less than that of soybean. Earlier reports have also revealed its nutritional potentials, (Apata and Ologhobo 1990), proximate analysis, mineral content and antioxidant properties (Nwokolo 1987). The major challenge with its preparation is the hard-to-cook characteristic which has been partly overcome by different processing methods (Njoku et al. 1989). The usual traditional practice is to convert bean seeds to flour, mix it with water to form a thick paste and prepare ‘akara’ (fried bean balls) and ‘moin moin’ (cooked bean paste) in order to serve as alternative methods of elevating the use of beans (Eke and Akobundu 1993). One of the current trends also experimented the inclusion of Sphenostylis stenocarpa water extractable proteins in baked products (Ade et al. 2012). Effort is continuously intensified to promote local crops in order to solve some nutrition-based challenges.
Bread is a popular confectionery baked product. However, the low protein content in commercial wheat bread has been a major concern as its consumption continues to rise in many developing nations (including Nigeria) (Onabolu et al. 2003). At this juncture, the combination of refined carbohydrate foods with lifestyle associated diseases has necessitated the need for modification of several food formulations to match everyday demands (Jideani and Onwubali 2009). The use of legume seed flour in WF would play an important role in uplifting the nutritional profile and also bringing in familiar taste in white bread (Dhingra and Jood 2001).
To fortify blends, the use of additives either singly or in combination is now common in the baking industry to produce bread of softer texture that will be appreciable to consumers priority (Ishida and Steel 2014). Earlier studies were majorly carried out on Sphenostylis stenocarpa flour highlighting its nutritional profile with a dearth of information on its incorporation in WF. There seems to be no information about its incorporation in WF with combined additives to substantiate its baking performance, consumer acceptability, nutritional characteristics and antioxidant properties. The exploration of this work sought to investigate (a) the optimized addition of Sphenostylis stenocarpa flour (ssF) in WF with variations from 0–20%, (b) effect of the additive(s) on the rheology of blends (c) the comparison between microstructure of control and best optimized composite dough with further analysis on the physico-sensory, antioxidant properties and nutritional characteristics of the produced bread.
Materials and methods
Sphenostylis stenocarpa was purchased from a local market in Akungba Akoko, Ondo State. Nigeria. The bean was identified at the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba Akoko. The bean was soaked until the coat was soft, sun dried and stored in an air-tight plastic container in the refrigerator before use. The bean was milled in a hammer mill 3,100 (Perten Instruments AB, Huddinge, Sweden) and sifted through a 250-μm sieve. Ingredients like compressed yeast (Tower brand, AB Mauri, India Pvt Ltd., Chennai, India), hydrogenated fat (Bunge India Pvt Ltd., Mumbai, India), water, salt (common food grade sodium chloride) and sugar were also used for the preparation of bread. Additives namely; dry gluten powder, DGP (5.0%), fungal from Aspergillus oryzae, FAA (0.002%) and sodium stearoyl-2-lactylate, SSL (0.5%) were procured from PD Navkar Bangalore, India.
Preparation of Blends
The WF used for this experiment had 11.32% of dry gluten, 29 ml Zeleny’s sedimentation value and 510 s Hagberg Falling number value. To produce bread with improved nutritive quality, different blends were prepared by increasing ssF levels (0–20%) in WF to determine the maximum level of incorporation without causing any adverse effect to the quality of bread. It was observed that 15% of ssF was the maximum level of incorporation. This preparation was determined during preliminary studies (data not shown). To further improve the quality of bread incorporated with 15% ssF, additives were incorporated (such as dry gluten powder, DGP (5.0%), fungal from Aspergillus oryzae, FAA (0.002%), sodium stearoyl-2-lactylate, SSL (0.5%) and in combination (GAS) to experiment with 15% ssF blends (Indrani et al. 2003).
The rheological characteristics
The effect of additives on the farinograph and extensograph characteristics of ssF blends was studied using the AACC method (AACC 2000).
Preparation of bread
The following formulation was used for the preparation of bread (all the measurements were in percentage). WF:ssF (100:0; 95:5; 90:10; 85:15 and 80:20), 15%ssF + dry gluten powder, DGP (5.0%), 15%ssF + fungal alpha amylase, FAA (0.002%) from Aspergillus oryzae, 15%ssF + sodium stearoyl-2-lactylate, SSL (0.5%) were experimented separately and in combination 15%ssF + GAS; compressed yeast, 2.0; salt, 1.5; sugar, 2.5; hydrogenated fat, 1.0; and water (optimum water absorption as determined with the farinograph). Breads in quadruplicate were prepared by mixing the ingredients in a Hobart mixer (Model N-50, Hobart, GmbH, Offenburg, Germany) with a flat blade for 4 min at 61 rpm. The dough was fermented in a chamber maintained at 30 °C and 75% relative humidity (RH) for 90 min, remixed, rounded and again fermented for 25 min, moulded, proofed for 55 min at 30 °C, 85% RH (relative humidity) and baked for 25 min at 220 °C, cooled and packed.
Scanning electron microscopic studies
The Scanning electron microscopic images (surface structure and cross section) of each dough taken from control, 15%ssF and 15%ssF + GAS were carried out according to the method of Sidhu et al. (1990). Developed dough was thinly sheeted and cut into pieces (size 20 × 20 mm) immediately after mixing. The sample pieces of the bread dough were defatted with hexane and freeze-dried using Heto freeze-dryer Model DW3 (Allerød, Denmark). Freeze-dried samples were separately placed on the sample holder using double sided scotch tape and was exposed to gold sputtering (2 min, 2 mbar). Finally, each sample was observed at 15 kV and 9.75 × 10−5using Leo scanning electron microscope model 435 VP (Leo Electronic System, Cambridge, UK. The collective results of this present research on dough in relation to the farinograph and extensograph revealed 15% ssF + GAS with the best attributes that competed favorably with control. SEM analysis was carried out by comparing the micrograph of control, 15%ssF and 15% ssF + GAS dough. Further studies on physico-sensory properties, instrumental colour measurement and texture analysis were also compared among them. Finally, the analysis on total phenol content, antioxidant activity and composition were evaluated and compared between control and 15% ssF + GAS.
Physical and sensory characteristics
Bread volume (ml) of control, 15%ssF and 15%ssF + GAS was determined by the rape seed displacement method (Chopin, S.A, France) and the weight (g) of bread sample was taken using weighing balance. Thereafter, the specific volume (cm3/g) of each bread was calculated. The sensory evaluation was carried out by twenty panellists of age between 25 to 55 years, including twelve male and 8 females, who have expertise in sensory evaluation of bakery products. The panellists were initially instructed about the product. Each bread in four replicates were evaluated by each panellist following a score card consisting of various quality parameters such as crust colour (1 = pale brown; 10 = golden brown); crust shape (1 = flat; 10 = convex shape); crumb colour (1 = dull white; 10 = golden brown); crumb grain (1 = closed and compact; 10 = fine and uniform); taste (1 = foreign; 10 = pleasant) and mouthfeel (1 = dry; 10 = easy breakdown and clean mouthfeel). The overall quality score (60) was taken as the combined score of all six quality attributes.
Instrumental color measurement
The crumb colour control, 15% ssF and 15% ssF + GAS bread was analyzed using the Hunter machine (Color measuring Labscan XE system, USA). Color readings were expressed by Hunter values for L, a and b. L values measure black to white (0–100), (a) values measure red (+ ve) to green (-ve) and (b) values measure yellow (+ ve) to blue (-ve).
Texture profile analysis (TPA)
Crumb texture analysis was measured on uniform slices of 10 mm thickness. Three slices from the center of control, 15%ssF and 15%ssF + GAS bread were taken for evaluation. Texture profile analysis (TPA) was performed using a universal testing machine TA-XT2i (Stable Micro Systems, Surrey, UK) equipped with a 30-kg load cell and 25-mm aluminum cylindrical probe. The settings used were test speed of 2.0 mm/s with a trigger force of 5 g to compress the middle of the bread crumb to 50% of its original height at a crosshead speed of 1 mm/s. TPA measured the hardness, cohesiveness and springiness. Gumminess and chewiness were derived from TPA parameters. Values were the mean of three replicates.
Extraction of phenolic compounds
The antioxidant components of bread samples were extracted following the method according to Li et al. (2007) with some modifications. One gram of sample was mixed with 10 mL 1 N HCl/95% ethanol (v/v, 15/85) solvent in an amber bottle. Then, extraction was performed in a temperature-controlled (65 °C) water bath shaker (VWR International, Radnor, PA, USA) at a constant speed for 80 min. The resulting mixture was centrifuged at 7800 × g (10,000 rpm at 5 °C for 15 min). The supernatant was collected and stored in the dark at − 20 °C until their use.
Determination of total phenol content (TPC)
Total phenolic content of sample extracts was determined using the Folin–Ciocalteu method. Freshly prepared diluted Folin–Ciocalteu reagent (1.5 mL) was used to oxidize 0.2 mL sample extracts. After allowing the mixture to equilibrate for 5 min, the reaction was then neutralized with 1.5 mL sodium carbonate solution (60 g/L) at room temperature. The absorbance of the resulting solution was measured at 725 nm. Gallic acid was used as the standard. The total phenolic content of samples was expressed as mg/gGAE (Li et al. 2007).
Determination of antioxidant activity
DPPH radical scavenging assay (RSA) of samples was evaluated according to the method of Cuendet et al. (1997).
Determination of proximate compositions
For control and 15% ssF bread samples, moisture content (AACC method 44–15), protein (AACC method 46–10), ash (AACCmethod 08–01), fat (AACC method 30–10) and dietary fibre (991.43) was determined according to the standard method (AOAC 2000). The carbohydrate was obtained by difference and energy was calculated by taking the values of 4, 4 and 9 kcal for carbohydrate, protein and fat in control and 15% ssF bread. Except for the energy value, all the proximate compositions were determined in the WF and Sphenostylis stenocarpa flour (ssF). In addition, Zeleny’s sedimentation value (AACC method 56–61) and Hagberg falling number (AACC method 56-81B) were determined in WF using standard methods AACC 2000).
Statistical analysis
The data were expressed as mean ± standard error. Results were analysed using Duncan’s new multiple range tests with different experiment groups appropriate to the completely randomised design with four replicates each, as described by Steel and Torrie (Steel and Torrie 1980). The significant level was established at p ≤ 0.05.
Results and discussion
Chemical composition of WF and Sphenostylis stenocarpa flour (ssF)
The chemical characteristics of WF and ssF are presented in Table 1. The reported value of protein (26.41%) in ssF was significantly (p ≤ 0.05) higher than that of WF (12.40%). The value of ash (3.38%), fat (1.61%) and moisture content (11.08%) in ssF were slightly higher than earlier values reported for chickpea, cowpea and horse gram flours that were examined with the prospect of using them as ingredients for food processing (Sreerama et al. 2012). The values for the moisture, fat and carbohydrate in the WF at 12.42, 3.03 and 66.86% respectively were significantly higher than that of ssF. The reported total dietary fibre (12.28%), insoluble dietary fibre (8.54%) and soluble dietary fibre (3.74%) values for ssF were higher than that of WF. The presence of fibre in legume has been reported to impart health benefits in human nutrition by maintaining normal functioning of intestine thus preventing gastrointestinal disorders (Sreerama et al. 2012). The total phenol content (TPC) was higher in ssF (0.35 mg/gGAE) in comparison to WF (0.08 mg/gGAE) while the evaluation of DPPH scavenging ability of ssF extract was also higher than that of WF considering the IC50 at 225 and 1078 µg/ml respectively. This result is in line with the earlier research of Cuendet et al. (1997) as a lower IC50 value is indicative of higher scavenging capacity.
Table 1.
Proximate composition of wheat flour (WF) and Sphenostylis stenocarpa flour (ssF)
| Parameters | WF | ssF |
|---|---|---|
| Moisture (%) | 12.42 ± 0.01a | 11.08 ± 0.03b |
| Ash (%) | 0.49 ± 0.06a | 3.38 ± 0.12b |
| Protein (%) | 12.40 ± 0.03b | 26.41 ± 0.05a |
| Fat (%) | 3.03 ± 0.20a | 1.61 ± 0.05b |
| Total dietary fibre (%) | 2.40 ± 0.02b | 12.28 ± 0.03a |
| Insoluble dietary fibre (%) | 1.95 ± 0.02b | 8.54 ± 0.02a |
| Soluble fibre (%) | 0.45 ± 0.02b | 3.74 ± 0.02a |
| CHO (%) | 66.86 ± 0.02a | 45.24 ± 0.05b |
| TPC (mg/g GAE) | 0.08 ± 0.02b | 0.35 ± 0.03a |
| IC50DPPH RSA (µg/ml) | 1078 ± 0.12a | 225 ± 0.05b |
WF wheat flour, CHO carbohydrate, TPC: total phenol content, ssF Sphenstylis stenocarpa flour, DPPH 1,1-diphenyl-2-picrylhydrazyl, RSA radical scavenging activity
Values in the row with the same superscript letters are not significantly different from each other at p ≤ 0.05. Values are means of three replicates ± standard deviation
Rheological properties of Sphenostylis stenocarpa flour supplemented WF blends
Farinograph characteristics
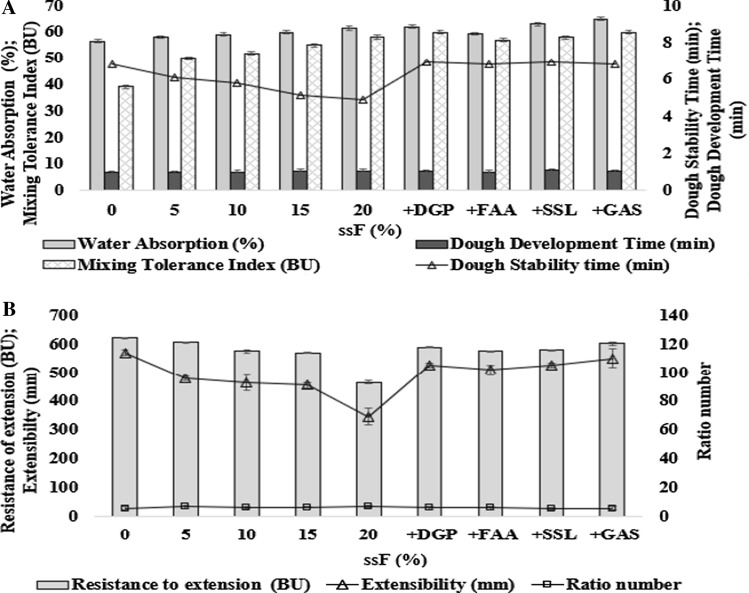
The rheological properties are mostly evaluated with the use farinograph. As shown in the farinographic data (Fig. 1a), the increased amount of ssF in the blend from 0 to 20% steadily increased water absorption from 56.60 to 61.50% and dough development time from 6.8 to 7.3 min. The reported higher fibre and protein content of ssF than WF (Table 1) could be responsible for the increased water absorption while it directly influenced the increased dough development time. According to Rosell et al. (2001), the higher number of hydroxyl groups present in fibre and the protein content allow more water interactions by hydrogen bonding. Similar work on the addition of defatted soya bean and sesame elicited an increase in water absorption and development time (Ribotta et al. 2005). The measurement of dough stability is used to determine the strength of dough. The higher it is, the stronger the dough. The stability time of dough was steadily decreased with increase in ssF from 0 to 20% at 6.8 to 4.9 min respectively (Fig. 1a). Meaning that the decrease in the stability time was in relation to the quantity of ssF replacement. The mixing tolerance index also displayed an increase in value from 39 to 58 BU with increase in ssF inclusion up 20%. These results of decreased stability time and increased mixing tolerance agree with the work reported by Abdalla (2003), in which an increase in the amount of sorghum flour lead to a decrease in stability time but increase in mixing tolerance index.
Fig. 1.
Effect of 0, 5, 10, 15 and 20% ssF on the farinographic (A) and extensographic (B) characteristics of wheat flour and the blend of 15% ssF with additive(s). Error bars are the mean of three replicates ± standard deviation. ssF: Sphenostylis stenocarpa flour, FAA: fungal α-amylase (0.002%), DGP: dry gluten powder (5.0%), SSL: sodium stearoyl-2-lactylate (0.5%) and GAS: combination of 5% DGP + 0.002%FAA + 0.5% + SSL
Furthermore, some researchers had experimented the use of single additive for improving the quality of dough for bread making (Miyazaki et al. 2004). The addition dry gluten protein (DGP) to 15% ssF caused an increase to water absorption (62%) while the dough development time was maintained at 7.3 min. There was a marginal increase of dough stability time at 6.9 min and a significant increase of mixing tolerance index (60 BU). Fungal alpha-amylase (FAA) addition on the contrary imparted slight decrease on dough development time (7.0 min) by the decreased water absorption capacity from 60.0% to 59.5% (Fig. 1a). The reducing effect may be attributed to the earlier report that α-amylase enzyme can quickly gain access to the active sites in starch molecules to catalyse saccharification to yield sugars and then hydrolyze them to low molecular weight dextrins that were produced from damaged starches hence, showing a low water binding capacity (Miyazaki et al. 2004). Addition of SSL to 15% ssF following the trend of gluten also slightly increased the water absorption capacity (63%), dough development time (7.5 min) and dough stability time (6.9 min) but maintained the mixing tolerance index (58 BU). Sodium stearoyl-2-lactylate (SSL) is a complex emulsifier with duo property that enhances dough stability. According to Chung and Tsen (1975), the addition of SSL eases the interaction of ionic surfactant with protein, lipids and starch. It also increases the interface activity between water and the non-aqueous phases of the bread dough thereby supporting the formation of blends and intense uniform film that will later imparts softness in bread crumb.
The effect of the combined additives (GAS) significantly improved farinographic parameters than other individual additive that favourably competed with the control (Fig. 1a). Regarding the interaction of these additives, the modifications promoted by gluten addition exerts its impact by fitting with the glutenic proteins to form a homogenous and stable gluten network that strengthens the technological potential of WF interaction. The action of α-amylase also imparts significant softness to dough through hydrolyzed α-(1–4) glycosidic bond of gelatinized starch Miyazaki et al. 2004). SSL (an anionic oil-in-water emulsifier) improves the quality of dough by facilitating the interaction between protein, lipids and starch thereby supporting the formation of blends and intense uniform film (Chung and Tsen 1975). The behavioural pattern of GAS could therefore be attributed to the synergistic action of the additives when added together.
Extensograph characteristics
Improvement in the value of extensograph is an indication of good handling property of dough for bread making (Brabender 1958). The determined extensographic parameters for this study are resistance to extension, extensibility and ratio number. As shown in Fig. 1b. There was an observed steady decrease in the values reported for resistance to extension with the increase in ssF inclusion in comparison to control. The values from 0–15% reduced from 623–570 BU. The decrease among the blends from 5–15% was not significant compared to the decrease reported for 20% inclusion of ssF at 466 BU. Ribotta et al. (2005) also reported a decrease in the extension on the inclusion of soy products. Moreover, the quantity of resistance of extension is dependent on the amount of gluten in dough. The observed significant decrease on 20% inclusion of ssF agrees with the reported work of Maforimbo et al. (2006) as decrease in the value of resistance to extension is an indication of difficulty in bread making. The decreased value of resistance to extension in relation to increased percentage of ssF inclusion also affected the extensibility. The increase in ssF inclusion from 0–20% reduced the extensibility value from 114–69 mm (Fig. 1b). This present study also confirms the direct relationship that exists between resistance to extension and extensibility (Enriquez et al. 2003). Ratio number values are derived through calculation in relation to resistance to extension and extensibility by division (Fig. 1b). The reported value for 5, 10 and 15% inclusion (6.33, 6.18 and 6.26) are higher in comparison to control (5.46). The ratio value for 20% (6.75) inclusion displayed the highest value as a result of its low resistance and extensibility values.
The effect of additives on extensographic parameters of 15% ssF incorporated dough revealed an increase in resistance of extension as reported for DGP, FAA, SSL and GAS to be 589, 575, 580 and 602 BU respectively (Fig. 1b). The increase in the individual and combined additives (GAS) could be attributed to the reported increase in farinographic water absorption capacity as revealed in Fig. 1a. Furthermore, the reported resistance to extension also influenced a decrease in their respective extensibility value for DGP (105 mm), FAA (102 mm), SSL (105 mm) and GAS (110 mm) in comparison to control (114 mm) but higher than that of 15% ssF (91 mm). There was also an observed decrease in their respective ratio number. Based on this present study, the dough with 15% ssF + GAS exhibited the best extensographic parameters with bread making quality than other individual additive that competed favourably with control. Again, the synergistic effect of the combined additives can be suggested to be responsible for the favourable improvements.
Scanning electron micrograph of dough
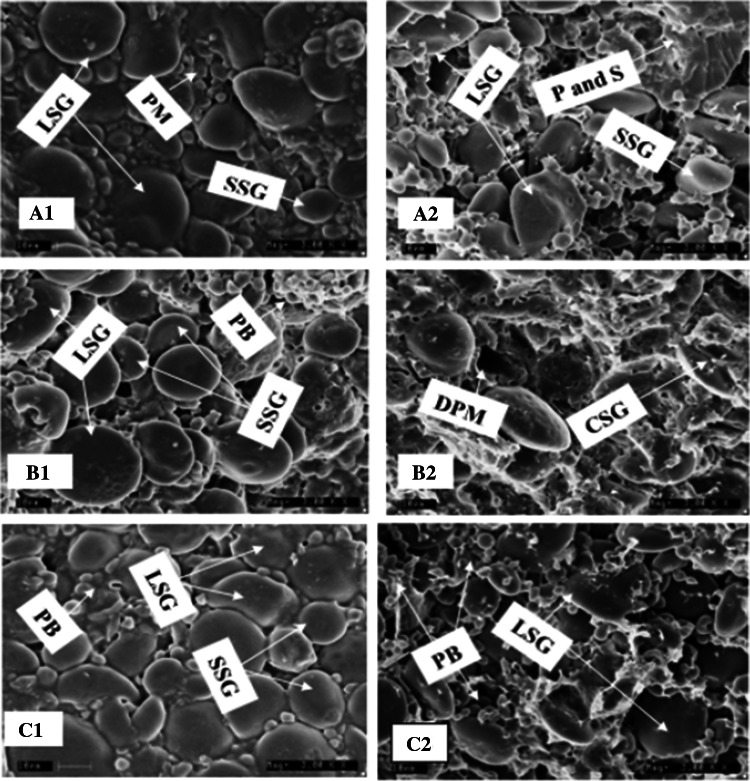
Two views of scanning electron micrograph (SEM) showing the surface structure and cross- section of control, 15% ssF and 15% ssF + GAS bread dough was examined and the results are presented in Fig. 3. The protein microstructure on the surface of the control dough depicts the thick protein matrix-starch interaction. The components appeared to be well compacted with no interspace between them as the starch granules were almost covered by the gluten film (Fig. 2(a1)). The cross-section image revealed an evenly distributed components of dough (Fig. 2(a2)). The observed continuous network of starch and protein matrix could be attributed to the presence of gliadins and glutenins as they confer viscosity and elasticity on gluten to maintain its holding capacity in dough (Miyazaki et al. 2004). On the other hand, the microscopic structure of dough prepared from 15% ssF (without additives) revealed the weak relationship between the protein and starch granules as they pooled back from one another leaving the starch granules to be exposed (Fig. 2(b1)). The cross section of the SEM also revealed the nature of loose protein-starch interaction as evident with the disrupted protein matrix (DPM) hollow (Fig. 2(b2)) The reason for this weak relationship could be linked to the fact that legume proteins do not have good dough forming properties and that the protein-starch complex in WF had been ruptured by supplemented protein (Rosell et al. 2001).
Fig. 3.
The representation of control (100% WF), ssBF and ssBF + additives bread. A (0, 5, 10, 15, and 20% inclusion of ssF without additives) and B (a: control, b: 15% ssBF, c: 15% ssF + DGP, d: 15% ssF + FAA, e: 15% ssF + SSL and f: 15% ssF + GAS. Please refer Fig. 1 for codes
Fig. 2.
Scanning electron micrograph of control (100% WF), 15% ssF and 15% ssF + GAS dough (× 3,000). a1, b1 and c1; surface structure of control, 15% ssF and 15% ssF + GAS respectively. a2, b2 and c2; cross section of control, 15% ssF and 15% ssF + GAS respectively. LSG: large starch granule, SSG: small starch granule, PM: protein matrix, PB: protein bodies, CSG: coated starch granules, DPM: disrupted protein matrix, P: protein, S: starch. Please refer Fig. 1 for codes
SEM of dough sample from 15% ssF + GAS revealed an improved relationship of protein and starch. The small and large starch granules were observed to be entangled in protein matrix to form a continuous network than that of control dough (Fig. 2(c1)). There was similarity between the observations in Fig. 2(c1), c2 as 15% ssF + GAS dough image showed a thicker, denser and tighter gluten film than control dough. The presence of the combined additives however exerted a protective effect against starch-protein complex disruption which eventually facilitated higher stability and elasticity of the dough.
Physico-sensory characteristics of bread
The physico-sensory characteristics was carried out to identify the quality of control, 15% ssF and 15% ssF + GAS bread and the results are presented in Table 2. The specific volume of the control, 15% ssF and 15% ssF + GAS bread was 3.80, 2.97 and 4.18 cm3/g respectively (Table 2). The observed reduction in the specific volume of 15% ssF agrees with the opinion of Ragaee et al. (2011) that the reduction of specific volume can be influenced by the substitution of composite flour as a result of gluten dilution in WF which affects the protein-starch interaction during mixing, fermentation and baking processes. The significant increase in the specific volume of 15% ssF + GAS bread may be likened to its inflating ability due to the improvement imparted by the additives (Rosell et al. 2001). The reported sensory scores for different parameters in control and 15% ssF showed a significant decrease for crust colour from 9 to 7, crust shape (9 to 6), crumb colour (9 to 8), crumb grain (8 to 6), mouthfeel (8.5 to 7) and taste (9 to 6). The overall quality score (OQS) which totalled 60 was reduced from 52.5 to 40 respectively (Table 2). The bread with 15% ssF + GAS displayed higher value of crust colour as indicated by the sensory score of 7.5, crust shape (8.5), crumb colour (8.5), crumb grain (8) mouthfeel (8), taste (8.5) and overall quality score (OQS) of 49. The clean mouthfeel and beany taste were observed in 15% ssF and 15% ssF + GAS but were still considered acceptable while the observed darker crust could have also occurred as a result of the non-enzymatic maillard reaction between protein and sugar (Fig. 3).
Table 2.
Physico-sensory characteristic and texture profile analysis of bread
| ssF (%) | |||
|---|---|---|---|
| Parameters | Control | 15% ssF | 15% ssF + GAS |
| Specific volume (cm3/g) | 3.80 ± 0.02 b | 2.97 ± 0.03c | 4.18 ± 0.03a |
| Hardness (N) | 64.84 ± 0.02c | 136.00 ± 0.03a | 77.00 ± 0.03b |
| Cohesiveness | 0.25 ± 0.02a | 0.18 ± 0.03c | 0.22 ± 0.03b |
| Springiness (mm) | 0.96 ± 0.03a | 0.79 ± 0.01c | 0.91 ± 0.02a |
| Gumminess | 16.21 ± 0.02c | 24.48 ± 0.03a | 16.94 ± 0.00b |
| Chewiness (N mm) | 15.56 ± 0.03b | 19.34 ± 0.03a | 15.41 ± 0.03b |
| Colour | |||
| L | 63.47 ± 0.13a | 50.78 ± 0.03c | 58.60 ± 0.03b |
| a | -0.19 ± 0.11a | 0.86 ± 0.14b | 0.60 ± 0.02c |
| b | 12.37 ± 0.03a | 11.75 ± 0.02c | 12.18 ± 0.01b |
| OQS (60) | 52.50 ± 0.03a | 40.00 ± .0.01c | 49.00 ± 0.03b |
ssF: Sphenostylis stenocarpa flour, overall quality score (OQS) is the combined score of sensory parameters such as crust colour, crust shape, crumb colour, crumb grain, mouthfeel and taste
Values in the row with the same superscript letters are not significantly different from each other at p ≤ 0.05. Values are means of three replicates ± standard deviation
Instrumental colour measurement
Crumb assessment is very important as it determines the quality of bread through the rheological and physical properties. It is also a parameter that influences the consumer priority. The crumb colour parameters of control, 15% ssF and 15% ssF + GAS bread was further analyzed using Hunger Lab machine and are presented in Table 2. The hunter Lab colour L* value representing lightness for control bread was 63.47. It significantly decreased to 50.78 in 15% ssF inclusion but upgraded to 58.60 with the incorporation of combined additives in 15% ssF + GAS. The −a* value, representing greenness increased from −0.19 in control bread to 0.86 in 15% ssF but reduced to 0.60 in 15% ssF + GAS bread. The b* value for control, 15% ssF and 15% ssF + GAS bread are 12.37, 11.75 and 12.18 respectively. The off white colour of bean bread crumb is in line with the opinion of MacDougall (2002) that the colour of processed product should still be as close as possible to the raw material.
Texture profile analysis
The texture profile analysis (TPA) of bread as presented in Table 2 showed that the hardness increased with the addition of 15% ssF (136.00 N) compared to control (64.84 N). According to Gomez et al. (2013), bread hardness occurs as a result of disruption that has taken place between gluten and fibrotic materials in flour. This eventually will hinder expansion during proofing to produce bread with low specific volume. However, a decrease in the value reported for 15% ssF + GAS (77.00 N) in comparison to 15% ssF can be linked to the effect of the combined additives as there was a compact relationship between the WF and ssF materials (Fig. 2(c1, c2)). Springiness is used to deduce the elastic nature of interaction between gelatinized starch and gluten in dough and it is carried on to the sponge structure of bread after heating (Hoseney et al. 1994). The reported lowest value of springiness in 15% ssF (0.79 mm) in comparison to control (0.96 mm) and 15% ssF + GAS (0.91 mm) is an indication of protein-starch disruption but was enhanced in 15% ssF + GAS. There was no significant difference in the values reported for springiness in 15%ssF + GAS and control. Cohesiveness in bread measures its ability to resist deformation by teeth. The value reported for 15% ssF (0.18) is the lowest in comparison to control (0.25) and 15% ssF + GAS (0.22) values with no significant difference. The formula for gumminess is derived by multiplying hardness and cohesiveness while chewiness is generated by multiplying hardness, cohesiveness and springiness. The result of this study revealed that 15% ssF had the highest value of gumminess (24.48) and chewiness (19.34 N mm). Again, there was no significant difference in the gumminess and chewiness values reported for control (16.21 and 15.56 N mm) and 15% ssF + GAS (16.94 and 15.41 N mm) respectively. Our findings are similar to the work of Wang et al. (2007) in which the tested composite bread with the least cohesive value displayed the highest values of gumminess and chewiness.
Antioxidant properties of bread
Free radical scavenging is the most critical step in the prevention of damage to body macromolecules through the powerful actions of antioxidants derived from natural sources. The phenolic acids exert antioxidant activities due to their redox properties which gives them the capacity to chelate metals and quench singlet oxygen reaction (Cuendet et al. 1997). The antioxidant properties of control and 15% ssF + GAS bread are presented in Table 3. The total phenol content of 15% ssF + GAS (4.84 mg/gGAE) was higher than that of control bread (1.49 mg/gGAE). DPPH (1,1-diphenyl-2-picrylhydrazyl) acts as a free radical by accepting electrons or hydrogen atoms to become a stable diamagnetic molecule (Je et al. 2009). The bread extract of 15% ssF + GAS scavenged DPPH radicals from visible deep purple colour to the yellow-coloured diphenyl picrylhydrazine more than the control bread as revealed through their reported IC50 value of 205 and 710 µg/ml respectively. The lower IC50 value signifies higher DPPH scavenging capacity (Cuendet et al. 1997). It could be inferred from the results that there is a direct correlation between total phenol content and radical scavenging ability which however raised the potency of 15% ssF + GAS bread to scavenge free radicals more than that of control bread. Hence, exhibiting higher antioxidant activity.
Table 3.
Proximate composition of bread
| Parameters | Control | 15% ssF + GAS |
|---|---|---|
| Moisture (%) | 31.55 ± 0.01b | 35.42 ± 0.03a |
| Ash (%) | 1.39 ± 0.03b | 4.25 ± 0.02a |
| Protein (%) | 7.36 ± 0.02b | 10.20 ± 0.03a |
| Fat (%) | 1.31 ± 0.03a | 1.26 ± 0.01b |
| CHO (%) | 56.04 ± 0.03a | 45.57 ± 0.01b |
| Energy (%) | 278.03 ± 0.02a | 255.34 ± 0.01b |
| Dietary fibre (%) | 2.35 ± 0.03b | 3.30 ± 0.02a |
| TPC (mg/g GAE) | 1.49 ± 0.06b | 4.84 ± 0.10a |
| IC50DPPH RSA (µg/ml) | 710.00 ± 0.05a | 205.00 ± 0.01b |
CHO carbohydrate, TPC total phenol content, DPPH 1,1-diphenyl-2-picrylhydrazyl, RSA radical scavenging activity, ssF + GAS Sphenostylis stenocarpa flour plus combination of 5% DGP + 0.002%FAA + 0.5% + SSL
Values in the row with same superscript letters are not significantly different from each other at p ≤ 0.05. Values are means of three replicates ± standard deviation
Chemical composition of bread
The chemical composition of control and 15% ssF + GAS bread is shown in Table 3. The moisture content value of 15% ssF + GAS bread (35.42%) was higher than that of control bread (31.55%). The protein content of the 15% ssF + GAS (10.20%) was also higher when compared to the control bread (7.36%). The supplementation of WF with legume increased the protein content of the bread because beans generally contain more proteins than cereals (Dhingra and Jood 2001). The same trend of value was also reported for the ash (4.25%) and dietary fibre (3.30%) of 15% ssF + GAS bread in comparison to the control at 1.39% and 2.35% respectively. These results show that there was an increase in the ash and dietary fibre contents of 15% ssF + GAS by 3.05 and 1.40 times respectively. The control bread had a higher content of fat, carbohydrate (CHO) and energy at 1.31, 59.20 and 278% respectively.
It is worthy of note that this present study has experimented the effect of an underutilized Sphenostylis stenocarpa flour (ssF) incorporation at optimum level of 15% in WF and additives (in single and combined forms). The blend with combined additives (GAS) produced bread that satisfactorily competed with control bread in sensory acceptance. However, the further elucidated nutritional content and functional properties was higher in 15%ssF + GAS bread in comparison to control bread. In view of this, our results have been able to report an increase in the protein, dietary fibre, total phenol content and antioxidant activity of 15% ssF + GAS bread than control bread thus, classifying it as bread with higher nutritional properties.
Conclusion
Sphenostylis stenocarpa popularly called African yam bean is an underutilized plant of numerous nutritional characteristics with potentials that can fortify the food base for human consumption. Our studies have been able to showcase the possibility of incorporating ssF at 15% optimum to produce bread with acceptable physico-sensory properties and increased nutritional properties. Therefore, the need to adopt the incorporation of ssF into baked product that is commonly consumed by the population will pave way to solving some nutrition-base challenges.
Acknowledgements
The Authors are grateful for the financial support provided by DBT-TWAS, Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko (Nigeria), Government of India and Director, CSIR-CFTRI for extending facilities.
Abbreviations
- DGP
Dry gluten powder
- FAA
Fungal α-amylase
- SSL
Sodium stearoyl-2-lactylate
- ssF
Sphenostylis stenocarpa flour
- WF
Wheat flour
Author’s contributions
SSA: conceptualization, methodology, validation, formal analysis and writing original draft. DI: designing the study, methodology, validation, writing/review. PP: Conceptualization, supervision, project administration, writing/review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Availability of data and material
The data that support the findings of this study are available from the corresponding authors on request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
This is an original work being done and it is not submitted to any other journals for publication. The data provided including figures and tables can be used for publishing in JFST and they are not being provided to any other journals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Methods 44–15, 46–10, ash, 08–01, 30–10, 991.43, 56–61. Approved methods of the AACC. 10. St. Paul: American Association for Cereal Chemists; 2000. [Google Scholar]
- Abdalla IA (2003) Biscuits from composite flour of wheat and sorghum. In: M.Sc. Thesis, University of Khartoum, Sudan
- Ade IC, Ingbian EK, Abu JO. Physical, chemical and sensory properties of baked products from blends of wheat flour and African yam Bean (Sphenostylis stenocarpa) water-extractable proteins. Nig Food J. 2012;30:109–115. doi: 10.1016/S0189-7241(15)30019-9. [DOI] [Google Scholar]
- Agunbiade SO, Longe O. The physico-functional characteristics of starches from cowpea (Vigna unguiculata), pigeon pea (Cajanus cajan) and African yam bean (Sphenostylis stenocarpa) Food Chem. 1999;65:469–474. doi: 10.1016/S0308-8146(98)00200-3. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersburg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Apata DF, Ologhobo AD. Some aspects of biochemistry and nutritive value of African yam bean seed (Sphenostylis stenocarpa) Food Chem. 1990;36:271–280. doi: 10.1016/0308-8146(90)90066-D. [DOI] [Google Scholar]
- Asogwa IS, Onweluzo JC. Effects of processing methods on the chemical composition of flour, 'Moin Moin' and 'akara' from Mucunapruriens. J Trop Agric Food Environ Ext. 2010;9:200–208. [Google Scholar]
- Brabender OHG (1958) Extensograph, instuction manual. Brabender OHG, Duisburg, No. 1702 E
- Chung OK, Tsen CC. Functional Properties of surfactants in relation to flour constituents in a dough system. Cereal Chem. 1975;52:832–843. [Google Scholar]
- Cuendet M, Hostettmann K, Potterat O. Iridoid Glycoside with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 1997;80:1144–1152. doi: 10.1002/hlca.19970800411. [DOI] [Google Scholar]
- Dhingra S, Jood S. Physico-chemical and nutritional properties of cereal-pulse blends for bread making. Nutr Health. 2002;163:183–194. doi: 10.1177/026010600201600304. [DOI] [PubMed] [Google Scholar]
- Eke OS, Akobundu ENT. Functional properties of African yam bean (Sphenostylis stenocarpa) seed flour as affected by processing. Food Chem. 1993;48:337–340. doi: 10.1016/0308-8146(93)90314-6. [DOI] [Google Scholar]
- Enriquez N, Peltzer M, Raimundi A, Tosi V, Pollio ML. Characterization of the wheat and quinoa flour blends in relation to their bread making quality. J Argent Chem Soc. 2003;91:47–54. [Google Scholar]
- Gómez M, OlieteB RCM, Pando V, Fernández E. Studies on cake quality made of wheat–chickpea flour blends. LWT Food Sci Technol. 2008;41:1701–1709. doi: 10.1016/j.lwt.2007.11.024. [DOI] [Google Scholar]
- Hoseney RC. Principles of cereal science and technology. 3. United Kingdom: American association of cereal chemists, Inc; 1994. pp. 203–206. [Google Scholar]
- Indrani D, Prabhasankar P, Rajiv J, Venkateswar Rao G. Scanning electron microscopy, rheological characteristics, and bread-baking performance of wheat-flour dough as affected by enzymes. J Food Sci. 2003;68:2804–2809. doi: 10.1111/j.1365-2621.2003.tb05809.x. [DOI] [Google Scholar]
- Ishida PM, Steel C. Physicochemical and sensory characteristics of pan bread samples available in the Brazilian market. Food Sci Technol Campinas. 2014;34:746–754. doi: 10.1590/1678-457X.6453. [DOI] [Google Scholar]
- Je JY, Park PJ, Kim EK, Ahn CB. Antioxidant and angiotensin 1 converting enzyme inhibitory activity of Bambusae caulis in liquamen. Food Chem. 2009;113:932–935. doi: 10.1016/j.foodchem.2008.08.022. [DOI] [Google Scholar]
- Jideani V, Onwublai F. Optimisation of wheat-sprouted soybean flour bead using response surface methodology. Afr J Biotechnol. 2009;8:6364–6373. doi: 10.5897/AJB09.707. [DOI] [Google Scholar]
- Li W, Pickard MD, Beta T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple wheat grain. J Agric Food Chem. 2007;55:8958–8966. doi: 10.1021/jf071715p. [DOI] [PubMed] [Google Scholar]
- MacDougall DB. Official methods of analysis. 1. Boca Raton, Fla: Woodhead Publishing; 2002. p. 378. [Google Scholar]
- Maforimbo E, Nguyen M, Skurray G. The effect of L-ascorbic acid on the rheological properties of soy–wheat dough: a comparison of raw and physically modified soy flours. J Food Eng. 2006;72:339–345. doi: 10.1016/j.jfoodeng.2004.12.013. [DOI] [Google Scholar]
- Malumba Doran L, Zanmenou W, Odjo S, Katanga J, Blecker C, Béra F. Morphological, structural and functional properties of starch granules extracted from the tubers and seeds of Sphenostylis stenocarpa. Carbohydr Polym. 2017;178:286–294. doi: 10.1016/j.carbpol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Maeda T, Morita N. Effect of various dextrin substitutions for wheat flour on dough properties and bread qualities. Food Res Int. 2004;37:59–65. doi: 10.1016/j.foodres.2003.08.007. [DOI] [Google Scholar]
- Njoku HO, Eli I, Ofuya CO. Effect of pre-treatment on the cooking time of the African Yam bean (Sphenostylis stenocarpa) J Food Sci. 1989;54:758–789. doi: 10.1111/j.1365-2621.1989.tb04700.x. [DOI] [Google Scholar]
- Nwokolo E. A Nutritional Assessment of African Yam Bean Sphenostylis stenocarpa Harms and Bambara Groundnut Voandzeia subterranea L. J Sci Food Agric. 1987;41:123–129. doi: 10.1002/jsfa.2740410205. [DOI] [Google Scholar]
- Onabolu A, Abass A, Bokanga M. New food products from Cassava. Ibadan, Nigeria: International Institute of Tropical Agriculture (IITA); 2003. p. 40. [Google Scholar]
- Ragaee S, Guzar I, Dhull K, Seetharaman K. Effects of fibre addition on antioxidant capacity and nutritional quality of wheat bread. J Food Sci Technol. 2011;44:2147–2153. [Google Scholar]
- Ribotta PD, Arnulphi S, León A, Añón MC. Effect of soybean addition on the rheological properties and breadmaking quality of wheat flour. J Sci Food Agric. 2005;85:1889–1896. doi: 10.1002/jsfa.2191. [DOI] [Google Scholar]
- Rosell CM, Rojas JA, Benedito de Barber C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001;15:75–81. doi: 10.1016/S0268-005X(00)00054-0. [DOI] [Google Scholar]
- Shelef O, Weisberg PJ, Provenza FD. The value of native plants and local production in an era of global agriculture. Front Plant Sci. 2017;8:2069. doi: 10.3389/fpls.2017.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu JS, Siebel W, Meyer D. Gelatinisation of starch during preparation of Indian unleavened flat breads. Starke. 1990;42:336–341. doi: 10.1002/star.19900420904. [DOI] [Google Scholar]
- Sreerama YN, Sashikala VB, Pratape VM, Singh V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chem. 2012;131:462–468. doi: 10.1016/j.foodchem.2011.09.008. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics. New York: McGraw-Hill; 1980. pp. 99–131. [Google Scholar]
- Wang J, Zhao M, Zhao Q. Correlation of glutenin macropolymer with viscoelastic properties during dough mixing. J Cereal Sci. 2007;45:128–133. doi: 10.1016/j.jcs.2006.07.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request.
Not applicable.