Abstract
Analysis of agrochemical residues in red chilli powder is always considered difficult because of higher matrix interference due to carotenoid pigments and other co-extractives. During the sample preparation, matrix components were co-extracted along with the target compounds leading to frequent source cleaning, changing of liner and column. Efforts were made to improve the chromatographic performance by optimizing sample preparation, choosing matrix-free transitions and introducing a retention gap. The Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) extraction was experimented using different dispersive adsorbents and the purified extract was analyzed by gas chromatography tandem mass spectrometry (GC–MS/MS) system. 84 pesticides under different class were validated and established a limit of quantification of 0.002–0.007 mgkg−1. The recovery was between 70 and 110% at 0.01, 0.025 and 0.05 mgkg−1 fortification level and corresponding precision was between 3 and 16% RSD. Suitability of the validated method was established through analysis of market samples of chilli powder for the quantitation of targeted pesticide residues.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-021-05177-3.
Keywords: QuEChERS, Multiresidue pesticides, Red chilli, GC–MS/MS Optimization, Retention gap, Spices
Introduction
Chillies are used as a spice in food recipes globally. Fresh chillies are dried under sunlight and powdered to get chilli powder. India holds prominent position contributing 25% of the world chilli production and exported worth approximately 1billion US$ in 2018–2019 (Spices Board India 2019). Though India has a favorable condition for chilli cultivation, one of the major problems faced by growers is the heavy infestation of various insect pests and diseases caused by various fungi and bacteria. More than 50 insecticide and 40 fungicide formulations are registered with the Central Insecticide Board and Registration Committee (CIB and RC) for the control of various pests and diseases encountered in chilli cultivation (CIB and RC 2021). However, use of these chemical formulations are not regulated and farmers use them non judiciously resulting pesticide residues in chilli powder. Processing of fresh commodities through drying may increases the residue concentration in the processed product (George et al. 2013; Shabeer et al. 2015a, b). The literature reports a concentration factor of 5.02–8.57 for pesticide residues in the process of drying of fresh chillies to dry chilli powder (Pathan et al. 2009; Patil et al. 2018a, b). Hence, the agro-chemicals contamination in dry chilli powder is more compared to fresh chillies and became a barrier in domestic and international trade.
Gas Chromatography and Liquid Chromatography-Tandem Mass spectrometers (GC–MS/MS and LC–MS/MS) have evolved as a selective reliable technology for targeted screening and quantitation of various pesticide residues in different agricultural commodities (Lehotay et al. 2010; Chaterjee et al. 2016; Jadhav et al. 2015, 2019; Shabeer et al. 2017; Patil et al. 2018a, b). Several researchers reported multi-residue method for analysis of pesticide residues in different complex spices or herbs matrices by GC–MS/MS and LC–MS/MS (Wong et al. 2010; Anagnostopoulos and Miliadis 2013; Bernardi et al. 2016; Taha and Gadalla 2017; Jadhav et al. 2017; Shabeer et al. 2018). Being a dried commodity, matrix interference associated with co-extractives are the major issue associated with pesticide residue analysis in spices and herbs. To reduce this matrix interferences, various strategies like dispersive solid phase extraction (d-SPE) clean-up (Jadhav et al. 2017; Shabeer et al. 2018), dilution of the sample extract before analysis (Amate et al. 2010; Kandaswamy et al. 2020),final extract subjected to solvent exchange (Shabeer et al. 2018), d-SPE clean up after freezing out (Rutkowska et al. 2018) and use of hydrophilic lipophilic balance (HLB) cartridge along with SPE cleanup (Goon et al. 2020) are employed. Analysts adopted these methods either individually or in combination in different spice matrices to reduce the volatile and non-volatile co-extractives. One of the challenges faced by analyst while analyzing the pesticide residue in chilli powder is the heavy matrix interference associated with its high pigmentation and capsinoid co-extractives. Chilli is known for its deep red colour due to the presence of carotenoids. During sample preparation the pigments and capsinoids interfere with the analytes and deposits in the liner and the column which affects the efficiency of the instrument. Though information about sample preparation is available in literature, sufficient data are not available to deal with pigment and co-extractives interference. So in this investigation, we focused on the removal of pigment and capsinoids using dispersive agents, to optimize the method for the reliable quantification of pesticide residues in chilli powder through enhanced chromatographic performance by introduction of retention gap and to validate a multi-residue method for analysis of pesticide residues in chilli powder. Finally, the optimized method was successfully applied for the analysis of chilli samples collected from Indian markets for the presence of targeted pesticide residues.
Materials and methods
Chemicals and reagents
The certified reference standards (CRMs) were procured from Dr.Ehrenstorfer GmbH (Augsburg, Germany). Extraction solvents such as acetonitrile (MeCN), acetic acid of analytical grade were purchased from Merck, Bangalore, India. Salts such as magnesium sulfate (MgSO4), sodium chloride (NaCl) and sodium acetate (CH3COONa) were purchased from Thomas Baker (Mumbai, India). Dispersive solid phase (d-SPE) adsorbents such as primary secondary amine (PSA), graphitized carbon black (GCB), bonded octadecyl silica (C18) were obtained from Agilent Technologies, Bangalore, India.
Apparatus
Centrifuge (Beckman Coulter, Allegra X-22RA, Atlanta, US), micro centrifuge (Eppendorf-5424, Germany), high-speed blender (IKA, Bangalore, India), analytical balance (Axis AGN2004PR, LC/GC, India) for sample weighing and 1.5 L capacity mixer grinder (GX7, India Ltd. India) for sample homogenization were used at different stages of sample preparation.
Preparation of calibration standard
Individual standard of stock solution (10,000 mgmL−1) of pesticides was prepared by dissolving 25 mg each in 25 mL acetonitrile. Adequate quantity of an intermediate mixture (10–15 compounds each) prepared by appropriate dilution. An intermediate total standard mixture of 10 µgmL−1 was prepared. A nine- point solvent calibration standard (0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, 100 ngmL−1) was prepared by serial dilution of appropriate stock solution using acetonitrile. The matrix-matched calibration standards of concentration (0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, 100ngmL−1) were prepared by successive dilutions in acetonitrile extract of the blank matrix.
GC–MS/MS analysis
Gas chromatography- tandem mass spectrometry
Gas chromatograph ( GC 7890A, Agilent Technologies, USA) coupled to a triple quadrupole mass spectrometer (7000B, Agilent Technologies, USA) was used for performing all experiments at electron ionization mode (EI, − 70 eV).The GC system was equipped with an Agilent 7693A autosampler, a temperature programmable multimode inlet (MMI) and a 4 mm internal diameter liner with glass wool. Two numbers of 15-m column of same chemical phase an dimension of 5% phenyl chemical phase of 0.25 mm ID, 250 µm film thickness connected with pressure module for performing backflush (Gray and Teale 2010). A 0.2 m of 0.25 mm ID uncoated retention gap was connected to the inlet before the first 15 m column by a connector. The conditions were optimized as injector set at cold splitless 70 °C (0.1 min), 450 °C min−1 to 325 °C, purge flow to inlet 50 mL at 1 min at 15 psi, oven temperature program 60 °C (1.0 min), 40 °C min−1 to 170 °C, 10 °C min−1 to 310 °C (3 min). The method was retention time locked using chlorpyrifos at 9.14 min. The collision cell gases were nitrogen (1.5 mL min−1) and helium (2.25 mL min−1). The electron multiplier (EM) gain was set to 25 and both MS resolutions were 1.2 atomic mass unit (amu) full width at half maximum. The dwell times were set at 10 milli-seconds, mass hunter quantitative analysis software (Version.B.04.04) was used for data processing. The temperatures of the transfer line and ion source were set at 310 °C, Quadrupole 1 and 2 were set at 150 °C. The mass spectrometer operated in Multi Reaction Monitoring (MRM) mode with acquisition starting from 4.4 min by 2ul sample introduction. The mid- point back flush was performed for 3.3 min at 60.0 psi post analysis.
Selection of two MRM transitions
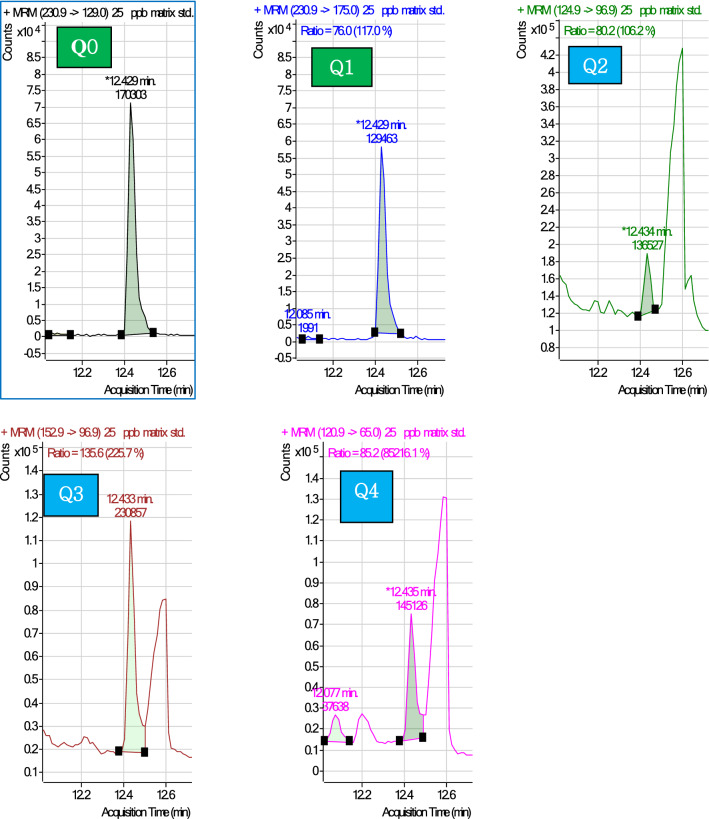
MRM database was provided by instrument vendor with optimized collision energy, precursor ions and product ions. The database provides the available transitions (for the targeted compounds) were added to the method. The matrix-matched calibration standards (0.39–100ngmL−1) and 0.01mgkg−1 fortified sample were acquired for selecting two ions for each compound in quant software on peak shape, ion ratio and abundance. For example, the analyte ethion MRM selection is given in Fig. 1. Among the five available ethion MRM transitions Q0 (230.9–120.9), Q1 (230.9–175.0), Q2 (124.9–96.9), Q3(152.9–96.9) Q4 (120.9–65.0), Q0 and Q1 were with highest abundance and ion ratio of 76%,58% respectively. Hence Q0 and Q1 were chosen for quantification and ion ratio. Similarly, two optimized MRM transitions were selected for all the targeted compounds (Supplementary Table-1).
Fig. 1.
Selection of quantifier and qualifier ions for the representative analyte ethion in MRM method
Sample preparation
1 kg organic certified red Chilli powder was received from Spice Board, India. Finely grounded and homogenized by using a mixer and grinder. The product was analyzed in triplicates for confirming incurred pesticides. No residues of targeted pesticides were present above the LOQ level in the sample. From this 0.5 kg of chilli powder was fortified with targeted test analytes at 0.010 mgkg−1 and the homogeneity was assessed by analyzing ten randomly selected portions (2 g each tested in a duplicate manner) for the corresponding residue content using QuEChERS sample preparation. The sample extraction and cleanup was done as per the procedure reported by Rejczak and Tuzimski, 2015.
Application of the method for analysis of market samples
25 market samples of chilli powder (Aprox. 250 g) was collected from nearby markets. Upon homogenization, 2 g of the sample was taken and undergoes extraction procedure as per the optimized method and the quantitation was done.
Results and discussion
Pesticide residue quantification in red Chilli powder is considered as complex due to the presence of co-extractives such as carbohydrates (40–50%), sugars (20–30%), volatile fiber (10%), protein (10%), capsaicin (10%), beta carotenes ( 0.05%) etc. Carotenoids are thermally labile and low volatiles (Rodriguez 2001) resulting in contamination of liner and the beginning of the column. Hence, sample preparation need to be optimized considering the removal of these interfering co-extractives from the acetonitrile extract. The results of optimization of sample preparation is presented below.
Optimization of sample preparation
Optimization of sample size
Sample size 1 g, 2 g and 3 g of red Chilli powder was weighed and transferred into a 50 mL plastic centrifuge tube. Sample size was evaluated for recovery and deposition of non-volatile co-extractives in the liner. In 1 g sample size, few compounds such as dieldrin, d-HCH, deltamethrin were not detected due to low response for qualifier ion at 0.010 mgkg−1 fortification level as the sample was diluted 5 times in the process of extraction. In case of 2 g and 3 g sample size, qualifier peak was detected for all targeted compounds and available for ion ratio calculation. However, 93% of compounds including the above compounds gave a recovery of 70–110% at 0.025 mgkg−1 spiking level. The recovery of analytes in case of 2 g and 3 g sample size were almost the same as 1 g sample size at 0.025 mgkg−1 fortification level. Further, it was observed more deposition of non-volatiles co-extractives on liner for 3 g sample size compared to 1 g and 2 g sample size. Hence, considering all the above observations, a samples size of 2 g was considered optimal for sample preparation.
Optimization of water addition
Penetration of water to the matrix is very much essential for the extraction of pesticide residues as the water act as a medium for residue exchange (Cajka et al. 2012). Minimum 6 mL water was required to soak 2 g of finely grounded red Chilli powder. Water addition was optimized based on recovery and relative standard deviation (RSD) target analytes. Upon comparing 6 ml, 8 ml and 10 mL of water, an average of 5% increase in recovery was observed for the majority of compounds in 10 ml of water addition. There was no significant improvement in recovery when 12 mL of water was added. Hence, 10 ml of water addition followed by soaking is considered optimal for sample preparation. The recovery percentage of each class of pesticides at different volumes of water addition is shown in Fig. 2.
Fig. 2.
Effect of different volume of water addition on recovery of selected pesticides
Cleanup optimization
The optimum level of d-SPE cleanup agents viz. GCB, C18 and PSA were finalized based on the deposition of non-volatile co-extractives in liner, analyte specific matrix interference and recovery.
Optimization of GCB
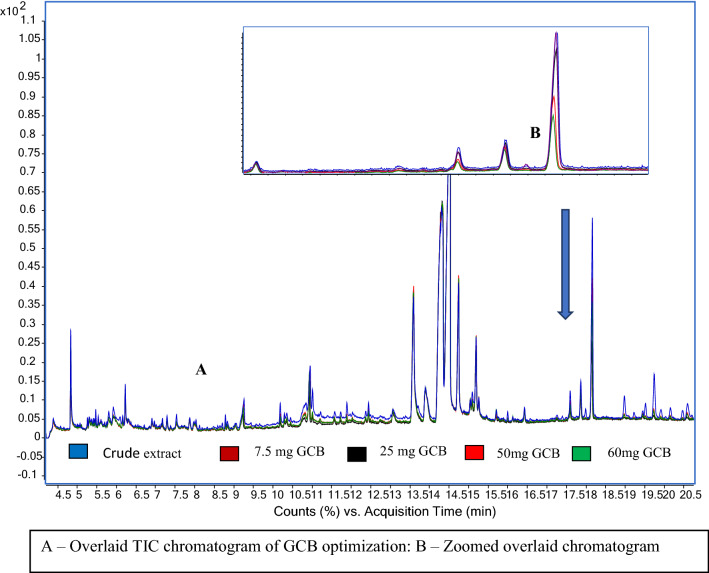
GCB is one of the most important d-SPE cleanup agents for removing pigments and planar matrix co-extractives like fatty acids. Different quantities of GCB, viz. 7.5 mg, 25 mg, 50 mg and 60 mg were tried for their cleanup efficiency. When 7.5 mg of GCB was used, higher background noise was observed. The recovery of some of the compounds like diniconazole and fenzaquin were 78% and 75% at 7.5 mg GCB cleanup. However, upon increasing the GCB amount to 50 mg, the recovery reduced only to 66% and 58%, respectively with acceptable repeatability (RSD < 15%). The average recovery of beta-BHC, pyrimethanil, fenchlorphos oxon, profenophos, mirex, cypermethrin III & IV isomers were 70 ± 5% in both 7.5 mg and 50 mg GCB cleanup. Upon increasing the GCB level to 60 mg, removed co-extractive peaks of tocopherol (18.1 Rt), propionic acid (17.9 Rt) and sitosterol (19.7) ethyl esters. Though further increase in GCB to 60 mg removed 15% more matrix co-extractive peaks and lesser liner deposition was observed, but the recovery was lesser than 30% for pyrimethanil, mirex and 5–20% average lower recovery observed for other compounds. In 50 mg GCB cleanup, the average recovery of 85% compounds were between 70–110% with acceptable repeatability. Hence, 50 mg GCB was considered optimal for further studies (Fig. 3).
Fig. 3.
Total Ion Chromatogram (TIC) of acetonitrile extract of chilli powder with varying amount of GCB cleanup
Optimization of PSA
Amount of PSA was optimized between 50 and 75 mg PSA to 1 ml of crude extract. d-SPE cleanup with 50 mg of PSA was removing more than 80% of ethenone (4.84 Rt), ethyl amphetamine (6.21 Rt) and hexadecanoic acid (9.2Rt) from the crude extract. Upon cleanup with 75 mg of PSA there was no significant improvement in removal of coextractives but observed reduction in the background noise (Fig. 4). The matrix effect was lowered by 5–12% for targeted pesticides in case of 50 mg PSA d-SPE cleanup. Where as in 75 mg of PSA cleanup matrix effect was not lowered for 97% of compounds and only 1.5% reduction in matrix effect for the remaining 3% compounds. Hence 50 mg of PSA addition per ml of crude extract was optimized for sample d-SPE cleanup.
Fig. 4.
Total Ion Chromatogram (TIC) of acetonitrile extract of chilli powder with varying amount of PSA cleanup
Optimization of C-18
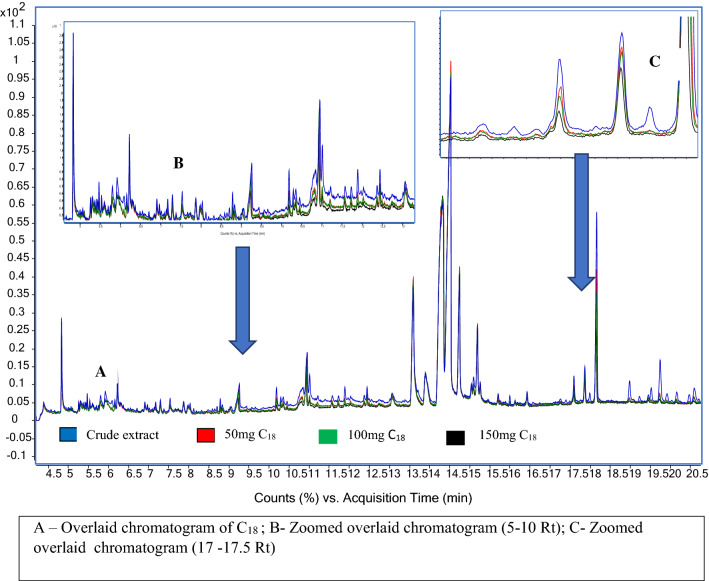
Amount of C-18 required for d-SPE cleanup was optimized between 50 mg, 100 mg and 150 mg of per 1 ml of crude extract. The matrix effect of all targeted compounds in crude extract was decreased upon C18 d-SPE cleanup. 150 mg of C18 cleanup reduces the background noise and removed 50% peak abundance of co-extractives. For example, propionic acid (17.9 Rt) was removed by 60% in 150 mg of C18 compared to 50% and 40% in 100 mg and 50 mg of C18 (Fig. 5). Hence 150 mg of C18 is considered as optimal for d-SPE cleanup.
Fig. 5.
Total Ion Chromatogram (TIC) of acetonitrile extract of chilli powder with varying amount of C18 cleanup
Optimized sample preparation method
2.0 ± 0.1 g of red chilli powder was weighed into a 50 mL plastic centrifuge tube,10 mL of distilled water added, shook well for one minute and soaked for 30 min for matrix swelling. Then, 10 mL of acidified acetonitrile (in 1% acetic acid) was added and shaken for one minute for extraction. To this sample, 6 g of MgSO4 (dehydrating agent) and 1.5 g of sodium acetate buffer (enables acetonitrile – water partition) was added. The samples were shaken vigorously for one minute followed by centrifugation at 5000 rpm for 5 min. 1 mL of supernatant acetonitrile layer from the above procedure was pipetted out and 50 mg of GCB, 150 mg of C18, 50 mg of PSA and 150 mg of MgSO4 added and undergone d-SPE cleanup. The content was vigorously shaken for a minute, then centrifuged at 10,000 RPM for 10 min and the supernatant was analyzed in GC–MS/MS.
Method validation
Method performance was evaluated according to SANTE guidelines. The parameters like linearity, limit of detection (LOD), the limit of quantitation (LOQ), matrix effect, recovery, precision and matrix effect were considered for method validation.
LOD, LOQ and linearity
The sensitivity of the instrument was expressed in terms of instrument limit of detection (iLOD). iLOD was calculated by injecting minimum concentration matrix matched standard six times. The iLOD was fixed by RSD% of six injections < 20% and deviation of back calculated concentration from true concentration in calibration linearity curve shall be ≤ ± 20% (SANTE/12682/2019, European Commission 2019). The matrix matched standard concentration of 0.39ngmL−1 repeatability in terms of RSD of all 84 compounds were < 20% and the difference in concentration was < + 20%. The method sensitivity was expressed in terms of method limit of quantification (mLOQ), determined for each compound by comparing the area of minimum concentration pesticides spiked as signal (S) and blank matrix signal as noise (N) at same retention time by S/N > 10 criteria. The method LOQ (S/N > 10) for all the target compounds was between 0.002 and 0.007 mgkg−1 (Supplementary Table 2). which was sufficient to achieve the minimum regulatory limit (MRL) of 0.01 mg kg−1 in chilli matrix.
The linearity of all compounds in solvent standard, matrix standard, was obtained by plotting the peak area against corresponding sample concentration at 0.002, 0.004, 0.008, 0.016, 0.032, 0.062, 0.125, 0.25, 0.5mgkg−1. Linearity regression co-efficient (R2) was shown between 0.9999 and 0.9990 for 88% compounds and 0.9990 to 0.9600 for remaining 12% of the compounds for the calibration range mentioned. The lowest R2 was chlorfenpyr with 0.9600, but the difference in concentration of expected and calculated was 18% and remaining compounds were between 5 and 18% which is lesser than the acceptance criteria of + 20%.
Recovery and precision
For recovery experiment, 2 g sample from homogenized material was spiked at 0.01, 0.025, 0.05 mgkg−1 (n = 6) of targeted analyte. Matrix match calibrations of respective matrices were used for calculation of recovery (%), and the repeatability of the method (precision) was estimated in terms of % RSD (n = 6).
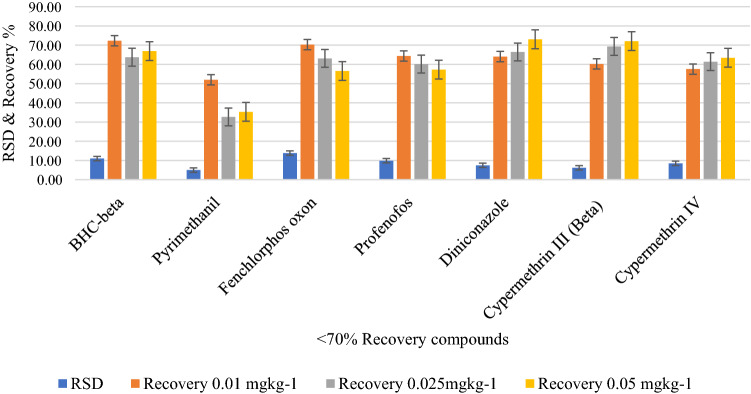
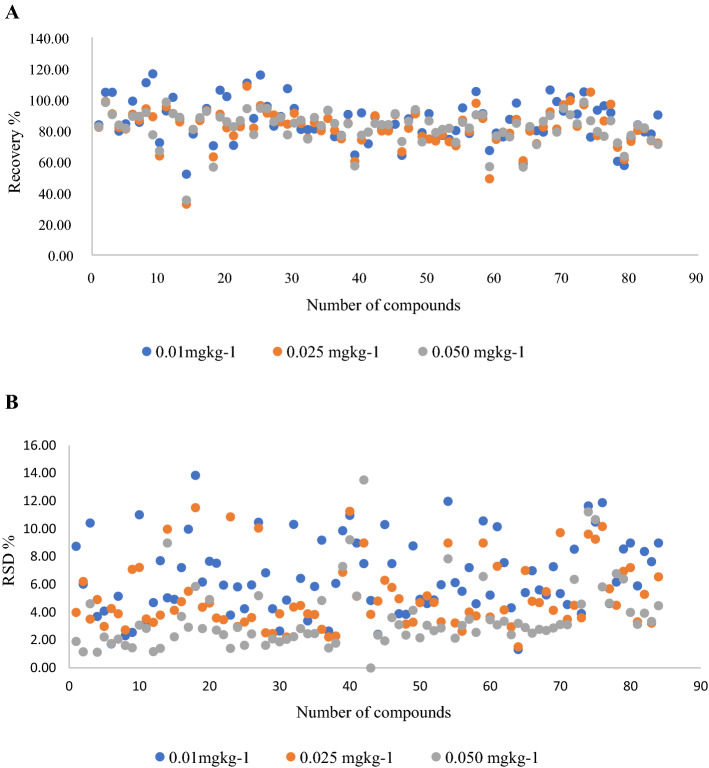
Recovery and associated RSD of target analytes at three different fortification levels are presented in Fig. 6A, B. 91% analytes gave satisfactory recovery of 70–110% at all three fortification levels (n = 6) and associated RSD was 3–16%. 9% of the analytes whose recovery was less than 70% gave a consistent and very low RSD (< 20% RSD) indicating repeatability. For example, pyrimethanil recovery was lesser than 70% and RSD was 5.04%, 9.98% and 8.98% at 0.01, 0.025 and 0.05 mgkg−1 fortification level, respectively. The compounds with low recovery and associated RSD are given in Fig. 7. the recovery of the compounds such as ethoxyquin, clomazone, alachlor and metalaxyl was 110.76%, 116.52%, 110.59% and 115.70%, respectively at 0.01mgkg−1 fortification level. The comparatively higher recovery of these analytes could be due to matrix induced signal enhancement or presence of these analytes at trace level (0.001–0.002 mgkg−1) in control sample used for recovery study which eventually contributed more at lowest fortification level of 0.01 mgkg−1 than 0.025 and 0.05 mgkg−1.
Fig. 6.
A Recovery (%) of different target analytes at three different spiking levels. B Associated standard deviation (%)
Fig. 7.
Recovery (%) and associated standard deviation (%) of analytes having less than 70% at three different spiking levels
Matrix effect
Matrix effect (ME) was determined by comparing the peak area of post-extraction spike standard with the corresponding area in a solvent standard of 100ngmL−1. The matrix effect was evaluated for representative class of pesticide given in (Supplementary Fig. 1). Significant reduction in matrix effect (%) was observed between crude extract and final optimized extract for mevinphos (40.26%), phorate (36.12%), bendiocarb (36.12%), thiobencarb (61.88%), BHC-alpha (31.78%), BHC-beta (47.05%), permethrin (45.08%) and cypermethrin (71.76%). Matrix suppression was observed for 6% of compounds such as beniocarb (− 21.56%), Terbufos (− 30.25%), fenchlorphosoxon (− 28.83%), phenothoate (− 32.58%) and pyriproxyfen (− 11.45%) and the remaining 94% observed matrix enhancement. Significant matrix effect in most of the cases emphasizes the importance of matrix match calibration standard for quantification.
Influence of retention gap in improving chromatographic performance
We observed during method development that the peak shape and sensitivity were lost after 75th injections for many compounds due to heavy deposition of co-extractive in liner and column. In MRM mode, these co-extractives were not monitored and resulted in liner change and column trimming. In order to improve the peak shape and sensitivity, a routine practice of changing the liner was carried out but was not successful. The second option of column trimming of 8 cm was done, which improved the peak shape but shift in retention time was observed. So to resolve this peak shifting, introduction of retention gap along with new a liner was tried. This configuration improved the peak shape and sensitivity without the shift in retention time. For example, dieldrin sensitivity and peak shape was lost after 75th injection. After replacing liner and introduction of retention gap, improvement in peak shape was observed (Fig. 8).
Fig. 8.
A Schematic diagram of column configuration with introduction of guard column. B Improvement in chromatographic performance of dieldrin upon replacement of liner and introduction of guard column
Analysis of market samples
The method developed was applied to quantify 25 real time market samples from different local brands. Thirteen pesticides were quantified above the LOQ level of 0.01mgkg−1. Among the targeted analytes, mirex was detected in 85% samples between 0.014 and 0.037 mgkg−1 and transfluthrin was detected in 20% samples between 0.028 and 0.057 mgkg−1. (Supplementary Fig. 2).
Conclusion
Complex matrix of red chilli powder pose a challenge for pesticide residue determination. An attempt was made to reduce co-extractives in order to extend the liner and column life without compromising the LOQ requirement. Acceptable recoveries within 70–110%, and its associated RSD < 20% RSD (n = 6) was obtained for most of the compounds at all three spiking levels. In terms of sensitivity all targeted analytes were quantified at lesser than required level of 0.01 mgkg−1. Lower recoveries, less than 70%, could be corrected for the known recovery factor in the analysis since the repeatability of the results were < 20% RSD. The introduction of retention gap was an alternative to conventional trimming of column to improve peak shape and sensitivity. GC–MS/MS method combined with optimized sample preparation gave reliable results and could be used by commercial laboratory for routine analysis of pesticide residues in chilli powder.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe
- GC–MS/MS
Gas Chromatography Tandem Mass spectrometry
- RSD
Relative standard deviation
- CIB and RC
Central Insecticide Board and Registration Committee
- LC–MS/MS
Liquid Chromatography Tandem Mass spectrometry
- d-SPE
Dispersive Solid Phase Extraction
- CRM
Certified reference standards
- MeCN
Acetonitrile
- MgSO4
Magnesium sulfate
- NaCl
Sodium chloride (NaCl)
- CH3COONa
Sodium acetate
- PSA
Primary secondary amine
- GCB
Graphitized carbon black (GCB)
- C18
Bonded octadecyl silica
- EI
Electron Impact ionization
- MMI
Multimode inlet
- MRM
Multi Reaction Monitoring
- Rt
Retention Time
- LOD
Limit of Detection
- LOQ
Limit of Quantification
Authors' contributions
Each authors contribution are as given below, CK: Conceptualization of the problem, Conducting the research and investigation process, specifically performing the experiments, initial draft preparation. DP: Data Curation, data analysis, writing original draft, experiment execution. TPSA: Ideas; formulation or evolution of overarching research goals and aims, manuscript review. SA: Ideas, Supervision, overall guidance, final manuscript review.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
As there was no animal or human cells were used in this study, no ethical approval was required. Hence, this declaration is “Not applicable”.
Consent to participate
No personally identifiable data presented in this manuscript, hence, “Not applicable”.
Consent for publication
No personally identifiable data presented in this manuscript, hence, “Not applicable”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anagnostopoulos C, Miliadis GE. Development and validation of an easy multiresidue method for the determination of multiclass pesticide residues using GC-MS/MS and LC-MS/MS in olive oil and olives. Talanta. 2013;112:1–10. doi: 10.1016/j.talanta.2013.03.051. [DOI] [PubMed] [Google Scholar]
- Bernardi G, Kemmerich M, Ribeiro LC, Adaime MB, Zanella R, Prestes OD. An effective method for pesticide residues determination in tobacco by GC-MS/MS and UHPLC-MS/MS employing acetonitrile extraction with low-temperature precipitation and d-SPE clean-up. Talanta. 2016;161:40–47. doi: 10.1016/j.talanta.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Cajka T, Sandy C, Bachanova V, Drabova L, Kalachova K, Pulkrabova J, Hajslova J. Streamlining sample preparation and gas chromatography-tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal Chim Acta. 2012;743:51–60. doi: 10.1016/j.aca.2012.06.051. [DOI] [PubMed] [Google Scholar]
- Chatterjee NS, Utture S, Banerjee K, Shabeer TPA, Kamble N, Mathew S, Kumar KA. Multiresidue analysis of multiclass pesticides and polyaromatic hydrocarbons in fatty fish by gas chromatography tandem mass spectrometry and evaluation of matrix effect. Food Chem. 2016;196:1–8. doi: 10.1016/j.foodchem.2015.09.014. [DOI] [PubMed] [Google Scholar]
- CIB & RC (2021) Central insecticide board and registration committee: major use of pesticides. http://www.ppqs.gov.in/divisions/cib-rc/major-uses-of-pesticides. Accessed Mar 2021
- European Commission Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. SANTE/11813/2017. Eur Comm Dir Heal Food Saf. 2017 doi: 10.13140/RG.2.2.33021.77283. [DOI] [Google Scholar]
- Ferrer Amate FC, Unterluggauer H, Fischer RJ, Fernández-Alba AR, Masselter S. Development and validation of a LC-MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal Bioanal Chem. 2010 doi: 10.1007/s00216-010-3526-x. [DOI] [PubMed] [Google Scholar]
- George T, Beevi SN, Xavier G, Kumar NP, George J. Dissipation kinetics and assessment of processing factor for chlorpyrifos and lambda-cyhalothrin in cardamom. Environ Monit Assess. 2013;185:5277–5284. doi: 10.1007/s10661-012-2943-z. [DOI] [PubMed] [Google Scholar]
- Goon A, Shinde R, Ghosh B, Banerjee K. Application of automated mini–solid-phase extraction cleanup for the analysis of pesticides in complex spice matrixes by GC-MS/MS. J AOAC Int. 2020;103:40–45. doi: 10.5740/jaoacint.19-0202. [DOI] [PubMed] [Google Scholar]
- Gray BP, Teale P. The use of a simple backflush technology to improve sample throughput and system robustness in routine gas chromatography tandem mass spectrometry analysis of doping control samples. J Chromatog A. 2010;1217:4749–4752. doi: 10.1016/j.chroma.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Jadhav MR, Shabeer TPA, Nakade M, Gadgil M, Oulkar DP, Arimboor R, Menon R, Banerjee K. Multiresidue method for targeted screening of pesticide residues in spice cardamom (elettaria cardamomum) by liquid chromatography with tandem mass spectrometry. J AOAC Int. 2017;100(3):603–609. doi: 10.5740/jaoacint.17-006. [DOI] [PubMed] [Google Scholar]
- Jadhav MR, Pudale A, Raut P, Utture S, Shabeer TPA, Banerjee K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC–MS/MS. Food Chem. 2019;272:292–305. doi: 10.1016/j.foodchem.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Kandaswamy C, Anandaram S, Presley SID, Shabeer ATP. Comparative evaluation of multi-residue methods for analysis of pesticide residues in black pepper by gas chromatography tandem mass spectrometry: critical evaluation of matrix co-extractives and method validation. J Food Sci Technol. 2020 doi: 10.1007/s13197-020-04605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A. 2010;1217:2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Pathan ARK, Parihar NS, Sharma BN. Short note effect of drying on the residues of Dicofol, Ethion and Cypermethrin in Chilli (Capsicum annum L.) Pest Manage Hortic Ecosyst. 2009;15:167–169. [Google Scholar]
- Patil VM, Singh S, Patel K, Patel Z. Effect of sun drying and grinding on the residues of six insecticides in chilli fruits. Pestic Res J. 2018;30:140. doi: 10.5958/2249-524x.2018.00023.7. [DOI] [Google Scholar]
- Patil R, Khan Z, Pudale A, Shinde R, Shabeer TPA, Patil A, Banerjee K. Comprehensive multiresidue determination of pesticides and plant growth regulators in grapevine leaves using liquid-and gas chromatography with tandem mass spectrometry. J Chromatog A. 2018;1579:73–82. doi: 10.1016/j.chroma.2018.10.025. [DOI] [PubMed] [Google Scholar]
- Rejczak T, Tuzimski T. Recent trends in sample preparation and liquid chromatography/mass spectrometry for pesticide residue analysis in food and related matrixes. J AOAC Int. 2015;98:1143–1162. doi: 10.5740/jaoacint.SGE1_Rejczak. [DOI] [PubMed] [Google Scholar]
- Rodriguez D (2001) A guide to carotenoid analysis in foods. Life Sci p 64
- Rutkowska E, Łozowicka B, Kaczyński P. Modification of multiresidue QuEChERS protocol to minimize matrix effect and improve recoveries for determination of pesticide residues in dried herbs followed by GC-MS/MS. Food Anal Methods. 2018;11:709–724. doi: 10.1007/s12161-017-1047-3. [DOI] [Google Scholar]
- Shabeer TPA, Banerjee K, Jadhav M, Girame R, Utture S, Hingmire S, Oulkar D. Residue dissipation and processing factor for dimethomorph, famoxadone and cymoxanil during raisin preparation. Food Chem. 2015;170:180–185. doi: 10.1016/j.foodchem.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Shabeer TPA, Girame R, Hingmire S, Banerjee K, Sharma AK, Oulkar D, Utture S, Jadhav M. Dissipation pattern, safety evaluation, and generation of processing factor (PF) for pyraclostrobin and metiram residues in grapes during raisin preparation. Environ Monit Assess. 2015 doi: 10.1007/s10661-015-4268-1. [DOI] [PubMed] [Google Scholar]
- Shabeer TPA, Jadhav M, Girame R, Hingmire S, Bhongale A, Pudale A, Banerjee K. Targeted screening and safety evaluation of 276 agrochemical residues in raisins using buffered ethyl acetate extraction and liquid chromatography–tandem mass spectrometry analysis. Chemosphere. 2017;184:1036–1042. doi: 10.1016/j.chemosphere.2017.06.086. [DOI] [PubMed] [Google Scholar]
- Shabeer TPA, Girame R, Utture S, Oulkar D, Banerjee K, Ajay D, Arimboor R, Menon KRK. Optimization of multi-residue method for targeted screening and quantitation of 243 pesticide residues in cardamom (Elettaria cardamomum) by gas chromatography tandem mass spectrometry (GC-MS/MS) analysis. Chemosphere. 2018;193:447–453. doi: 10.1016/j.chemosphere.2017.10.133. [DOI] [PubMed] [Google Scholar]
- Spices Board India (2019) Statstics on item wise export of spices from India. Spices Board India, Ministry of Commerce, Governement of India. http://indianspices.com/export/major-itemwise-export.html. Accessed Oct 2020
- Taha SM, Gadalla SA. Development of an efficient method for multi residue analysis of 160 pesticides in herbal plant by ethyl acetate hexane mixture with direct injection to GC-MS/MS. Talanta. 2017;174:767–779. doi: 10.1016/j.talanta.2017.06.080. [DOI] [PubMed] [Google Scholar]
- Wong JW, Zhang K, Tech K, Hayward DG, Krynitsky AJ, Cassias I, Schenck FJ, Banerjee K, Dasgupta S, Brown D. Multiresidue pesticide analysis of ginseng powders using acetonitrile- or acetone-based extraction, solid-phase extraction cleanup, and gas chromatography-mass spectrometry/selective ion monitoring (GC-MS/SIM) or -tandem mass spectrometry (GC-MS/MS) J Agric Food Chem. 2010;58:5884–5896. doi: 10.1021/jf903851h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.