Abstract
In this study, raw fruits of important olive cultivars mostly used in the Turkish table (and oil) olive sector, Ayvalik, Gemlik, Domat, Memecik and Uslu, were investigated based on their biophenolic profiles by a HPLC–DAD method. Biophenolic compounds have great importance in olive processing (table and oil) technology and human nutrition physiology and are commonly found in natural products obtained from fruits and vegetables, including table olives and olive oil. Raw olive fruits samples, grown in Bornova and Kemapaşa which are the experimantal areas of Olive Research Institute (İzmir-Turkey), were harvested in different maturity stages during two corp years (2007–2008). The total phenolic content (TPC) and the simple biophenolic profile analysis of raw olive samples were carried out using UV/VIS spectroscopic and HPLC–DAD methods, respectively. It was showed that domestic olive cultivars, mostly used in table and oil technologies, exposed great differences in biophenolic profiles due to the cultivar and harvest time according to the results of this study. TPC data for all raw samples varied from1 89.8 mg GAE /100 g (Domat) to 421 mg GAE /100 g (Uslu). Hydroxytyrosol (HT) was the major phenolic compound for all raw olive samples and it varied from 58.70 mg/100 g (Uslu) to 27.53 mg/100 g (Memecik). The highest amount of tyrosol (TY) compound was found Uslu (21.23 mg/100 g) while Ayvalik had the lowest amount of this compound (6.13 mg/100 g). In this study, the raw fruits of the domestic five table cultivars were characterized and classified chemometric methods (Principal Component Analysis, PCA and Hierarchical Cluster Analysis, HCA) based on their simple phenolic compounds. Luteloin (LT) was effective on the characterization of Uslu cultivar while Gemlik was classified with the apigenin (APG). The Hydroxytyrosol (HT) was discriminative in classification of Memecik cultivar whereas the tyrosol (TY) played role in characterization of Ayvalik cultivar.
Keywords: Biophenolics, Olive fruit, Turkish olive cultivars, HPLC–DAD, Chemometry, PCA, HCA
Introduction
The fruits of olive trees, growing especially well in locations having a Mediterranean climate, are rarely consumed as a natural fruit due to its extreme bitterness originated from phenolic compounds. The olive fruits are exposed to various processes for direct human consumption (Bianco and Ucella, 2000; Ucella, 2001; Rivas et al. 2000). Phenolic compounds not only have antimicrobial and antioxidant properties but also contribute to the prevention of Dacus olea infestation in olives (Aktaş et al. 2014).
The table olive and its derivate virgin olive oil are defined as a typical example of a "natural" functional food due to their remarkable nutritional properties (fatty acid composition and presence of bioactive ingredients originated from mostly its phenolic substances). Polar biophenols (hydroxytrosol [HT] and its derivatives) and biophenolic acids are the most bioactive ingredients, present in raw olive fruit (Bianco and Ucella, 2000; Ucella, 2001; Rivas et al. 2000; Goulas et.al. 2012).
The Mediterranean diet, covering daily consumption of table olive, is declared as the one of cultural heritage of the world by UNESCO (2014). As a traditional and natural product fruit source, the olive fruit, especially an end product of fermented foods, has a specific role in daily nutrition of people of Mediterranean basin since ancient period due to its special nutritional, cultural and hedonic aspects (Uylaşer 2015; Beb Ghorbal et al. 2018). Several epidemiological studies suggest that the table olive and olive oil significantly contributes to the well known effects of the Mediterranean diet in lowering the incidents of degenerative pathologies, including coronary heart disease and cancer. The protective effects could be ascribed to the fatty acid composition of the oil and also to minor constituents (especially, phenolic compounds having effects antioxidant and free radical-scavenging activities), as indicated by recent biochemical pharmacological and other studies (Bianco and Ucella, 2000; Ucella, 2001; Rivas et al. 2000; Goulas et.al. 2012).
Olive is one of those fruits containing high amount of phenolic substances. It is known that the phenolic compounds are secondary metabolites and their compositions are complex for olive fruits. The phenolic profile of olive fruits has a large diversity of molecular structures. Olive fruit is a significant and remarkable natural source of biophenolic compounds (Brenes-Balbunes et al. 1992; Vinha et al., 2005; Gomez–Rico et al. 2009; Arslan and Özcan, 2011; Goulas et.al., 2012; Uylaşer 2015).
There are many well known local olive cultivars used for olive processing (table and oily) industry) in Turkey, some of them are specific for main growing zone and its sub-zones. Among these olive cultivars, Ayvalik ([oily + table] in North Aegean zone), Gemlik ([table + oily] in Marmara region), Domat ([table] in East Agean zone), Memecik ([oily + table] in South Aegean zone) and Uslu ([table] in East Agean zone) olive varieties are mostly used for table olives production technology in Turkey. A number of studies have focused on the phenolic profiles of raw olive fruits from several countries (Brenes-Balbunes et al. 1992; Bastoni et al. 2001; Campastre et al. 2002; Vinha et al. 2005; Ben Othman et al. 2008; Gomez–Rico et al. 2009; Romeo et al. 2009; Marsilio et al. 2011; Bolandnazar et al. 2014). In recent years, there have been few works published on the concentrations of biophenolics compounds of Turkish monovarietal olive fruits (Arslan and Özcan, 2011; Aslan 2012; Dağdelen et al. 2013; Şahan et al. 2013; Aktaş et al., 2014; Uylaşer 2015; Ben Ghorbal et al., 2018). Although, Turkey is the world’s second largest producer of table olive (especially the world’s first in table black olive production); there is a lack of data elucidating on the characteristics of biophenolic profiles of economically important table olives grown in Turkey. For this reason, the aim of the study is to determine and characterize the profile of individual biophenolic compounds of major domestic table olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) cultivated in the same pedoclimatic conditions and also, to obtain a better knowledge of the qualitative attributes and quantitative amounts of these phenolic compounds.
Materials and methods
Olive samples
In this study, important five domestic olive cultivars (Ayvalik, Gemlik, Domat, Memecik, and Uslu), mostly used in the Turkish table olive sector, were investigated based on their biophenolic profiles. Raw olive samples grown in Bornova and Kemalpaşa experimental areas Research Institute for of Olive Culture (Izmir, Turkey) in the same pedoclimatic conditions were harvested in different ripening stages (green–purple–black) based on colour changes of the olive fruits according to the ripening conditions of olive cultivars. All olive samples were manually collected in the single harvest period (between October and November) during two corp years (2007–2008).
Total phenolic contents of olive samples
Total phenolic contents (TPC) of raw olive samples were by the Folin-Ciocalteu method (at 760 nm using a UV/ VIS spectrophotometer and the result as gallic acid [mg GAE/100 g]) according to Skerget et al. (2005).
Determination of biophenolic profiles of table olives by HPLC–DAD
The biophenolic profiles of raw five olive samples (Ayvalik, Gemlik, Domat, Memecik and Uslu) were examined by a HPLC–DAD method (Morello et al. 2005a, b). For the extraction of phenolic compounds, 5 g of olive flesh sample was mixed with (80:20)% methanol: water (400 ppm Sodium metabisulfite) and the mixture was centrifuged at 4000 rev/min for 20 min. The collected methanol phases were evaporated at 35 °C in a rotary evaporator. Extractions with n-hexane and ethyl acetate were carried out for 3 times. The collected ethyl acetate phase was evaporated at 35 °C in a rotary evaporator. After evaporation, the residue was solved with 2.5 ml methanol and filtrated through 0.45 µm.
The all analyses were complicated on Agilent HPLC–DAD 1100 (USA) and the method conditions were designed as follows; mobile phase (solvent A): 5% formic acid, mobile phase (solvent B): methanol and flow rate: 0.9 ml/min. Determinations of biophenolic compounds in olive samples were carried out via DAD detector on 280 nm and 320 nm with the use of Phenomenex column (C18 RP, 250 mm × 4,6 mm, 5 µm) having the injection volume of 20 µl. The temperature of the column was at ambient temperature. The gradient elution program was changed as follows: to 98% (A) and (2%) for 3 min, 95% (A) and 5% (B) in 2 min, 90% (A) and 10% (B) in 5 min, 85% (A) and 15% (B) in 5 min, 80% (A) and 20% (B) in 15 min, 75% (A) and 25% (B) in 6 min, 65% (A) and 35% (B) in 3 min, 60% (A) and 40% (B) in 4 min, 55% (A) and 45% (B) in 6 min, 53% (A) and 47% (B) in 3 min, 50% (A) and 50% (B) in 17 min, 33% (A) and 67% (B) in 4 min and 100% solvent B in maintained for 10 min. Phenolic compounds were identified by comparing their retention times with those of commercial standards. Phenolic standards had linear calibration curves through the origin (R2 = 0.98). The HPLC–DAD method was validated for phenolic determination of raw olive samples within 95% confidence limits. Reference phenolic standards, except for HT (Extrasyntese, Genay–France), were used as primer standard substances (Sigma Aldrich Chemicals, Germany).
Chemometric analysis
Characterization and classification of the all data were carried out using chemometric methods, Principal Componenet Analysis [PCA (Ward Method)] and Hierarchical Cluster Analysis [HCA (Euclidian Distance)] based on cultivars and their biophenolic profiles. Multivariate analysis was performed using the Matlab 7.5.0 (R2007b, Mathworks Inc, Boston, USA). Cultivars and biophenolics data was normalized prior to all chemometric analysis. Data were normalized prior to chemometric analysis. To this end, mean of each olive cultivar sample and biophenolic profile parameter was substracted from the correspoding sample and parameter, and the computed values for each cultivar sample and biophenolic parameter were divided by their standard deviation so as to obtain zero-mean and unit-variance data.
Statistical analysis
Biophenolic data obtained from raw fruits of olive varieties were subjected to the analysis of variance (ANOVA). Among olive varieties, significant means were compared by Duncan’s new multiple range test. Statistical analyses were performed using the SPSS 12 A statistics software (SPSS Inc., Chicago, IL, USA).
Results and discussion
Table 1 shows the total phenolic contentes and the biophenolic profiles belonging to the raw fruits of domestic olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) mostly used in table and oil technologies in Turkey. In raw fruits of olives were identified as fractions of biophenolic profiles following: hydroxytyrosol (HT), tyrosol (TY), gallic acid (GA), chlorogenic acid (CLG), vanillic acid (VA), caffeic acid (CA), syringic acid (SA), p-coumaric acid (p-Q), ferulic acid (FA), sinamic acid (SN), quercetin (Q), luteolin (LT) and apigenin (APG) (Table 1).
Table 1.
The Phenolic Profiles of Important Turkish Table Olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) **
| Ayvalık | Gemlık | Domat | Memecık | Uslu | |
|---|---|---|---|---|---|
| Fruit color in harvest | Purple | Black | Green | Green | Black |
|
Total phenolic contents (mg GAE /100 g) |
250.8 c ± 0.15 |
274.9 b ± 0.34 |
189.8 e ± 0.22 |
208.2 d ± 0.19 |
421.0 a ± 0.23 |
| Simple phenolic fractions (mg/100 g) | |||||
| Hydroxytyrosol |
42,46 d ± 0.45 |
47,57 c ± 0.66 |
55,61 b ± 0.50 |
27,53 c ± 0.35 |
58,7 a ± 0.85 |
| Tyrosol |
6,13 e ± 0.15 |
18,25 b ± 0.85 |
11,21 c ± 0.15 |
9,75 d ± 0.20 |
21,23 a ± 0.55 |
| Luteolin |
3,66 d ± 0.05 |
4,72 c ± 0.15 |
2,27 e ± 0.25 |
55,73 a ± 0.95 |
6,87 b ± 0.45 |
| Apigenin |
7,54 c ± 0.03 |
5,58 d ± 0.15 |
3,64 e ± 0.25 |
24,43 a ± 0.95 |
8,41 b ± 0.05 |
| Gallic acid | < d.l | < d.l | < d.l |
4,48 b ± 0.55 |
6,58 a ± 0.65 |
| Caffeic acid |
4,25 c ± 0.03 |
5,08 b ± 0.02 |
7,14 a ± 0.04 |
< d.l |
6,72 a ± 0.09 |
| Ferulic acid |
3,85 d ± 0.03 |
5,25 b ± 0.07 |
4,41 c ± 0.02 |
1,13 e ± 0.02 |
5,84 a ± 0.06 |
| Quercetine |
3,74 cd ± 0.15 |
6,17 b ± 0.35 |
7,97 a ± 0.50 |
< d.l |
4,12 c ± 0.11 |
| p-Qoumaric acid |
3,19 b ± 0.03 |
4,36 a ± 0.05 |
3,47 b ± 0.10 |
1,7 c ± 0.03 |
4,91 a ± 0.05 |
| Syringic acid | < d.l | < d.l | < d.l | < d.l |
5,21 a ± 0.25 |
| Cinnamic acid |
2,13 c ± 0.04 |
3,05 b ± 0.02 |
2,74 b ± 0.03 |
1,26 d ± 0.05 |
10,97 a ± 0.45 |
| Vanilic acid |
3,13 d ± 0.05 |
4,32 c ± 0.07 |
6,55 a ± 0.35 |
< d.l |
5,65 b ± 0.55 |
| Clorogenic acid |
5,9 b ± 0.45 |
< d.l |
2,38 c ± 0.05 |
< d.l |
6,99 a ± 0.55 |
**Each value is an average of four determinations, and values in the same column with different letters show statistically significant differences (P < 0.05)
d.l.(detection limit)
HT Hydroxytyrosol, TY Tyrosol, GA Gallic acid, CLG Chlorogenic acid, VA Vanillic acid, CA Caffeic acid, SA Syringic acid, p-Q p-coumaric acid, FA Ferulic acid, SN Sinamic acid, Q Quercetin, LT Luteolin, APG Apigenin
The total phenolic contents (TPC) in raw fruits of domestic olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) were determined by spectroscopic method and the results are presented in Table 1. The findings showed wide variation from 189.8 mg GAE/100 g (Domat) to 421 mg GAE /100 g (Uslu). The variation and significant (P < 0.01) differences were determined among TPC of table olive cultivars according to the results of the Duncan’s Multiple Range Test (Table 1). The TPC data are agree with the findings given by Tunisia cultivars (144–674 mg GA/100 g) (Ben Othman et al. 2008) Portugal olives (120–230 mg GAE/100 g) (Cabral et al. 2018) and the results of domestic olives (Ayvalık, Domat, Gemlik, Erkence and Sarı Ulak) (Arslan and Özcan, 2011; Aslan, 2012; Şahan et al. 2013), but lower than those (4317.69 mg/kg GA–6042.46 mg/kg) determined for Gemlik olive cultivar from Turkey (Uylaşer, 2015) and (3670–9135 mg GAE/ kg) recorded for Italian olive cultivars (Romeo et al. 2018) and (315–3579 GAE mg/kg) reported for Algerian olive cultivars (Medjkouh et al. 2018).The TPC differences among the domestic table olive cultivars may be due to several factors such as genetics of cultivar, climate conditions in harvest years and agronomic techniques (Ben Othman et al., 2008; Uylaşer 2015; Cabral et al. 2018; Romeo et al. 2018).
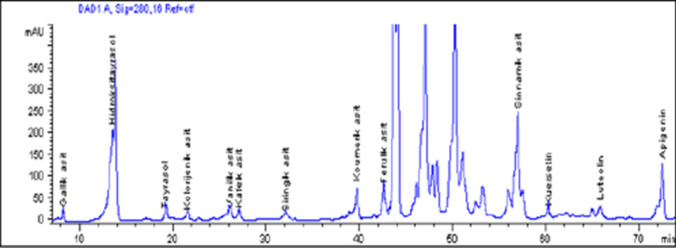
Chromatogram of the phenolic profiles of raw Uslu olive fruit is shown in Fig. 1.The concentrations of individual biophenolics for all olive samples is given in Table 1. It was showed that domestic five olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) had great differences in biophenolic profiles due to the cultivar and harvest time according to the results of analysis (Table 1). The HT, TY, LT, APG, p-Q, FA and SN were determined in raw samples of five olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) while only in one variety (Uslu), the SA was found (Table 1).
Fig. 1.
HPLC Choromatograms showing biophenolic profiles relatated to raw Uslu olive fruit
The TY and HT are phenylethanoids—a type of phenolic compound characterized by a phenethyl alcohol structure (D’Angelo et al. 2005). The HT, from the most abundant phenolic alcohols in olives (Medjkouh et al. 2018), was the major phenolic compound for all olive samples and it varied from 27.53 mg/100 g (Memecik) to 58.70 mg/100 g (Uslu).These values for Domat, Gemlik and Ayvalik cultivars were found as 55.61, 47.57 and 42.46 mg/100 g, respectively (Table 1). The HT findings for five olive cultivars are lower than those(400- 9500 mg/kg) reported for European olive cultivars (Brenes–Balbuens, 1992; Vinha et al., 2005; Gomez–Rico et al. 2009; Romeo et al. 2009) and Tunisia cultivar (135–257 mg/100 g) (Ben Othman et al. 2008) but higher than some values (0.54 and 3.08 mg/100 g) reported by Campastre et al. (2002) for Italian cultivar (Douro). Our findings on HT agree with Sarı Ulak and Gemlik olive cultivar, Turkish domestic variety (30–550 mg/kg) Arslan and Özcan, 2011; Aslan 2012; Ben Ghorbal et al. 2018) and (14.7–295 mg/100 g) Algerian olive cultivars reported by Medjkouh et al. (2018), but notably lower when compared with findings (75–8200 mg/kg) of domestic olive cultivars (Ayvalik, Domat, Hurma, Erkence and Gemlik) (Aktaş et al. 2014; Dağdelen et al. 2013; Uylaşer 2015).
The highest amount of TY compound, the one other important phenolic alcohol in olives, was determined Uslu (21.23 mg/100 g) while Ayvalik had the lowest amount (6.13 mg/100 g). The TY was the second major compound in all raw olive samples (Table 1). The TY contents of five domestic table olive cultivars in this study were generally similar to the findings (30–300 mg/kg) of various domestic olive (Hurma, Erkence, Ayvalik, Domat and Gemlik) cultivars (Yorulmaz et al. 2012; Dağdelen et al., 2013; Şahan et al., 2013; Aktaş et al. 2014; Uylaşer 2015; Ben Ghorbal et al., 2018) and another studies of Mediterranean (Italy, and Algeria) olive cultivars (Brenes-Balbuens et al. 1992; Medjkouh et al. 2018). The TY levels determined for the five table olive cultivars in this study are lower than those reported (400–880 mg/kg) for olive cultivars (Douro and Sari Ulak) by Campastre et al. (2002) and Morello et al. (2005a). In addition, the TY contents found for these olive cultivars in this study are higher than those reported (20–72 mg/kg) for foreign cultivars by Marsilio et al. (2001) and Ben Othman et al. (2008).
As shown in Table 1, the GA, from remarkable phenolic acids, was determined only two olive varieties, Uslu and Memecik. The GA contents of raw olive fruit samples were as Uslu 6.58 mg/100 g and Memecik 4.48 mg/100 g. The GA compound data in this study are higher than those (0.4–3.5 mg/kg) reported by Dagdelen et al. (2013) for Turkish domestic olive cultivars (Ayvalik, Domat, Gemlik). Our findings for GA were generally similar to the data (6.1 mg/100 g) recorded by Ben Othman et al. (2008) for the Tunisia cultivar.
The LT is a flavone—a type of flavonoid with potential for cancer prevention (Lin et al. 2008). As shown in Table 1, luteloin, from important flavonoid compound in olives, ranged from 2.27 mg/100 g (Domat) to 55.73 mg/100 g Memecik when the APG, one other flavonoid compound, were between 3.64 mg/100 g (Domat) and 24.43 mg/100 g (Memecik). The LT contents of five domestic table olive cultivars in this study were generally agree with the findings (12–846 mg/kg) of some domestic olive (Sarı Ulak, Hurma, Erkence, Ayvalik, Domat and Gemlik) cultivars (Dağdelen et al. 2013; Şahan et al. 2013; Aktaş et al. 2014; Ben Ghorbal et al. 2018) and another studies of Mediterranean (İtaly and Portugal) olive cultivars (Brenes-Balbuens et al. 1992; Vinha et al. 2005). In addition, the LT data for all raw samples were lower than those reported by Campastre et al. (2002). Our APG data were agree with the findings given by Vinha et al. (2005). The APG results in five table olive samples were higher than those reported by some researchers (Campastre et al. 2002; Yorulmaz et al. 2012; Dağdelen et al. 2013; Ben Ghorbal et al. 2018) but APG data recorded by Aktaş et al. (2014) were higher than our findings.
The FA, p-Q and SN contents, from phenolic acids, in all raw olive samples ranged from 1.13 mg/100 g (Memecik)–5.84 mg/100 g (Uslu), 1.7 mg/100 g (Memecik)–4.91 mg/100 g (Uslu) and 26 mg/100 g (Memecik)–10.97 mg/100 g (Uslu), respectively (Table 1). These findings coming from phenolic acids (FA,p-Q and SN compounds) may be characteristic biomarkers for these varieties. These data determined from three phenolic acids were generally similar to the findings of some studies (Ben Othman et al. 2008; Arslan and Özcan, 2011; Aslan 2012; Yorulmaz et al. 2012; Aktaş et al. 2014; Ben Ghorbal et al. 2018). Our data for FA,p-Q and SN compounds were higher than some results (Dağdelen et al. 2013).
There is no determined some phenolic acid compounds (CA, Q,SA, VA and CLG) only in Memecik olive samples (Table 1), these findings may be useful for characterization of Memecik cultivar based on biophenolic profile. In addition, the SA was determined only in Uslu variety as 10.97 mg/100 g (Table 1). This finding may be characteristic property for this cultivar. As shown in Table 1 and Fig. 2, the CA contents of raw olive fruits were from 4.25 mg/100 g (Ayvalik) to 7.14 mg/100 g (Domat) when the VA and CLG accounted for 3.13 mg/100 g (Ayvalik)–6.55 mg/100 g (Domat) and 2.83 mg/100 g (Domat)–6.99 mg/100 g (Uslu), respectively. The CA and SN data of all raw olive samples were agree with Sarı Ulak cultivar (Arslan and Özcan, 2011), Algerian olive cultivars –for only the CA–(Medjkouh et al. 2018) and Hurma, Erkence and Gemlik olives results (Aktaş et al. 2014). Our data for CA,VA and SN compounds were higher than some findings (Dağdelen et al. 2013). The Q, a remarkable flavonoid compound in olive fruit, varied from 3.74 mg/100 g (Ayvalik) to 7.97 mg/100 g (Domat) according to olive cultivars (Table 1). This data was lower than Erkence and Gemlik olives results (Aktaş et al. 2014).
Fig. 2.

The Principal Component Analysis (PCA) scores concerning the distribution of economically importance domestic table olive cultivars (the first two PC which account for 96.38% of the totally variability in the data from the olive samples [PC1 86.87% and PC2 9.71%])
It was observed fas a remarkable property that Uslu cultivar, in general as known gave the oil in the lowest oxidative stabilitiy by national olive oil sector, have rich in all biophenolic compounds (Table 1). All data on biophenolic profiles of five Turkish table olive cultivars (Ayvalik, Gemlik, Domat, Memecik and Uslu) are lower than those reported by Vinha et al. (2005) for 29 different Portugal cultivars, Brenes-Balbuens et al. (1992) and Gomez–Rico et al. (2009) for Spain cultivars (Ascolana Tenera and Cornicobra). In general, the all findings for raw olive samples were agree with some studies (Bolendnazar et al. 2014; Ben Othman et al. 2008), except the highest HT data in Chemlali cultivar.
There was a significant (p < 0.01) variation among simple biophenolic compounds of olive fruits. In addition, there are some notable differences in biophenolic composition among the green and black stages of olive fruit samples (Table 1). Several factors are known to affect the quality and quantitative phenolic profiles of olives. TPC and simple phenolic contents of olive fruits is closely related to cultivar, agronomic traits, climatic conditions and ripening level of the fruit, especially harvest years (Ben Othman et al., 2008; Arslan and Özcan, 2011; Goulas et.al. 2012; Dağdelen et al. 2013; Şahan et al. 2013; Aktaş et al. 2014; Bolandnazar et al. 2014; Uylaşer 2015; Ben Ghorbal et al. 2018).
In this study, the domestic table olive cultivars were classified chemometric methods (Principal Component Analysis, PCA and Hierarchical Cluster Analysis, HCA. Some phenolic compounds of economically importance Turkish table olive cultivars (Figs. 2 and 3) played a remarkable role in the characterization of their cultivars. The data matrix of variables analysed (table olive samples and their biophenolic compounds) was subjected to PCA. Applying PCA to the table olive samples (n = 5) data determined the percentage of total variance explained by the first two PCs was 96.38% [PC1 86.87% and PC2 9.71%] (Fig. 2) while biophenolic compounds determined the percentage of total variance explained by the first two PCs was 98.44% [PC1 74.84% and PC2 23.6%] (Fig. 3).
Fig. 3.

The Principal Component Analysis (PCA) scores generated using biophenolic profile data taken from economically importance domestic table olive cultivars
The results were graphically represented by PCA score and loading plots (Fig. 2 and 3). Visualization of the discrimination among table olive cultivars on the plane of the first two PCs led to a fairly good separation (Fig. 2). According to PCA biplot analysis, the LT was was effective on the characterization of Uslu cultivar while Gemlik was classified with the APG. The HT was discriminative in classification of Memecik cultivar. The VA, Q, CA, FA, CLG, p-QA, SNA, GA and SA aided in the classification of Domat olive cultivar whereas the TY played role in characterization of Ayvalik cultivar. (Fig. 2 and 3). PC1 for table olive cultivars have positive loadings with LT (0.83), APG (0.30) while PC2 for so-called olive cultivars have mainly negative loadings with HT (−0.53), LT (−0.31), GA (−0.29) and SNA (−0.36) based on biophenolic profile. Also, PC1 for their biophenolic compounds is composed of remarkable positive loadings which includes prominent contributions from Ayvalik (0.39), Gemlik (0.46), Domat (0.52) and Uslu (0.53) while PC 2 has a significance negative loading (−0.95) for Memecik cultivar based on table olive cultivars.
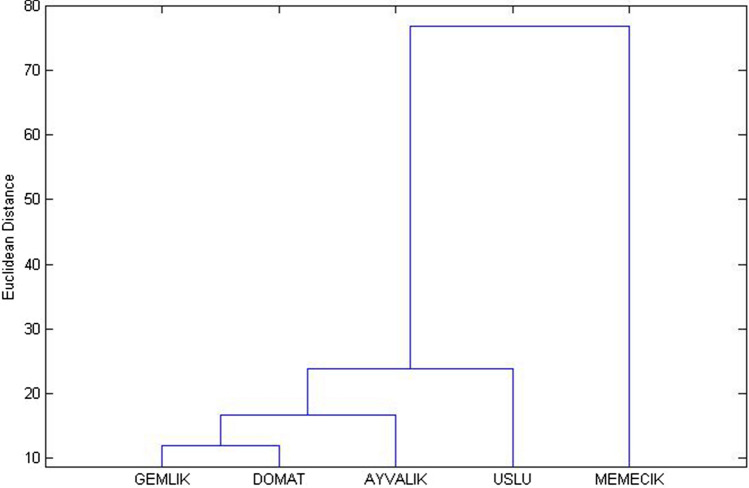
In this study, the economically important cultivars of Turkish table olive sector (Gemlik, Memecik, Ayvalik, Uslu, and Domat) were classified by mostly used a chemometric method Hierarchical Cluster Analysis (HCA). The dendrogram (Fig. 4) based on the HCA results (Euclidian method) of domestic table olive samples could be separated into four groups based on some important phenolic profile parameters. Group 1: Gemlik and Domat olive cultivars, Group 2: Ayvalik olive cultivar, Group 3: Uslu olive cultivar and Group 4: Memecik olive cultivar.
Fig. 4.
The dendrogram on Hierarchical Cluster Analysis (HCA) results concerning the classification of economically importance domestic table olive cultivars
Some phenolic compounds were used to identify the economically Turkish table olive cultivars (Gemlik, Memecik, Ayvalik, Uslu, and Domat) (Fig. 5). The HT, LT, APG and TY compounds of domestic table olive cultivars grouped to the left in the dendrogram. The CLG, SNA, SA and GA parameters were grouped on middle dendrogram. The Q, VA, CA, p-QA and FA compounds grouped to the right of dendrogram (Fig. 5). There are similar chemometric (including PCA and HCA) investigations based only on fruit profiles (especially total or individual phenolics) data for Turkey (Yorulmaz et al. 2012; Aktaş et al. 2014) and Algeria olives (Medjkouh et al. 2018).
Fig. 5.
The dendogram on HCA results concerning the distribution of the biophenolic profile data of economically importance domestic table olive cultivars
Conclusion
It is determined that the five Turkish table olive cultivars (Ayvalik, Domat, Gemlik, Memecik and Uslu) contained different classes of phenolic compounds such as phenolic acids (GA, CLG,VA, CA,SA, p-Q, FA and SN), phenolic alcohols (TY,HT), flavonoids (LT, APG). All the olive varieties were generally found to be rich in the phenolic compounds such as HT, TY, LT and APG. Uslu cultivar had higher amount of TPC and simple biphenolic fractions than other table olive cultivars. HT was the most abundant biophenolic compound for Ayvalik, Domat, Gemlik and Uslu cultivars while LT was major biophenolic compound in Memecik cultivar. The data, as observed in the present study, may also provide a database for food scientists, food technologists, and the Turkish Table Olive Industry to develop polyphenol-rich (functional) products. Also, it is thougt that more detailed studies covering location, harvest time and different olive processing (for table and oil properties) techniques are needed to exhibit the phenolic propeties of Turkish table olive cultivars.
Acknowledgements
The authors would like to express their thanks to the Ministry Food, Agriculture and Livestock of Turkey (especially Dr. Seyfi Özışık, Director of Inst. Res. Olive Culture, Bornova, Izmir/ Turkey) Project No TAGEM 06/11/01/118, for financial support. Also, we thanks to Asst. Prof. Dr. Hamdi Dibeklioğlu (Dep. Food Eng. Bilkent University –Ankara / TURKEY) for his helping in chemometric analysis.
Authors’ contribution
ŞI— providing materials, carrying out the the all spectroscopic and HPLC–DAD analysis and drafting the manuscript, HD supervised the work and carrying out scientific comments with writing/revisions of the text.
Funding
The project was supported by General Directorate of Agricultural Research of Ministry Ministry Food, Agriculture and Livestock with a project (No: TAGEM / 06/11/01/118).
Data availability
The data in the article was firstly published and could not be available somewhere else.
Declarations
Conflict of interest
There is no professional or other personal interest of any service and/or institution that could be construed as influencing the position presented in the manuscript. The work has not been published before.
Ethical approval
There is no ethics interest related in research.
Consent for participate
No conflict of interest exits in the submission of this manuscript. All the authors listed have participated the research and approved the manuscript that is enclosed.
Consent for publication
The paper was approved by all authors for publication. All the authors agree to publish the paper in ‘‘Journal of Food Science and Technology’’. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aktas AB, Ozen B, Tokatli F, Sen I. Phenolics profile of a naturally debittering olive in comparison to regular olive varieties. J Sci Food Agric. 2014;94:691–698. doi: 10.1002/jsfa.6308. [DOI] [PubMed] [Google Scholar]
- Arslan D, Ozcan MM. Phenolic profile and antioxidant activity of olive fruits of the Turkish variety “Sarıulak” from different locations. Grasas Aceites. 2011;62:453–461. doi: 10.3989/gya.034311. [DOI] [Google Scholar]
- Aslan D. Physico-chemical characteristics of olive fruits of Turkish varieties from the province of Hatay. Grasas Aceites. 2012;63(2):158–166. doi: 10.3989/gya.071611. [DOI] [Google Scholar]
- Bastoni L, Bianco A, Piccioni F, Uccella N. Biophenolic profile in olives by NMR. Food Chem. 2001;73:145–151. doi: 10.1016/S0308-8146(00)00250-8. [DOI] [Google Scholar]
- Ben Ghorbal A, Leventdurur S, Agirman B, Boyaci-Gunduz CP, Kelebek H, Carsanba E, Darici M, Erten H. Influence of geographic origin on agronomic traits and phenolic content of cv. Gemlik olive fruits. J Food Compos Anal. 2018;74:1–9. doi: 10.1016/j.jfca.2018.08.004. [DOI] [Google Scholar]
- Ben Othman N, Roblain D, Thonart P, Hamdi M. Tunisian table olive phenolic compounds and their antioxidant capacity. J Food Sci. 2008;73:235–239. doi: 10.1111/j.1750-3841.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- Bianco A, Uccella N. Biophenolic components of olives. Food Res Int. 2000;33:475–485. doi: 10.1016/S0963-9969(00)00072-7. [DOI] [Google Scholar]
- Bolandnazar SZ, Hoseini E, Servili M, Ghavami M. Evaluation of changes in phenolic compounds of two varieties of olives during the course of maturation. J Food Biosci Tech. 2014;4:69–73. [Google Scholar]
- Brenes-Balbuens M, Garcia-Garcia P, Garrido-Fernandez A. Phenolic compounds related to the black color formed during the processing of ripe olives. J Agric Food Chem. 1992;40:1192–1196. doi: 10.1021/jf00019a023. [DOI] [Google Scholar]
- Campestre C, Marsilio V, Lanza B, Lezzi C, Bianchi G. Phenolic compounds and organic acids change in black oxidized table olives. Acta Hortic. 2002;586:575–578. doi: 10.17660/ActaHortic.2002.586.120. [DOI] [Google Scholar]
- Cabral P, Barros T, Nunes P, Quintas C. Physicochemical, nutritional and microbiological characteristics of traditional table olives from Southern Portugal. Emirates J Food Agric. 2018;30:611–620. doi: 10.9755/ejfa.2018.v30.i7.1747. [DOI] [Google Scholar]
- Angelo D´S, Ingrosso D, Migliardi V, Sorrentino A, Donnarumma G, Baroni A, Masella L, Tufano MA, Zappia M, Galletti P. Hydroxytyrosol, a natural antioxidant from olive oil, prevents protein damage induced by long-wave ultraviolet radiation in melanoma cells. Free Radic Biol Med. 2005;38:908–919. doi: 10.1016/j.freeradbiomed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Dağdelen A, Tumen G, Ozcan MM, Dundar E. Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalık, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013;136:41–45. doi: 10.1016/j.foodchem.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Gomez–RicoInarejos–GarciaDesamparadosFregapane AAMMSG. Effect of malaxation conditions on phenol and volatile pofiles in olive paste and the corresponding virgin olive oils (Olea europaea L. Cv. Cornicabra) J Agric Food Chem. 2009;57:3587–3595. doi: 10.1021/jf803505w. [DOI] [PubMed] [Google Scholar]
- Goulas V, Charisiadis P, Gerothanassis IP, Manganaris AG. Classification, biotransformation and antioxidant activity of olive fruit biophenols: a review. Curr Bioact Compd. 2012;8:232–239. doi: 10.2174/157340712802762465. [DOI] [Google Scholar]
- Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsilio V, Campestre C, Lanza B. Phenolic compounds change during California-style ripe olive processing. Food Chem. 2001;74:55–60. doi: 10.1016/S0308-8146(00)00338-1. [DOI] [Google Scholar]
- Medjkouh L, Tamendjari A, Alves CR, Laribi R, Oliveira MBPP. Phenolic profiles of eight olive cultivars from Algeria: effect of Bactrocera oleae attack. Food Funct. 2018;9:890–897. doi: 10.1039/c7fo01654a. [DOI] [PubMed] [Google Scholar]
- Morello JR, Vuorela S, Romero M, Motilva MJ, Heinonen M. Antioxidant activity of olive pulp and olive oil phenolic compounds of the Arbequina cultivar. J Agric Food Chem. 2005;53:2002–2008. doi: 10.1021/jf048386a. [DOI] [PubMed] [Google Scholar]
- Morello J, Romero M, Ramo T, Motilva J. Evaluation of L-phenyl alanine ammonia-lyase activity and phenolic profile in olive drupe (Olea europaea L.) from fruit setting period to harvesting time. Plant Sci. 2005;168:65–72. doi: 10.1016/j.plantsci.2004.07.013. [DOI] [Google Scholar]
- Rivas CS, Espin JC, Wichers HJ. Review: oleuropein and related compounds. J Sci Food Agric. 2000;80:1013–1023. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1013::AID-JSFA571>3.0.CO;2-C. [DOI] [Google Scholar]
- Romeo FV, De Luca S, Piscopo A, Perri E, Poiana M. Effects of post-fermentation processing on the stabilisation of naturally fermented green table olives (cv Nocellara etnea) Food Chem. 2009;116:873–878. doi: 10.1016/j.foodchem.2009.03.037. [DOI] [Google Scholar]
- Romeo FV, Timpanaro N, Intelisano S, Rapisarda P. Quality evaluation of Aitana, Caiazzana and Nocellara del Belice table olives fermented with a commercial starter culture. Emirates J Food Agric. 2018;30:604–610. doi: 10.9755/ejfa.2018.v30.i7.1748. [DOI] [Google Scholar]
- Şahan Y, Cansev A, Gülen H. Effect of processing techniques on antioxidative enzyme activities, antioxidant capacity, phenolic compounds, and fatty acids of table olives. Food Sci Biotechnol. 2013;22:613–620. doi: 10.1007/s10068-013-0122-9. [DOI] [Google Scholar]
- Skerget M, Kotnik P, Hadolin M, Hras A, Simonic M, Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89(2):191–198. doi: 10.1016/j.foodchem.2004.02.025. [DOI] [Google Scholar]
- Uccella N. Olive biophenols: novel ethnic and technological approach. Trends Food Sci Tech. 2001;11:328–339. doi: 10.1016/S0924-2244(01)00028-0. [DOI] [Google Scholar]
- Uylaşer V. Changes in phenolic compounds during ripening in Gemlik variety olive fruits obtained from different locations. CyTA - J Food. 2015;13(2):167–173. doi: 10.1080/19476337.2014.931331. [DOI] [Google Scholar]
- Vinha AF, Ferreres F, Silva BM, Valentao P, Gonçalves A, Pereira JA, Oliveira MB, Seabra RM, Andrade PB. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): influences of cultivar and geographical origin. Food Chem. 2005;89:561–568. doi: 10.1016/j.foodchem.2004.03.012. [DOI] [Google Scholar]
- Yorulmaz A, Poyrazoglu ES, Özcan MM, Tekin A. Phenolic profiles of Turkish olives and olive oils. Eur J Lipid Sci Technol. 2012;114:1083–1093. doi: 10.1002/ejlt.201100186. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in the article was firstly published and could not be available somewhere else.