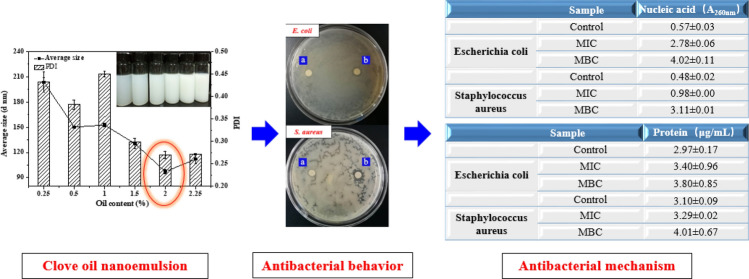
Abstract
Clove oil has many functions such as antibacterial, anti-inflammatory, anti-oxidation. In this experiment, a self-emulsification method was used to prepare clove oil nanoemulsion. And then filter paper diffusion method, minimum inhibitory concentration, and minimum bactericidal concentration were used to study the inhibitory behavior of clove oil nanoemulsion on Escherichia coli and Staphylococcus aureus. And explore the antibacterial mechanism by dynamically testing the content of nucleic acid and protein in the culture solution during the antibacterial process. The results show that when the surfactant content is 10 wt%, the hydrophile-lipophile balance (HLB) is 13.93, and the oil phase content is 2 wt%, a clove oil nanoemulsion with better dispersion and smaller average particle size can be prepared. The minimum inhibitory concentration (MIC) of clove oil nanoemulsion against Escherichia coli and Staphylococcus aureus is 0.5, 0.25 mg/mL, and the minimum bactericidal concentration (MBC) is 1, 2 mg/mL. The increase in protein content and the exponential growth of nucleic acid release also indicated that the clove oil nanoemulsion destroys the integrity of the cell membrane. The experimental results can provide a reference for the application of clove oil nanoemulsion in food, medicine and other fields.
Graphic abstract
Keywords: Clove oil, Nanoemulsion, Antibacterial, Antibacterial mechanism
Introduction
Essential oil is a natural hydrophobic liquid and a rich source of a variety of biologically active compounds, such as terpenes, terpenoids and phenolic aromatic components (Nakhli et al. 2018, Shetta et al. 2019). Clove essential oil is a volatile aromatic substance extracted from the Myrtaceae tree Clove, which mainly contains eugenol, acetyl eugenol, carnation, etc. These components determine the anti-inflammatory, analgesic, antibacterial and antiseptic effects of clove oil (Zhou et al. 2000, Li et al. 2006, Pan et al. 2016). Clove oil is usually prepared into an antibacterial film with other substances to improve its antibacterial effect (Wei et al. 2018, Song et al. 2020), and can also be prepared into emulsion products to exert other beneficial effects (Liang et al. 2016, Feng et al. 2020, Hosseini and Rajaei 2020). The development of essential oil products has broadened the research content of nutrition and biomedicine, and has also increased the scope of the use of essential oils.
Emulsions, which can be divided into large emulsions, nanoemulsions and microemulsions, are a common delivery system for encapsulating lipophile biological active ingredients (McClements et al. 2017, Xu et al. 2018, Chuesiang et al. 2019, Ghiasi et al. 2019). Nanoemulsions have been proven to be very effective delivery systems for encapsulating, protecting and improving the bioavailability of lipopilic bioactive nutrients and drugs, etc. (Huang et al. 2010, Silva et al. 2012). Due to the characteristics of small particle size, transparent appearance and stable system, nanoemulsion is widely used in food, cosmetics, and other aspects (Yang et al. 2021). For example, nanoemulsion, due to its controllable particle size distribution and stability of ultra-fine droplets, has been used as the delivery system of active substances or effective ingredients in the field of cosmetics and has shown a good application prospect (Tong et al. 2019). The solubility and bioavailability of lycopene were significantly improved when nanoemulsion was used as a transport system (Wu et al. 2019). In addition, the research and application of essential oil nanoemulsions are also very extensive. For example, peppermint oil nanoemulsion was prepared using soy protein isolate (SPI), phosphatidylcholine (PC) as emulsifiers and studied for its stabilization mechanism and bacteriostatic properties (Zhang et al. 2020). In this paper, sweet orange oil emulsion was prepared with tea saponin as an emulsifier and the effects of sweet orange oil and rosin glyceride content on the emulsion properties were investigated (Peng et al. 2020). Some scholars used sodium caseinate as an emulsifier to prepare seabuckthorn fruit oil nanoemulsion and studied the characteristics, stability, and cytotoxicity of the emulsion (Jiang et al. 2019). In addition, there are some effects of cinnamon essential oil, camellia oil, etc., which are not listed here (Hou et al. 2020, Sun et al. 2020). It can be seen that the construction of essential oil nanoemulsions also provides opportunities for the use of emulsions.
Among the numerous reports on essential oil nanoemulsions, there are few studies on clove oil nanoemulsions, especially the research on the antibacterial and antibacterial mechanism of clove oil nanoemulsions. In this paper, clove oil nanoemulsion was prepared by the spontaneous emulsification method. The effects of surfactant and oil phase content on clove oil nanoemulsion were studied, and the antibacterial properties and antibacterial mechanism of clove oil nanoemulsion were determined. These results provide a theoretical reference for the application of clove oil nanoemulsion in various fields.
Materials and methods
Materials
Clove oil, Tween 80, Span 80, Coomassie Brilliant Blue G250 were all purchased from Sinopharm Chemical Reagent Co., Ltd. The nutrient broth (NB) and nutrient agar (NA) medium were purchased from Qingdao Haibo Biotechnology Co., Ltd. The Escherichia coli and Staphylococcus aureus used in the experiment were provided by the microbiology laboratory of the college, and the strains were stored on the agar slope at 4 °C.
Preparation of clove oil nanoemulsion
The spontaneous emulsification method is a kind of low-energy emulsification method, and it is prepared by referring to the spontaneous emulsification method of Xu et al. 2019 and Zhang and Qin 2019. Different concentrations of Tween 80 and span 80 were mixed first to obtain three surfactants with 9:1, 8:2, 7:3 mass ratios of Tween and span 80, and their hydrophilic-lipophilic equilibrium constants (HLB) were 11.79, 12.36, 13.93. The emulsion was prepared by mixing clove oil with the mixture surfactant, adding deionized water, and stirring at 400 rpm at room temperature. In addition, two sets of clove oil nanoemulsions were prepared. The HLB value of the mixed surfactant in a group of emulsions is 11.79, 12.36, 13.93, and the content of the mixed surfactant is 5, 7.5, 10, 12.5, and 15%. In another group of emulsions, the content of mixed surfactants with an HLB value of 13.93 is 10%, and the content of clove oil is 0.25, 0.5, 1, 1.5, 2, 2.25%, respectively.
Characterization of clove oil nanoemulsion
The particle size of the clove oil nanoemulsion was measured by dynamic light scattering (Zetasizer Nano ZS, UK), the scattering angle of the sample was 173° and the measurement temperature was 25 °C. The average particle size and polydispersity coefficient (PDI) of the emulsion are statistically analyzed through the software, and each sample is tested 5 times repeatedly.
Determination of inhibition zone
The antibacterial properties of clove oil nanoemulsion in vitro were evaluated by the paper diffusion method on agar medium, and the antibacterial properties in vitro were demonstrated by measuring the size of the inhibition zone (Zhang et al. 2015). Two typical pathogenic bacteria, including Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, were selected as experimental models of in vitro bacteriostatic activity. When the strains were cultured in sterilized NB medium at 37 °C for 24 h, the total number of Escherichia coli and Staphylococcus aureus detected by plate was 1.5 × 106 CFU and 1.8 × 105 CFU, respectively. Sterilized NA medium (18 mL) was poured into a Petri dish (9 cm) and allowed to cool to set statically, and 100 μL of the above bacterial liquid was spread evenly in the cooled NA medium. Filter papers were punched into 6 mm diameter discs for sterilization. A further round filter paper sheet containing 6 μL clove oil nanoemulsion was placed on the surface of Na medium, which was then incubated at 37 °C for 24 h to measure the size of the inhibition zone using vernier calipers.
Determination of MIC and MBC
The double dilution method reported previously was used to determine MIC and MBC (Zhang et al. 2015). The clove oil nanoemulsion was diluted with pure water into a series of concentration gradients, and then added into 10 mL NB medium tubes, respectively. The final series concentrations of clove oil nanoemulsion were 0.125, 0.25, 0.5, 1.0, 2.0, 4.0 μL/mL. 50 μL of Escherichia coli and Staphylococcus aureus bacteria liquid was added to each of the above tubes for sufficient shaking, and further incubated at 37 °C in a shaker for 24 h. The minimum inhibitory concentration (MIC) was determined when turbidity was not visible to the naked eye in the test tube. 100 μL of the mixed bacterial liquid was spread evenly on a nutrient agar medium, and the minimum bactericidal concentration (MBC) was determined by incubating the mixed bacterial liquid at 37 °C for 48 h without the growth of bacteroids.
Determination of protein and nucleic acid in bacterial liquid
Determination of nucleic acid content: the above-mentioned Escherichia coli and Staphylococcus aureus were cultured in a shaker at 37 °C for 12 h, and the bacteria were collected after centrifugation at 4000 r/min for 10 min. The volume of the bacteria was fixed with PBS buffer (pH 7.0) for 100 mL. The absorbance of the supernatant was measured at 260 nm after centrifugation at 37 °C for 3 h. Determination of protein content: the protein content in the supernatant was determined by the coomassie brilliant blue staining method according to the previous method (Nie et al. 2019). Weigh 50 mg of Coomassie Brilliant Blue in a 1 L volumetric flask, add 40 mL of 95% ethanol to dissolve, then add 120 mL of 85% phosphoric acid, add distilled water to a constant volume, mix well, remove the residue by suction and set aside. Take bovine serum albumin (BSA) to prepare a BSA standard solution with a concentration of 0.1 mg/L. Then prepare a series of standard concentrations of the standard solution, respectively take 1 mL into a clean test tube, add 4 mL of the above dye reagent, vortex to mix, and react at room temperature for 5 min. Use the blank solution as the reference solution and perform the spectrophotometric test. Measure the absorbance of the sample group at a wavelength of 595 nm. The protein content in the sample is obtained by drawing the standard curve.
Results and discussion
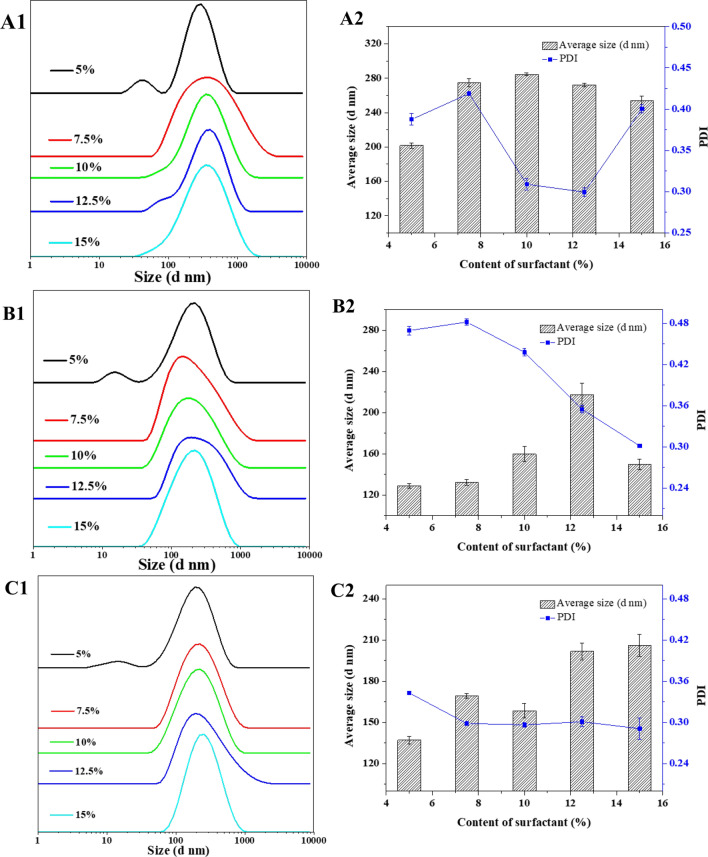
Effect of HLB and surfactant content on the particle size of clove oil nanoemulsion
The HLB value is an important parameter for evaluating the emulsifying ability of surfactants and the performance of preparing emulsions. According to the above preparation method, the mixed surfactants with different contents and HLB values of 11.79, 12.36, and 13.93 are obtained to determine the effect of the HLB value and content of the surfactant on the particle size and particle size of the emulsion (Fig. 1). When the HLB value was 11.79 (Tween80: Span80 = 9:1), the average particle size of clove oil nanoemulsion increased from 201.65 nm to 284.50 nm as the surfactant content increased from 5 to 10%. When the surfactant content continued to increase to 15%, the average particle size of the emulsions decreased to 254.15 nm, showing the overall trend of first increasing and then decreasing, with a wide range of PDI values (0.30–0.42). When the HLB value is 12.36 (Tween80: Span80 = 8:2), the variation trend of the average particle size of the emulsion with the surfactant content is consistent with the HLB value of 11.79. The average particle size of the emulsion increased from 128.80 nm to 217.35 nm and then decreased to 149.70 nm, and the PDI value showed a decreasing trend (0.48–0.30). When the HLB value was 13.93 (Tween80: Span80 = 7:3), with the increase of surfactant content, the average particle size of the emulsion showed an overall increasing trend, from the initial 137.10 nm to 206.05 nm. The PDI values were generally unchanged after decreasing (0.34–0.29). With the increase of HLB value, the average particle size and PDI value of clove oil nanoemulsion decreased, which was consistent with the results of Xu's study, confirming that the mixed surfactants had a significant effect on the emulsion (Xu et al. 2019). Similar to the results obtained by An et al., the emulsion particle size showed an initial increase followed by a decrease and a gradual increase in the range of HLB values 8–16 (An et al. 2014). When the mixed surfactant HLB value was 13.4, the emulsion particle size was the smallest (An et al. 2014). PDI characterizes the distribution of particle size in the dispersed system. The smaller the PDI value indicates that the smaller the size distribution range of the emulsion system, the more uniform the droplet dispersion (Primozic et al. 2017). The PDI value of clove oil nanoemulsion was the smallest and dispersion was the most uniform when the HLB value was 13.93.
Fig. 1.
Effects of different HLB (A1 11.79, B1 12.36, C1 13.93) and surface-active content on particle size distribution (A1, B1 and C1) and average particle size (A2, B2 and C2) of clove oil nanoemulsion
The emulsions with different surfact analyzed on the basis of HLB values of 13.93. When the surfactant content increased from 5to 7.5%, the average particle size increased from 137.10 to 169.20 nm, and the PDI value decreased from 0.34 to 0.30. When the surfactant content increased to 10%, the average particle size was slightly reduced to 158.55 nm, and when the surfactant content exceeded 10%, the average particle size increases to 206.05 nm, and the PDI value was unchanged (Fig. 1). This is similar to the findings of An and Xu that the particle size of the emulsion increases when the surfactant content exceeds 10% (An et al. 2014, Xu et al. 2019). This may be due to the different adsorption behavior of mixed surfactants on the oil–water interface. When the emulsifier content is small, the emulsifier is not enough to cover the surface of all oil droplets, and the unembedded oil droplets gather between them, forming oil droplets of different sizes, leading to the increase of PDI value (Sun et al. 2020). With the increase of surfactant content, the adsorbed surfactant on the oil–water interface increases, and the surface tension of the interface decreases, which is beneficial to the formation of an emulsion with a smaller particle size and more uniform dispersion (Wang et al. 2009). When the surfactant content is too high, the viscosity of the continuous phase and the hardness of the interface increase so that the resistance of the surfactant to enter the oil phase increases, which in turn leads to the formation of large droplets (Gursoy et al. 2004). Therefore, the mixed surfactant with an HLB value of 13.93 and a content of 10% was finally selected for the next study.
Effect of oil phase content on the particle size of clove oil nanoemulsions
As shown in Fig. 2, the influence of different clove oil content on the particle size and polydispersion index of nanoemulsion was observed. With the increase of clove oil content, the average particle size of nanoemulsion showed a decreasing trend from 203.50 nm to 112.00 nm, and the minimum average particle size of emulsion was 97.22 nm when the oil content was 2%. As for the PDI value, as the content of clove oil increased from 0.25 to 2.25%, the PDI value showed a decreasing trend, from 0.43 to 0.27. In addition to the increase in clove oil content from 0.5 to 1%, the PDI value increased from 0.38 to 0.45. This indicates that the higher oil load will lead to more homogenized droplets with smaller particle sizes, which will make the interface film surrounding the oil droplets more stable and stronger coalesce force. The continuously decreasing PDI value also indicates that the increase of clove oil content will make the emulsion more uniform (Qi et al. 2019). When the clove oil content increased from 0.5 to 1%, the PDI value increased. This may be because with the increase of oil content, the density difference between the oil phase and the water phase in the emulsion becomes larger, and the emulsion is more prone to delamination, leading to the uneven distribution of particles (Sun et al. 2020). Based on the analysis of average particle size and PDI value, under the condition that the HLB value is 13.93 and the content of the mixed surfactant is 10%, the oil phase content of 2% is selected as the next research object.
Fig. 2.
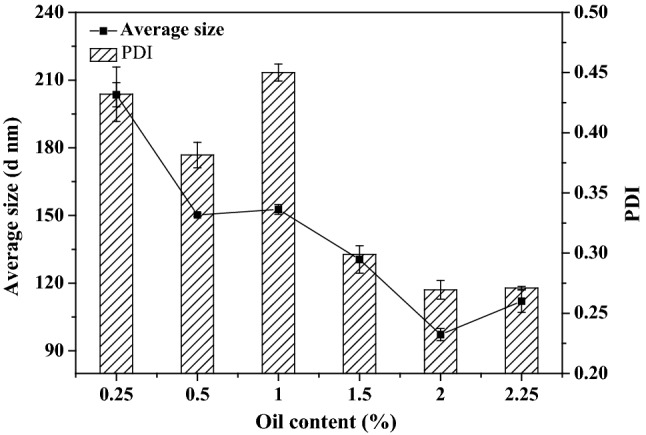
Effect of clove oil content on the average particle size of nano emulsion under HLB 13.93 and surfactant 10 wt%
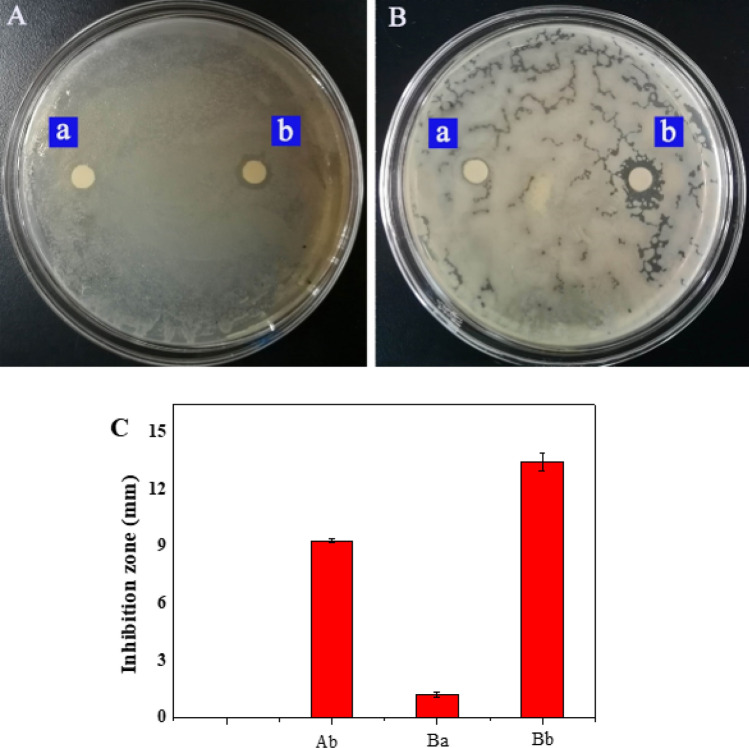
Antibacterial behavior of clove oil nanoemulsion
Clove oil has anti-inflammatory and bacteriostatic effects, and Escherichia coli and Staphylococcus aureus are the most common bacterial species in wound infection. They can represent Gram-negative and Gram-positive bacteria, so the bacteriostatic behavior of clove oil nanoemulsion can be evaluated. As shown in Fig. 3A and B, the bacteriostatic zone of the filter paper sheet containing clove oil nanoemulsion is obvious, indicating that clove oil nanoemulsion has inhibitory behavior on both bacteria, and the antibacterial effect of clove oil nanoemulsion on Staphylococcus aureus is more obvious. The inhibition zones of the clove oil nanoemulsion against Escherichia coli and Staphylococcus aureus were 9.32 and 13.47 mm, respectively, while the inhibition zones of the blank group against these two bacteria were 6.42 and 7.23 mm (Fig. 3C). This may be because Gram-negative bacteria (Escherichia coli) have a double-layer membrane structure with high lipopolysaccharide content, which is more resistant to the invasion of clove oil nanoemulsion than the single-layer membrane of Gram-positive bacteria (Staphylococcus aureus) (Hsouna et al. 2011). The antibacterial behavior of clove oil has been reflected in many articles, including the effect of clove oil alone on pathogens and the inhibitory effect of clove oil in combination with drugs or atmospheric pressure plasma on pathogens (Kunicka et al. 2020, Yoo et al. 2021, Qiu et al. 2019).
Fig. 3.
The inhibitory and bacteriostatic size of clove oil nanoemulsion (HLB 13.93, surfactant 10%, clove oil 2 wt%) on Escherichia coli (A) and Staphylococcus aureus (B) and its inhibitory size (C), a blank, b clove oil nanoemulsion
In addition to the bacteriostatic zone, MIC and MBC can also demonstrate the antibacterial effect of clove oil nanoemulsion on Escherichia coli and Staphylococcus aureus. As shown in Table 1, the minimum inhibitory concentrations of clove oil nanoemulsion against Escherichia coli and Staphylococcus aureus were 0.50 and 0.25 mg/mL, and the minimum bactericidal concentrations were 1 and 2 mg/mL, respectively. This was similar to the antibacterial results of clove oil against Escherichia coli and Staphylococcus aureus in the study of Ji et al., the MIC and MBC of clove oil against Escherichia coli O157:H7 and Staphylococcus aureus were 0.05and 0.10%, respectively (Yoo et al. 2021). However, in the study on the preparation and antibacterial activity of clove oil microemulsion by Liang et al., the MIC of clove oil microemulsion against Escherichia coli was similar to the experimental results, while the MIC of clove oil microemulsion against Staphylococcus aureus was significantly different (Liang et al. 2016). This may be due to the different preparation methods of the emulsion which limited the release of clove oil, thus affecting the antibacterial effect of clove oil. In addition, according to the MIC and MBC of the two bacteria, the clove oil nanoemulsion had a better bacteriostatic effect on Staphylococcus aureus than Escherichia coli, which was consistent with the previous results of the bacteriostatic zone, but Staphylococcus aureus was more tolerant to the bactericidal effect than Escherichia coli, which was similar to the research results of Hou et al. (2020). It may be because Gram-negative bacteria are more sensitive than Gram-positive bacteria. Both the outer and inner membranes of Gram-negative bacteria have target sites for the main components of essential oils. Once the outer membrane is destroyed, the cytoplasmic membrane is easily destroyed and cytoplasm is leaked, thereby achieving a stronger bactericidal effect.
Table 1.
Antibacterial activity of clove oil nanoemulsion against Escherichia coli and Staphylococcus aureus
| MIC (mg/mL) | MBC (mg/mL) | |
|---|---|---|
| Escherichia coli | 0.5 | 1 |
| Staphylococcus aureus | 0.25 | 2 |
Antibacterial mechanism of clove oil nanoemulsion
The release of the endolysates of the nucleic acid and protein of the bacterial cell can indicate the influence of the clove oil nanoemulsion on the integrity of the bacterial cell membrane, and it can also reflect the antibacterial mechanism of the clove oil nanoemulsion on the bacterial cell. As shown in Table 2, for the absorbance of Escherichia coli and Staphylococcus aureus at 260 nm, when the MIC concentration of clove oil nanoemulsion was added, the absorbance of them increased from 0.57 and 0.48 in the blank group to 2.78 and 0.98, respectively. When MBC concentration was added, it increased to 4.02 and 3.11. The protein release also showed an increasing trend (Table 3), from 2.97, 3.10 μg/mL in the original blank group to 3.40, 3.29 μg/mL (MIC) and 3.80, 4.01 μg/mL (MBC). Although the release of protein was not obvious with the change of the concentration of clove oil nanoemulsion, the release of nucleic acid doubled with the increase of the concentration of clove oil nanoemulsion. In conclusion, clove oil nanoemulsions can destroy the integrity of Escherichia coli and Staphylococcus aureus. Previous research results also confirmed that clove oil can destroy the integrity of bacterial cell membrane, so as to enter the cells and play a role (Yoo et al. 2021). Nucleic acid is the genetic material of organisms, and protein is the material basis of life. These two types of biological macromolecules are very important unit material structures in the entire cell (Sharma et al. 2013). This indicates that the clove oil nanoemulsion can destroy the integrity of the bacteria, damage the cell membrane and cause the release of nucleic acids and proteins, which can affect the growth of bacteria, inhibit the reproduction of bacteria, and have the effect of antibacterial and sterilization.
Table 2.
Effect of clove oil nanoemulsion on nucleic acid release from Escherichia coli and Staphylococcus aureus
| Sample | Nucleic acid (A260nm) |
|---|---|
| Escherichia coli | |
| Control | 0.57 ± 0.03 |
| MIC | 2.78 ± 0.06 |
| MBC | 4.02 ± 0.11 |
| Staphylococcus aureus | |
| Control | 0.48 ± 0.02 |
| MIC | 0.98 ± 0.00 |
| MBC | 3.11 ± 0.01 |
Table 3.
Effect of clove oil nanoemulsion on protein release from Escherichia coli and Staphylococcus aureus
| Sample | Protein (μg/mL) |
|---|---|
| Escherichia coli | |
| Control | 2.97 ± 0.17 |
| MIC | 3.40 ± 0.96 |
| MBC | 3.80 ± 0.85 |
| Staphylococcus aureus | |
| Control | 3.10 ± 0.09 |
| MIC | 3.29 ± 0.02 |
| MBC | 4.01 ± 0.67 |
Conclusion
Using the self-emulsification method, the particle size of clove oil nanoemulsion was 97.22 nm when HLB of the mixed surfactant was 13.93, the content was 10 wt%, and the content of clove oil was 2 wt%. The clove oil nanoemulsion had an obvious antibacterial effect on Escherichia coli and Staphylococcus aureus, the minimum inhibitory concentration was 0.5, 0.25 mg/mL, the minimum bactericidal concentration was 1, 2 mg/mL, respectively. The inhibition mechanism of clove oil nanoemulsion was initially investigated by the release amount of nucleic acid and protein, which was greatly released due to the broken integrity of the bacteriophage so that the growth and reproduction of the bacteriophage were blocked. In this study, clove oil was used as the main antimicrobial substance, and the emulsion was used as the carrier to load and release clove oil, which not only expanded the application of clove oil in food, cosmetics, etc., but also provided a basis for further exploration of various characteristics and applications of clove oil nanoemulsion.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. U2004160 and No. 31701647), Natural Science Foundation of Henan province (Grant No. 162300410229), High level talent research foundation of Xinyang Normal University, and Nanhu Scholars Program for Young Scholars of XYNU. This research was also kindly supported by the Analysis & Testing Center of Xinyang Normal University.
Abbreviations
- HLB
Hydrophile-lipophile balance
- MIC
Minimum inhibitory concentration
- MBC
Minimum bactericidal concentration
Authors' contributions
HS carried out the work and wrote the MS. DL supervised the work. SZ carried out sectional work. ZL revised the paper. WX supervised the work and revised the paper.
Funding
The research was financially supported by Wei Xu through the National Natural Science Foundation of China (Grant No. U2004160 and No. 31701647) and Natural Science Foundation of Henan province (Grant No. 162300410229).
Declarations
Conflict of interest
There is no professional or other personal interest of any service and/or company that could be construed as influencing the position presented in the manuscript. The work has not been published before.
Ethics approval
There is no ethics interest related in research.
Consent to participate
No conflict of interest exits in the submission of this manuscript. All the authors listed have participated the research and approved the manuscript that is enclosed.
Consent for publication
The paper was approved by all authors for publication. All the authors agree to publish the paper in “Journal of Food Science and Technology”. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
Availability of data and material—(data transparency)
The data in the paper was firstly published and could not be available somewhere else.
Code availability—(software application or custom code)
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Denglin Luo, Email: luodenglin@163.com.
Wei Xu, Email: toxuwei1986@163.com.
References
- An YP, Yan XX, Li B, Li Y. Microencapsulation of capsanthin by self-emulsifying nanoemulsions and stability evaluation. Eur Food Res Technol. 2014;239:1077–1085. doi: 10.1007/s00217-014-2328-3. [DOI] [Google Scholar]
- Chuesiang P, Siripatrawan U, Sanguandeekul R, McClements DJ, McLandsborough L. Antimicrobial activity of PIT-fabricated cinnamon oil nanoemulsions: effect of surfactant concentration on morphology of foodborne pathogens. Food Control. 2019;98:405–411. doi: 10.1016/j.foodcont.2018.11.024. [DOI] [Google Scholar]
- Feng WX, Wu DH, Cai GL, Wang L, Zhai XC, Lu J. The inhibitory effect of essential oil nanoemulsions on Fusarium graminearum. Food Ferment Ind. 2020;46:94–100. [Google Scholar]
- Ghiasi Z, Esmaeli F, Aghajani M, Ghazi-Khansari M, Faramarzi MA, Amani A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: an in vivo study. Int J Pharm. 2019;559:341–347. doi: 10.1016/j.ijpharm.2019.01.043. [DOI] [PubMed] [Google Scholar]
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hosseini RS, Rajaei A. Potential Pickering emulsion stabilized with chitosan-stearic acid nanogels incorporating clove essential oil to produce fish-oil-enriched mayonnaise. Carbohydrate Polym. 2020;241:116340. doi: 10.1016/j.carbpol.2020.116340. [DOI] [PubMed] [Google Scholar]
- Hou KH, Feng X, Gao CC, Tang XZ. Antibacterial activity and stability of cinnamon essential oil nanoemulsion. J Chin Cereals Oils Assoc. 2020;35:86–92. [Google Scholar]
- Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of ceratonia siliquaessential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Micobiol. 2011;148:66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Huang QR, Yu HL, Ru QM. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:50–57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- Jiang ZR, Zhang T, Wang T, Chang M, Liu RJ, Jin QZ, Wang XG. Properties and antioxidant activities of seabuckthorn fruit oil nanoemulsions. China Oils Fats. 2019;44:59–64. [Google Scholar]
- Kunicka SA, Tyfa A, Laskowski D, Plucińska A, Rajkowska K, Kowal K. Clove oil (Syzygium aromaticum L.) activity against alicyclobacillus acidoterrestris biofilm on technical surfaces. Molecules. 2020;25:3334. doi: 10.3390/molecules25153334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Ji BP, Zhou F, Li B. Study on extraction of two essential oils, analysis of major components and antimicrobial activities. Food Sci. 2006;08:64–68. [Google Scholar]
- Liang R, Zhong F, Wang H, Zhang ML. Study on the preparation and antibacterial activity of clove oil microemulsions. Food Mach. 2016;32:127–131. [Google Scholar]
- McClements DJ, Bai L, Chung C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu Rev Food Sci Technol. 2017;8:205–236. doi: 10.1146/annurev-food-030216-030154. [DOI] [PubMed] [Google Scholar]
- Nakhli A, Mbouga MGN, Bergaoui M, Khalfaoui M, Cretin M, Huguet P. Modeling of essential oils adsorption onto clays towards a better understanding of their interactions. J Mol Liq. 2018;249:132–143. doi: 10.1016/j.molliq.2017.11.012. [DOI] [Google Scholar]
- Nie CH, Zheng X, Ayijulaike KDE, Li XR, Adilijiang YMT, Guo W, Gao XL. Determination of whey protein content in different kinds of milk by coomassie brilliant blue method. J Food Saf Qual. 2019;10:1138–1142. [Google Scholar]
- Pan XC, Fu YS, Xu JF, Zhu JH. Study on the active ingredients for antibacterial activities of volatile oils from 17 kinds of natural plants. Sci Technol Food Ind. 2016;37:107–112. [Google Scholar]
- Peng Q, Duan HY, Wang C. Preparation and characterization of sweet orange oil nanoemulsion. Food Ferment Ind. 2020;46:148–153. [Google Scholar]
- Primozic M, Duchek A, Nickerson M, Ghosh S. Effect of lentil proteins isolate concentration on the formation, stability and rheological behavior of oil-in-water nanoemulsions. Food Chem. 2017;237:65–74. doi: 10.1016/j.foodchem.2017.05.079. [DOI] [PubMed] [Google Scholar]
- Qi YM, Xun CR, Che JL, Jiang LZ, Ma WJ, Zhang XY, Li Y, Wang ZJ. Preparation and properties of peppermint oil nanoemulsions. Food Sci. 2019;40:29–35. [Google Scholar]
- Qiu M, Long NN, Gao MX, Zhou YY, Sun FH, Lin L, Dai M. In vitro anti-MRSA effect of clove oil combined with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Chin Trad Herbal Drugs. 2019;50:1629–1635. [Google Scholar]
- Sharma A, Bajpai VK, Baek KH. Determination of antibacterial mode of action of allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J Food Saf. 2013;33:197–208. doi: 10.1111/jfs.12040. [DOI] [Google Scholar]
- Shetta A, Kegere J, Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int J Biol Macromol. 2019;126:731–742. doi: 10.1016/j.ijbiomac.2018.12.161. [DOI] [PubMed] [Google Scholar]
- Silva HD, Cerqueira MA, Vicente AA. Nanoemulsions for food applications: development and characterization. Food Bioprocess Technol. 2012;5:854–867. doi: 10.1007/s11947-011-0683-7. [DOI] [Google Scholar]
- Song LL, Cui SN, Xie W, Zhang Y. Study on bacteriostasis performance of natural preservative and starch compound film. Cereals Oils. 2020;33:86–90. [Google Scholar]
- Sun YX, Zheng XY, Zheng LL, Yang Y, Xiao D, Ai BL, Zhang WM, Sheng ZW. Study on preparation of camellia oil nanoemulsion by soy protein isolate-tea saponin compound emulsifier and properties of nanoemulsion. Sci Technol Food Ind. 2020;41:27–34. [Google Scholar]
- Tong KF. Advances in research of nanoemulsions and their applications in cosmetics. Detergent Cosmet. 2019;42:48–56. [Google Scholar]
- Wang LJ, Dong JF, Chen J, Eastoe JL, Li XF. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330:443–448. doi: 10.1016/j.jcis.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Wei XY, Chang G, Cui LH, Jing W, Li JH, Wang HJ. Preservation performance of clove oil/ pineapple leaf fiber composite antibacterial membrane on pork. Food Ind. 2018;39:198–200. [Google Scholar]
- Wu WY, Li L, Li DH, Xie XN, Li Y. Stability studies for lycopene-load nanoemulsions system. J Chin Inst Food Sci Technol. 2019;19:96–103. [Google Scholar]
- Xu W, Jin WP, Huang KL, Huang L, Lou YC, Li J, Liu XF, Li B. Interfacial and emulsion stabilized behavior of lysozyme/xanthan gum nanoparticles. Int J Biol Macromol. 2018;117:280–286. doi: 10.1016/j.ijbiomac.2018.05.187. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu DD, Xu B, Huang L, Xiong YZ, Ge PP, Wang Z. Preparation, antibacterial and antioxidant properties of green tea seed oil nanoemulsions by self-emulsification method. Micro Nano Lett. 2019;14:1219–1222. doi: 10.1049/mnl.2019.0185. [DOI] [Google Scholar]
- Yang F, Yang JC, Qiu S, Xu W, Wang YT. Tannic acid enhanced the physical and oxidative stability of chitin particles stabilized oil in water emulsion. Food Chem. 2021;346:128762. doi: 10.1016/j.foodchem.2020.128762. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Baek KH, Heo YS, Yong HI, Jo C. Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157:H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 2021;93:103611. doi: 10.1016/j.fm.2020.103611. [DOI] [PubMed] [Google Scholar]
- Zhang ML, Qin Y. Research progress in nanoemulsion prepared by low-energy methods. Deterg Cosmet. 2019;42:37–42. [Google Scholar]
- Zhang XY, Pan Y, Wang ZJ, Jiang LZ, Che JL, Zhu YF, Zhong CY. The stability mechanism and antimicrobial property of peppermint oil nanoemulsion. J Chin Inst Food Sci Technol. 2020;20:34–43. [Google Scholar]
- Zhang YB, Liu Y, Jiang PP, Li WD, Wang YF. Mechanism and antibacterial activity of cinnamaldehyde against Escherichia coli and Staphylococcus aureus. Modern Food Sci Technol. 2015;31:31–35. [Google Scholar]
- Zhou JX, Xu H, Jin H. Study on the antibacterial effect and antibacterial components of clove oil. Food Ind. 2000;03:24–25. [Google Scholar]