Abstract
The development of novel and sustainable food products, such as cheese- and meat analogues, requires a better understanding of the use of less refined ingredients. We investigated the distribution of water between the protein and starch phase of doughs and heat-induced gels made from air-classified faba bean fractions by developing a method suited for investigation of such multi-component ingredients. The moisture contents of the protein and starch phases in the dough were determined using a method based on partial sorption isotherms of mixed doughs of protein- and starch-rich fractions at high water activity. Water content of the protein phase is higher than that of the starch phase in dough, showing that protein takes up more water than starch at room temperature. Also, the moisture content of the protein phase in the gels was calculated using a model based on the denaturation temperature of legumin. From the experiments and the modelling, it became evident that the moisture content of the protein phase in the gel is lower than the moisture content of the protein phase in the dough, showing the importance of considering moisture migration from the protein to the starch during heating.
Keywords: Starch gelatinization, Protein denaturation, Faba bean concentrate, Mild processing, Moisture distribution, Starch swelling
Abbreviations: DSC, Differential Scanning Calorimetry; FM, Faba bean meal; PF, Protein fraction; SF, Starch fraction; TD-NMR, Time Domain Nuclear Magnetic Resonance
Graphical abstract
Highlights
-
•
Water distribution among phases in doughs and gels is measured “in-situ”.
-
•
Addition of starch increases water content of protein phase in doughs.
-
•
Water migrates from protein to starch after initial starch gelatinization.
1. Introduction
To make attractive new food products, we have to understand how sensory properties of the product depend on components and their interactions with water. This understanding is especially important in soft-solid products like cheeses and meat but even more so for the plant-based alternatives of those products. Thus far, the products are mainly based on soy, pea or wheat, but interest in the use of other raw materials is increasing. Faba beans are considered as one of the promising new crops to provide healthy plant proteins (Multari et al., 2015). It is therefore taken as an exemplary material of a starch-containing crop in this study.
Meat and cheese analog products are characterized by having a dry matter content of around 20–40% in the water phase, which gives high water activity. Besides, multiple aqueous phases can be present in those products. The water content in each phase of such materials has a large influence on properties such as gelling behavior and thereby on the properties of the final product. Thus far, research on the influence of starch on protein gelation and vice-versa, as well as research on the influence of water content on product properties of soft-solid products, is often limited to the use of pure components, meaning isolates (Dekkers et al., 2016; Aguilera and Baffico, 1997; Eliasson, 1983; Walsh et al., 2008; Zhang et al., 2016; Fernández-Gutiérrez et al., 2004). The use of less refined ingredients would improve the sustainability and cost efficiency of these products, for example meat analogues (Berghout et al., 2015; Schutyser et al., 2015; van der Goot et al., 2016). In order to use less refined ingredients for making food products, the interactions of the components present in less refined ingredients needs to be understood. This can only be achieved by performing experiments directly on the less refined ingredients instead of mixtures of highly purified ingredients, where protein and starch structure and therefore functionality have been modified during the purification process applied to make the ingredients (Bühler et al., 2021).
Starch gelatinization is a clear example of the effect of water content on food properties. Although the initial gelatinization of starch occurs at a gelatinization temperature independent of the moisture content, the magnitude of gelatinization at said temperature decreases with the water content (Donovan, 1979; Eliasson, 1980; Tananuwong and Reid, 2004). In case of incomplete gelatinization of starch, a second transition occurs at higher temperatures. This transition is the melting of starch crystals, and the melting temperature depends on the available water (Tananuwong and Reid, 2004). Additives that modify the water activity (and therefore the water availability) also influence the gelatinization parameters of starch (Wootton and Bamunuarachchi, 1980). Similarly, the gelling parameters of protein are influenced by the available water as well. Barker (1933) found a linear correlation between the decrease of the denaturation temperature of egg albumin and the moisture content. Since then, similar relations have been established for many proteins, including vicilin and legumin in faba bean (Arntfield et al., 1985). As the gelling properties of protein and the gelatinization properties of starch both depend on the available moisture, determining this available moisture is essential to understand the behavior of food products that contain both starch and proteins. In a mixed dough or gel, starch and protein are often seen as individual phases that do not mix on a molecular level due to thermodynamic incompatibility (Grinberg and Tolstoguzov, 1997; Tolstoguzov, 1997, 2006; Bot and De Bruijne, 2003). Due to different affinities for water, the available moisture in these two phases will differ from the overall moisture content, which is an average value of the different moisture contents in the different phases. Often this water distribution is not measured, but obtained via fitting the water distribution between phases in a biopolymer blend or assumed from the overall moisture content when describing for example rheological properties by applying the polymer blending law to starch-protein mixtures or mixed protein blends (Aguilera and Baffico, 1997; Shrinivas and Kasapis, 2009; Fitzsimons et al., 2008; Clark et al., 1983). In addition, Dekkers et al. (2016) developed a method based on TD-NMR to determine water distribution in mixed protein gels. However, it requires the availability of and information about the pure components used to make the mixed gels. For many food products, it is not possible to obtain the components in pure form, especially in the same physical state as in the product. For example, a purification process might involve a heating step, which changes properties of starches and proteins, which can change the distribution of water among them. The use of model mixtures of protein isolates and starch isolates will therefore not accurately reflect the behavior of real ingredients (Bühler et al., 2021).
The aim of this research is to determine how starch and protein content influence the water distribution in unheated doughs and heat-induced gels that are prepared from mixtures of mildly refined ingredients. For this, we developed new methods for the determination of the water distribution in doughs at room temperature and for gels at elevated temperatures that do not depend on pure components that are otherwise difficult to obtain in the same, native form in which they are present in the mildly refined fractions. The method for the doughs combines fundamental principles of sorption isotherms with mass balances. Modelling is used to calculate the contribution of each pure component, which is then transferred into a sorption isotherm for the pure component at high water activity (aw > 0.96). This high aw is relevant when considering products like meat and cheese analogues that have a moisture content between 40 and 80% Kyriakopoulou et al. (2019); Cornet et al. (2021); Grasso et al. (2021). However, this range of water activity is rarely investigated, causing a limitation of available data to compare to for both faba bean (Alpizar-Reyes et al., 2018; Menkov, 2000) and starch in general (Al-Muhtaseb et al., 2004). The method to determine the distribution of moisture among the phases of gels at elevated temperatures is based on the denaturation temperature of the protein, measured with DSC. These methods enable us to understand the changes in water distribution due to a thermal treatment under the relevant conditions. These changes might have implications for research on the properties of such mixed doughs and gels that depend on the water distribution, for example determining and predicting sensory attributes of protein/starch gel products (meat analogues, cheese analogues, sauces, dairy analogues).
Faba bean Meal (FM) is taken as an exemplary material. FM can be dry fractionated, delivering protein and starch enriched fractions with native properties. This ensures that neither protein nor starch are denatured or otherwise influenced by the fractionation method, therefore retaining their native functionality. Furthermore, FM contains enough starch to allow a starch content range in mixed doughs of 0.09–0.66 g g−1 (db.) while ensuring that protein content is high enough to allow the detection of its denaturation.
2. Materials & methods
Faba bean Meal HOMECRAFT®Pulse 3101 (FM) was supplied by Ingredion (Hamburg, Germany). FM had a protein content of 29% (N-conversion factor = 6.25). FM was air classified using a Hosokawa Multi Mill (Alpine, Augsburg, Germany). The classifier wheel speed was set to 7000 rpm and the air flow to 70 m3h−1. The composition of the protein-rich fine fraction (Protein Fraction - PF) and the starch-rich coarse fraction (Starch Fraction - SF) is shown in Table 1.
Table 1.
Composition of PF and SF in mass %. Starch content was determined using MegaZyme Starch Kit, protein content was determined using DUMAS (N-conversion factor = 6.25). Moisture content was determined using oven drying at 105 °C until the weight remained constant.
| Starch content / % | Protein content / % | Moisture content / % | |
|---|---|---|---|
| PF | 8.5 | 58.8 | 8.2 |
| SF | 66.3 | 15.5 | 6.79 |
PF and SF were mixed at different ratios to vary starch content (9–70% d.b.). Starch content will be expressed in g g−1 as w/w % on d.b., unless specified otherwise. Mixtures were combined with distilled water to create doughs with different moisture content (36–66% wet basis), for which the moisture content of the powders was taken into account. Moisture content or dry matter content are expressed in g g−1 on wet basis, unless specified otherwise. Doughs were stored in vacuum-sealed bags at 4 °C for 48 h to allow water distribution to reach equilibrium.
2.1. Water activity
Water activity (aw) of the doughs was measured using an Aqualab TDL water activity meter (METER Group, Pullman, USA). Approximately 3 g of sample were used for each measurement. The measuring temperature was set to 25 °C with a deviation of 0.05 °C, ensuring constant measuring conditions.
2.2. Moisture content
Moisture content (mc) of all doughs was determined by oven drying. Dough samples were dried in aluminum cups at 105 °C for 24 h. Moisture content was calculated according to Equation (1):
| (1) |
Where mwet is the weight of the wet sample and mdry is the weight of the dry sample. Moisture contents are always expressed on a wet basis (wb.), unless specified otherwise.
2.3. Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) was used to determine the denaturation temperature of the proteins and the degree of starch pasting. Dough samples were degassed in an ultrasonic water bath for 15 min 60 mg was transferred to High Volume Pans (100 μl, TA Instruments, New Castle, USA). The pans were placed in the DSC (DSC-250, TA Instruments, New Castle, USA) where they were first equilibrated at 20 °C until the temperature was constant. Samples were then heated with a ramp of 5 °C min−1 to 160 °C. After cooling, the cycle was repeated. TRIOS software was used to analyze the obtained thermograms and to identify the peak temperatures of the protein denaturation and the enthalpy change of the initial peak of starch pasting (G peak). ΔH was obtained by adjusting for the amount of starch present in the sample using
| (2) |
where ΔH is the enthalpy change per overall sample mass in J g−1, mc is the overall moisture content in g g−1 (wb.) and sc is the starch content in g g−1 (db.).
2.4. Modelling and statistics
All measurements are shown as individual data points in the graphs. Multiple Linear Regression was used to fit the data in R (Version 3.6.1). For the dough, the model Equations (3), (4), (5), (6)) were used:
| (3) |
| (4) |
| (5) |
| (6) |
where mc is the overall moisture content of the sample in g g−1 (wb.), aw is the water activity, sc is the starch content in g g−1 (db.) and a, b, c and d are model parameters. For the model of the gel, the same method was used with the model Equations (7)–(10).
| (7) |
| (8) |
| (9) |
| (10) |
where Td is the denaturation temperature of legumin in °C, mc is the overall moisture content of the sample in g g−1 (wb.), sc is the starch content in g g−1 (db.) and e, f, g and h are model parameters. The residuals of each fit were checked for correlation in R. The summary tables of each model can be found in the Supplementary information. The best fit was chosen based on the value of and the significance of the independent variables (p-value).
3. Theory
Starting point of the method to determine the distribution of moisture among phases of a starch-protein blend at room temperature (called “dough”) presented in this paper is the fact that the water activity of such a blend is determined by both the overall moisture content and the composition of the dough (Barbosa-Cánovas et al., 2007) (Fig. 1). The assumption of full phase separation between starch and protein makes it possible to use the sorption isotherm of the blend to obtain the isotherms of the individual phases (Labuza and Hyman, 1987). The moisture content of the individual phases in the dough can then be read from the sorption isotherm of the individual phases at the measured aw of the dough. At moisture contents higher than 40%, the water activity of the product is between 0.9 and 1 and their relation can be approximated well by a straight line. The shape of the sorption isotherm of starch and protein only deviates from a linear relation at aw < 0.95 (Lomauro et al., 1985; Xu et al., 2019). This simple method to determine the water available to starch and protein can however only be carried out when the mixture is in equilibrium. Therefore, it is not suitable at high temperatures due to evaporation and reactions taking place (e.g. gelation). To determine the distribution of moisture at higher temperatures, a method based on the denaturation temperature of the protein is proposed. As the denaturation temperature of protein depends on the moisture content of the protein phase, the first can be used to determine the latter if the relation is known. We model the relation by extrapolating the measured denaturation temperature of protein of the mixed gels to a protein phase containing no starch, yielding the moisture content of the protein phase in the mixed, heat-induced gel.
Fig. 1.
Sketch of sorption isotherms of protein (dotted line), starch (dashed line) and a mixture of the two (solid line), adapted from Barbosa-Cánovas et al. (2007). Arrows indicate the method to obtain the moisture content of the individual phases in the mixture. The red square indicates the area of the curve that is investigated in this study. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next to the denaturation temperature of protein, the gelatinization of starch can also be monitored. If sufficient water is available, starch granules fully gelatinize at the initial gelatinization temperature (Biliaderis et al., 1980; Haase and Shi, 1991), resulting in the peak of gelatinization (G peak) (Tananuwong and Reid, 2004) in a DSC thermogram. Under low moisture conditions, the amorphous regions of the starch granule are not fully hydrated and hence the crystalline regions initially stay partly intact. The crystalline regions of the starch granule then melt at a higher temperature, also resulting in a peak in the DSC thermogram (M1 peak: high temperature, low moisture melting peak) (Donovan, 1979; Hoseney et al., 1986). The position of this peak depends on the amount of water that can be taken up by the granules during the initial gelatinization. When no water is available, only the M1 peak is present (Biliaderis et al., 1980). According to Tananuwong and Reid (2004), the sum of the enthalpy change of the G and M1 peak is independent of the overall moisture content. At high moisture contents, the peak temperature of the M1 peak decreases in a magnitude that it overlaps the G peak. Here, this fact is used to take the enthalpy change of the G peak of the starch phase (ΔH) as a measure of the moisture content of starch in the dough.
4. Results & discussion
4.1. Moisture distribution in starch-protein doughs at room temperature
In this study, protein-rich fractions (PF) and starch-rich fractions (SF) were mixed to obtain doughs that vary in protein and starch content. Besides, water addition was used to change the water contents. Since PF and SF used in this study were obtained via dry fractionation, it is assumed that starch granules will remain intact (Vogelsang-O’Dwyer et al., 2020). This prevents mixing with the other components on a molecular level and starch can therefore be regarded as a separate phase. The other phase consists of proteins and other components such a fibres and sugars. Also these components will bear native properties, due to the lack of heating in dry fractionation. Despite the presence of the other components, we will describe the latter phase as protein phase, and study how the water will distribute between the starch and this so-called protein phase (Eliasson, 1983).
The overall moisture content (mc) and water activity (aw) of 155 dough samples with different starch and protein contents and added amounts of water were measured (Fig. 2) at 25 °C. The data obtained represents the section of the sorption isotherm where the shape of the curve becomes asymptotic (Baucour and Daudin, 2000), indicated by the red square in Fig. 1. The partial sorption isotherm therefore becomes linear at these high moisture contents. Therefore, the data in Fig. 2 shows linear relations for each starch concentration linking water activity to moisture content. The slopes differ depending on the starch content, leading to a larger difference in aw at the lower moisture contents than at the highest moisture content.
Fig. 2.
Partial sorption isotherms showing the influence of starch content of the correlation between overall moisture content and water activity. The dotted line represents the protein phase (Equation (12)), the solid line the starch phase (Equation (13)).
The potential influence of the starch content on the correlation between the moisture content and the water activity can be incorporated in a model via Equations (3)–(6). It is important to note that the dry based protein content (pc) and the dry based starch content (sc) of the samples can be expressed as sc + pc = 1. This means that protein content was not varied independently of starch content and is therefore not used as an independent variable. Equation (6) was found to give the best fit based on the highest , while the p-value was below 0.001 for all variables used, showing that they are significant. Summary tables of all fitted models can be found in the Supplementary information (Tables S1–S4). Fitting resulted in the following equation describing the relation between water activity, starch content and moisture content in starch-protein doughs:
| (11) |
where mc is the overall moisture content of the sample in g g−1 (wb.), aw is the water activity, and sc is the starch content in g g−1 (db.). This optimized fit resulted in . The investigated range is limited to the moisture content relevant for protein/starch gel products such as meat- and cheese analogues.
The moisture content of the protein phase as well as the starch phase in a sample at a given water activity can be calculated from Equation (11) by setting the starch content or respectively the protein content to zero. This yields the moisture content of the protein phase in the dough (mcp(dough), Equation (12)) and the moisture content of the starch phase in the dough (mcs(dough), Equation (13)):
| (12) |
| (13) |
where mci(dough) is the moisture content of the protein or starch phase in the dough in g g−1 (wb.) and aw is the water activity (−) of the sample. These equations are given as lines in Fig. 2. The linear relation from Equation (11) is supported by the fact that the shape of the sorption isotherm of starch and protein only deviates from a linear relation at lower aw, according to literature: In a study on several raw legume flours (chickpea, lentil and yellow pea), sorption isotherms were measured, for all of which this deviation only occurred at aw < 0.8 and mc < 0.13 g g−1 (wb.) (Xu et al., 2019). For corn starch the relation seems to be linear at aw > 0.9 and mc > 0.15 g g−1 (wb.) at 30 °C (Peng et al., 2007). All samples used in the further analysis of this paper had an aw of over 0.97.
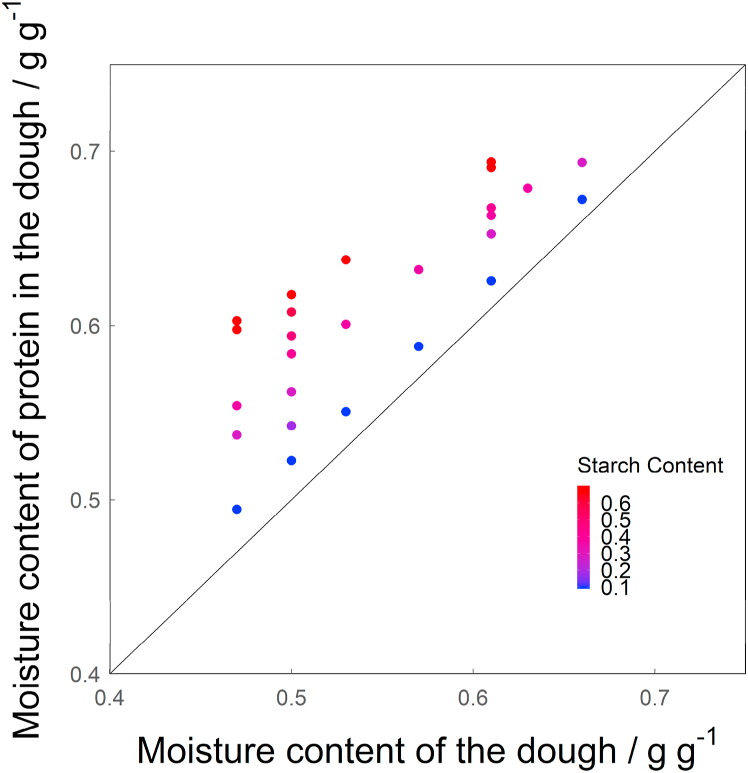
In Fig. 3, the calculated moisture content in the protein phase at room temperature is shown over the overall moisture content for all used starch contents (db.). The moisture content of the protein phase in the dough increases with starch content, depending on the overall moisture content. At lower moisture contents (0.47 g g−1), an increase of the starch content from 0.09 to 0.7 g g−1 (d.b.) leads to an increase in moisture content of the protein phase of 0.11 g g−1 (22%). The same increase in starch content at higher overall moisture content (0.61) only causes an increase of 0.07 g g−1 (9%). As the overall moisture content increases and the aw approaches 1, the sample approaches a regime of excess water, where a change in starch content will not have an influence on the moisture content in protein, as both the moisture content in starch and protein will be equal to the overall moisture content.
Fig. 3.
Modelled moisture content in the protein phase in the dough (mcp(dough)) over the moisture content of the dough. The solid line represents where the moisture content of the dough and the moisture content of the protein phase in the dough would be the same.
4.2. Moisture distribution in starch-protein mixtures at increasing temperature
4.2.1. DSC thermograms of starch/protein blends
The starch gelatinization as well as the protein denaturation were analyzed using DSC. From the thermograms of the DSC (Fig. 4) we can gain information about changes that occur, such as the peak temperature (T) and the enthalpy change (ΔH) associated with the reaction. Fig. 4 shows three exemplary thermograms, one with a low mcs(dough) (red), one with an intermediate mcs(dough) (green) and one with a high mcs(dough) (blue). There are three peaks visible in all three thermograms. The first peak is the initial gelatinization of starch, also called the G peak (Tananuwong and Reid, 2004), which occurs when starch is heated above the pasting temperature in the presence of excess water (Biliaderis et al., 1980; Haase and Shi, 1991). For most starches this peak lies between 60 °C and 80 °C (Copeland et al., 2009). The second peak, around 90 °C, was credited to the denaturation of vicilin, while the third peak was credited to the denaturation of legumin at around 106 °C (Arntfield et al., 1985). DSC measurements of starch can also give rise to another peak (M1), especially at low moisture content, which represents the melting of the starch. This peak was not identified in this research.
Fig. 4.
Exemplary DSC thermograms of three samples with (red) 0.28 g g−1 starch content (d.b.), 0.51 g g−1 moisture content resulting in a mcs(dough) = 0.34 g g−1, with (green) 0.37 g g−1 starch content (d.b.), 0.53 g g−1 moisture content resulting in a mcs(dough) = 0.41 g g−1, and with (blue) 0.66 g g−1 starch content (d.b.), 0.61 g g−1 moisture content resulting in a mcs(dough) = 0.57 g g−1. Curves are offset in y-direction to display all three graphs in one figure without overlapping. Tick-marks on y-axis represent steps of 0.02 W g-1. All curves shown are in the range of −0.28 to −0.20 W g-1. Please note that the Heat Flow Q shown here is only normalized for the overall mass, not the mass of starch in the sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.2.2. Starch gelatinization
For the samples analyzed, the G peak (the excess water gelatinization peak) occurred at 73.4 °C (±2.4 °C) (data not shown). The peak temperature did not correlate with the overall moisture content or the moisture content of starch in the dough (Equation (13)) ( < 0.1), as is expected from literature (see Section 1). van der Sman and Meinders (2011) modelled the initial gelatinization of starch using free volume extension of Flory-Huggins theory and constructed a state diagram for starch, which shows limited influence of the mass fraction of water (yw) (which is equivalent to mcs(dough)) on the gelatinization temperature for yw > 0.3 g g−1. They do, however, show a linear relation of the melting temperature of starch to the mass fraction of water, which describes the peak temperature of the M1 peak. In this paper, the M1 peak could not be identified, as its position (T) and size (ΔH) depend on the moisture content and it can overlap with the denaturation peak of vicilin and potentially also legumin. The possible effects of this on the interpretation of the denaturation temperature of legumin (Td) are discussed towards the end of Section 4.2.3.
In Fig. 5, the ΔH over the overall moisture content of the dough is shown. There is no clear correlation of the two, resulting in an of the linear regression of 0.63. It is quite obvious, however, that ΔH depends on the starch content as well, since the high starch content samples (red) are underestimated by the linear regression, while the low starch content samples are mostly overestimated (purple, blue). Therefore, in Fig. 5b, ΔH is shown over mcs(dough), which is derived from Equation (13) which in turn depends on the overall moisture content and the starch content of the sample (Equation (11)). The use of a linear regression to correlate ΔHStarch and mcs(dough) yields an of 0.87. This underlines that Equations (12), (13)) give a reasonable approximation of the distribution of moisture between the protein and starch phase. It is important to note that at high mcs(dough) starch always fully gelatinizes during the initial gelatinization and therefore ΔH becomes constant (Baks et al., 2008). This most likely occurs outside the regime of mcs(dough) investigated here, as (Habeych et al., 2009) and (Baks et al., 2007) found a ΔH of 13.4 ± 0.6 J g−1 and 13.4 ± 2.0 J g−1 for wheat starch at a moisture content of 0.91 g g−1 and 0.9 g g−1 respectively. The extrapolation of the current data set to mcs(dough) = 0.9 g g−1 yields a ΔH of 14.68 J g−1, indicating the validity of our data and the linear correlation of mcs(dough) and ΔH. For samples with low moisture content of starch in the dough, the starch does not fully gelatinize at the temperature of the G peak but undergoes further transition at higher temperatures. The degree to which starch gelatinizes at this temperature depends on the initial water content of the starch (Tananuwong and Reid, 2004), or in this case mcs(dough). At mcs(dough) below 0.3 g g−1, no gelatinization occurred at the initial gelatinization temperature.
Fig. 5.
The enthalpy of the initial starch gelatinization (ΔHStarch) over (a) the overall moisture content of the dough and (b) the modelled moisture content of starch in the dough (mcs(dough)). Dotted lines are linear fits of ΔHStarch with (a) mc and (b) mcs(dough) as variables, resulting in = 0.63 and = 0.87, respectively.
This also suggests that the often described swelling of starch granules before or during initial gelatinization (Ai and Jane, 2015; Palanisamy et al., 2020) does not affect the moisture content of the protein phase or starch phase. Since the moisture content of the starch phase remains the same during swelling, the swelling of starch granules is caused by a migration of water within the starch phase. It is therefore likely that the moisture content of the starch phase consists of an extra-granular and an intra-granular water population, changing to only an intra-granular water population through swelling (Tananuwong and Reid, 2004).
4.2.3. Protein denaturation temperature of mixed starch-protein gels
As mentioned in Section 4.2.1, three peaks were found in the thermograms depending on starch and moisture content. The second and third peak at around 90 °C and 106 °C respectively represent vicilin and legumin (Arntfield et al., 1985). As the third peak was the most distinct and with good resolution, it was chosen to represent the denaturation of protein. Besides, the effect of moisture content on denaturation temperature (Td) seems to be comparable for vicilin and legumin in the studied range (Arntfield et al., 1985), and the composition of the protein in the protein phase is unlikely to change.
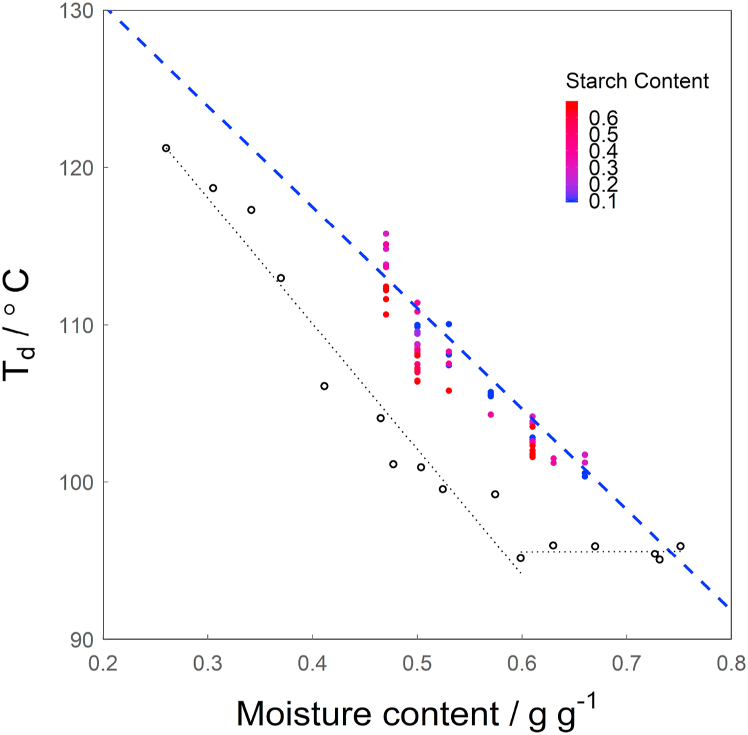
Fig. 6 shows the denaturation temperature of legumin (Td) against the overall moisture content of the sample. By changing the starch content, the denaturation temperature of legumin decreased by maximally 5.12 °C, when using blends with an overall moisture content of 0.47 g g−1.
Fig. 6.
Td measured using DSC over the overall moisture content of the sample (wb.). The open black symbols show data for legumin in faba bean protein isolate, taken from Arntfield et al. (1985). The blue dashed line is Td for sc = 0, according to Equation (14), the black dashed lines are linear regressions of the data reproduced from (Arntfield et al., 1985) in the range where Td depends on mc and where Td remains constant irrespective of mc. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The potential influence of the starch content on the correlation between the moisture content and Td can be incorporated in a model via Equations (7)–(10). Equation (9) was found to give the best fit based on the highest , while the p-value was below 0.001 for all variables used in this model, showing that they are significant. Equation (10) resulted in the same , but the p-values show that not all variables are significant. Summary tables of all fitted models can be found in the Supplementary information (Tables S5–S8). Fitting resulted in the following equation describing the relation between moisture content, starch content and the denaturation temperature of legumin in starch-protein gels:
| (14) |
where Td is the denaturation temperature of legumin in °C, mc is the overall moisture content of the sample in g g−1 (wb.) and sc is the starch content in g g−1 (db.), resulting in an = 0.88.
Similarly to the approach used to determine the mcp(dough), setting sc to 0 after solving for mc yields the moisture content of the protein phase in the gel (mcp(gel)):
| (15) |
where mcp(gel) is the moisture content of the protein phase in the gel in g g−1 (wb.) and Td is the denaturation temperature of legumin in °C. This relation is shown in Fig. 6 by the dashed blue line. Furthermore, the values of denaturation temperature of legumin found by (Arntfield et al., 1985) using DSC are shown as well (open symbols). The relation of the moisture content of faba bean protein and these values can be split in two regimes: a linear decrease of Td with increasing moisture content until 0.6 g g−1 and a constant Td above 0.6 g g−1. Here we found a similar dependency of the denaturation temperature on moisture content in the protein phase as reported by Arntfield et al. (1985). They do, however, differ from our results in temperature, which could be due to possible differences in origin of the raw materials and the purification process used by them. In our paper, a less refined material is used, leaving the possible influence of other components like sugars, fibres and salts on the denaturation temperature. The purification of the faba bean protein used by Arntfield et al. (1985) could have partially denatured the protein, causing the shift of the curve to lower temperatures observed here. Most likely, the Td of mildly refined faba bean fractions also does not decrease for moisture contents above the maximum moisture content used in this study.
In Fig. 7, the modelled moisture content in the protein phase at denaturation is shown over the overall moisture content for all starch contents used (db.). Moisture content in protein at denaturation increases with starch content. At overall moisture contents >0.6 g g−1 the trend becomes less evident. This either means that the influence of starch on the moisture content of protein at denaturation is limited at high moisture contents (as is the case at room temperature), or that the influence of moisture content in protein on denaturation temperature becomes smaller at overall moisture contents >0.6 g g−1. The latter was also found to be true by Arntfield et al. (1985), who showed that the decrease of denaturation temperature of legumin with increasing moisture content levels off at 1.5 g g−1 (db.), which is equal to 0.6 g g−1 (wb.).
Fig. 7.
Modelled moisture content in the protein phase in the gel (mcp(gel)) over the moisture content of the gel. The solid line represents where the moisture content of the gel and the moisture content of the protein phase in the gel would be the same.
It is important to note that the starch melting peak (M1 peak) was not identified in this research. It potentially overlaps with the Td peak and interferes with the analysis. Hoseney et al. (1986) and Tananuwong and Reid (2004) show that the M1 peak is rather broad and not a sharp peak like the G peak or the peaks for protein denaturation observed in this study. Such overlap is most likely at the lowest overall moisture content and starch content, as these conditions lead to the lowest values of mcs(dough)), which in turn lead to a high M1 temperature. However, this also decreases the overall enthalpy of the starch gelatinization (since there is less starch in the sample), thereby decreasing the influence of the M1 peak on the analysis of the denaturation peak of legumin and likely making this overlap less relevant. There is, however, the possibility of relevant overlap of the legumin peak and the M1 peak at low moisture contents (0.47 g g−1) and intermediate starch contents (purple points), as these conditions lead to a relatively low mcs(dough), causing the M1 peak to increase in height and appear at higher temperatures, and also lead to a smaller peak for the denaturation of legumin, as there is less protein present as the starch content increases. This could be the cause for the deviation of these samples from the rest of the data, observed in Figs. 6 and 7.
4.3. Comparing water distribution in doughs and gels
The comparison of the moisture content in the protein phase in the ingredients studied at room temperature (Fig. 3) with the moisture content in the protein phase at denaturation (Fig. 7) revealed that the latter is lower by up to 21.15%. The difference between them is larger for lower moisture contents and increases with starch content. Therefore, moisture had migrated from the protein phase to the starch phase during the heating process. A similar conclusion was drawn by Eliasson (1983) after studying the influence of wheat gluten on the gelatinization of wheat starch, using isolated ingredients. Since we have established that the moisture content of protein at room temperature is not influenced by the swelling of starch granules during their initial gelatinization (G peak), the redistribution of water must occur later, at higher temperatures. We suggest that this occurs during starch melting, which takes place at those higher temperatures. This is represented by the M1 peak that most likely overlaps with the denaturation peaks of vicilin and legumin and can therefore not be detected. Melting of starch modifies the affinity of starch for water, creating a force “pulling” the water to the starch phase. Simultaneously, denaturation of protein exposes hydrophobic sites, creating a force “pushing” the water to the starch phase.
5. Conclusions
The distribution of water between the starch and protein phase in doughs and gels of mixed mildly-refined fractions of faba bean flour was determined in a moisture content range relevant for soft-solid foods such as plant-based meat and cheese alternatives. Quantification of the moisture content distribution was achieved by applying models based on established concepts such as sorption isotherms, mass balances and the moisture dependence of starch gelatinization and protein denaturation. The model based on sorption isotherms was used to determine the water distribution in a dough at room temperature, while the model based on the moisture dependence of protein denaturation determines the water distribution at elevated temperatures when protein denatures and gelatinizes. In the dough, protein takes up between 0.12 g g−1 and 0.28 g g−1 more water than starch. The amount of water associated with the protein phase in the gel is up to 0.12 g g−1 less than in the dough, showing the amount of water that migrated from the protein phase to the starch phase upon heating. The enthalpy change of the initial starch gelatinization correlates reasonably well with the calculated moisture content of the starch phase in the dough. Therefore, no or limited redistribution of moisture occurs between the starch and protein phase during the initial starch gelatinization, but at higher temperatures, during the melting of starch crystals.
This paper shows that it is possible to derive properties of components present in a flour or enriched fraction without having the components as pure ingredients. Such an approach will become more important when focusing on the use of enriched fractions rather than pure ingredients in future food applications. These findings have implications for the further development and design of production processes for aforementioned plant-based meat and cheese alternatives. The methods presented here can be used to predict and control ingredient properties that determine process parameters such as processing temperature. Furthermore, they underline the limitations of using overall moisture content without understanding water uptake and distribution in the individual components, all of which becomes even more important when using less refined ingredients.
CRediT authorship contribution statement
Jan M. Bühler: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Atze Jan van der Goot: Conceptualization, Supervision, Writing – review & editing. Marieke E. Bruins: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is part of the project Plant Meat Matters, which is co-financed by Top Consortium for Knowledge and Innovation Agri & Food by the Dutch Ministry of Economic Affairs; the project is registered under contract number TKI-AF-16011.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.03.013.
Contributor Information
Jan M. Bühler, Email: jan.buhler@wur.nl.
Atze Jan van der Goot, Email: atzejan.vandergoot@wur.nl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aguilera J., Baffico P. Structure-mechanical properties of heat-induced whey protein/cassava starch gels. J. Food Sci. 1997;62:1048–1054. doi: 10.1111/j.1365-2621.1997.tb15035.x. [DOI] [Google Scholar]
- Ai Y., Jane J.L. Gelatinization and rheological properties of starch. Starch Staerke. 2015;67:213–224. doi: 10.1002/star.201400201. [DOI] [Google Scholar]
- Al-Muhtaseb A.H., McMinn W.A., Magee T.R. Water sorption isotherms of starch powders: Part 1: mathematical description of experimental data. J. Food Eng. 2004;61:297–307. doi: 10.1016/S0260-8774(03)00133-X. [DOI] [Google Scholar]
- Alpizar-Reyes E., Castaño J., Carrillo-Navas H., Alvarez-Ramírez J., Gallardo-Rivera R., Pérez-Alonso C., Guadarrama-Lezama A.Y. Thermodynamic sorption analysis and glass transition temperature of faba bean (Vicia faba L.) protein. J. Food Sci. Technol. 2018;55:935–943. doi: 10.1007/s13197-017-3001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntfield S., Murray E., Ismond M. The influence of processing parameters on food protein functionality III. Effect of moisture content on the thermal stability of fababean protein. Can. Inst. Food Sci. Technol. J. 1985;18:226–232. doi: 10.1016/S0315-5463(85)71920-7. [DOI] [Google Scholar]
- Baks T., Bruins M., Janssen A., Boom R. Effect of pressure and temperature on the gelatinization of starch at various starch concentrations. Biomacromolecules. 2008;9:296–304. doi: 10.1021/bm700814a. [DOI] [PubMed] [Google Scholar]
- Baks T., Ngene I., van Soest J., Janssen A., Boom R. Comparison of methods to determine the degree of gelatinisation for both high and low starch concentrations. Carbohydr. Polym. 2007;67:481–490. doi: 10.1016/j.carbpol.2006.06.016. [DOI] [Google Scholar]
- Barbosa-Cánovas G.V., Fontana A.J. Jr., Schmidt Shelly J., Labuza T.P., editors. Water Activity in Foods. 1 ed. Blackwell Publishing Ltd; 2007. [Google Scholar]
- Barker B.Y.H.A. The effect of water contetn upon the rate of heat denaturation of crystallizable egg albumin. J. Gen. Physiol. 1933;17:21–34. doi: 10.1085/jgp.17.1.21. URL: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucour P., Daudin J.D. Development of a new method for fast measurement of water sorption isotherms in the high humidity range. Validation on gelatine gel. J. Food Eng. 2000;44:97–107. doi: 10.1016/S0260-8774(99)00171-5. [DOI] [Google Scholar]
- Berghout J., Pelgrom P., Schutyser M., Boom R., van der Goot A. Sustainability assessment of oilseed fractionation processes: a case study on lupin seeds. J. Food Eng. 2015;150:117–124. doi: 10.1016/j.jfoodeng.2014.11.005. [DOI] [Google Scholar]
- Biliaderis C.G., Maurice T.J., Vose J.R. Starch gelatinization phenomena studied differential scanning calorimetry. J. Food Sci. 1980;45:1669–1674. https://doi-org.ezproxy.library.wur.nl/10.1111/j.1365-2621.1980.tb07586.x URL: [Google Scholar]
- Bot A., De Bruijne D.W. Osmotic properties of gluten. Cereal Chem. 2003;80:404–408. https://onlinelibrary.wiley.com/doi/abs/10.1094/CCHEM.2003.80.4.404 URL: [Google Scholar]
- Bühler J.M., Schlangen M., Möller A.C., Bruins M.E., van der Goot A.J. Starch in plant-based meat replacers: a new approach to using endogenous starch from cereals and legumes. Starch - Stärke. 2021 doi: 10.1002/star.202100157. [DOI] [Google Scholar]
- Clark A.H., Richardson R.K., Stubbs J.M. Structural and mechanical properties of agar/gelatin Co-gels. Small-deformation studies. Macromolecules. 1983;16:1367–1374. https://pubs-acs-org.ezproxy.library.wur.nl/doi/abs/10.1021/ma00242a019 URL: [Google Scholar]
- Copeland L., Blazek J., Salman H., Tang M.C. Form and functionality of starch. Food Hydrocolloids. 2009;23:1527–1534. doi: 10.1016/j.foodhyd.2008.09.016. [DOI] [Google Scholar]
- Cornet S., Snel S., Schreuders F., van der Sman R., Beyrer M., van der Goot A. Thermo-mechanical processing of plant proteins using shear cell and high-moisture extrusion cooking. Crit. Rev. Food Sci. Nutr. 2021:1–18. doi: 10.1080/10408398.2020.1864618. [DOI] [PubMed] [Google Scholar]
- Dekkers B.L., de Kort D., Grabowska K., Tian B., van As H., van der Goot A. A combined rheology and time domain NMR approach for determining water distributions in protein blends. Food Hydrocolloids. 2016;60:525–532. doi: 10.1016/j.foodhyd.2016.04.020. arXiv:arXiv:1011.1669v3. [DOI] [Google Scholar]
- Donovan J.W. Phase transitions of the starch–water system. Biopolymers. 1979;18:263–275. doi: 10.1002/bip.1979.360180204. [DOI] [Google Scholar]
- Eliasson A.C. Effect of protein on the gelatinization of starch. Starch Staerke. 1980;32:270–272. doi: 10.1002/star.19800320806. URL: [DOI] [Google Scholar]
- Eliasson A.C. Differential scanning calorimetry studies on wheat starch—gluten mixtures: I. Effect of gluten on the gelatinization of wheat starch. J. Cereal. Sci. 1983;1:199–205. doi: 10.1016/S0733-5210(83)80021-6. [DOI] [Google Scholar]
- Fernández-Gutiérrez J., San Martín-Martínez E., Martínez-Bustos F., Cruz-Orea A. Physicochemical properties of casein-starch interaction obtained by extrusion process. Starch - Stärke. 2004;56:190–198. doi: 10.1002/star.200300211. [DOI] [Google Scholar]
- Fitzsimons S.M., Mulvihill D.M., Ã E.R.M. Co-gels of whey protein isolate with crosslinked waxy maize starch: analysis of solvent partition and phase structure by polymer blending laws. Food Hydrocolloids. 2008;22:468–484. doi: 10.1016/j.foodhyd.2007.01.011. [DOI] [Google Scholar]
- van der Goot A., Pelgrom P., Berghout J., Geerts M., Jankowiak L., Hardt N., Keijer J., Schutyser M., Nikiforidis C., Boom R. Concepts for further sustainable production of foods. J. Food Eng. 2016;168:42–51. doi: 10.1016/j.jfoodeng.2015.07.010. [DOI] [Google Scholar]
- Grasso N., Roos Y., Crowley S., Arendt E., O'Mahony J. Composition and physicochemical properties of commercial plant-based block-style products as alternatives to cheese. Future Foods. 2021;4 doi: 10.1016/j.fufo.2021.100048. https://linkinghub.elsevier.com/retrieve/pii/S2666833521000381 [DOI] [Google Scholar]
- Grinberg V., Tolstoguzov V. Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocolloids. 1997;11:145–158. doi: 10.1016/S0268-005X(97)80022-7. [DOI] [Google Scholar]
- Haase N.U., Shi H.L. A characterization of faba bean starch (Vicia faba L.) Starch - Stärke. 1991;43:205–208. doi: 10.1002/star.19910430602. [DOI] [Google Scholar]
- Habeych E., Guo X., van Soest J., van der Goot A.J., Boom R. On the applicability of Flory-Huggins theory to ternary starch-water-solute systems. Carbohydr. Polym. 2009;77:703–712. doi: 10.1016/j.carbpol.2009.02.012. [DOI] [Google Scholar]
- Hoseney R.C., Zeleznak K.J., Yost D.A. A note on the gelatinization of starch. Starch - Stärke. 1986;38:407–409. doi: 10.1002/star.19860381203. [DOI] [Google Scholar]
- Kyriakopoulou K., Dekkers B., van der Goot A.J. Elsevier Inc; 2019. Plant-Based Meat Analogues. [DOI] [Google Scholar]
- Labuza T., Hyman C. Moisture migration and control in multi-domain foods. Trends Food Sci. Technol. 1987;9:47–55. [Google Scholar]
- Lomauro C., Bakshi A., Labuza T. Evaluation of food moisture sorption isotherm equations part I: fruit, vegetable and meat products. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1985;18:111–117. [Google Scholar]
- Menkov N.D. Moisture sorption isotherms of broad bean seeds at different temperatures. Food Nahrung. 2000;44:443–446. doi: 10.1002/1521-3803(20001201)44:6<443::AID-FOOD443>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Multari S., Stewart D., Russell W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015;14:511–522. doi: 10.1111/1541-4337.12146. [DOI] [Google Scholar]
- Palanisamy A., Deslandes F., Ramaioli M., Menut P., Plana-Fattori A., Flick D. Kinetic modelling of individual starch granules swelling. Food Struct. 2020;26 doi: 10.1016/j.foostr.2020.100150. [DOI] [Google Scholar]
- Peng G., Chen X., Wu W., Jiang X. Modeling of water sorption isotherm for corn starch. J. Food Eng. 2007;80:562–567. doi: 10.1016/j.jfoodeng.2006.04.063. [DOI] [Google Scholar]
- Schutyser M., Pelgrom P., van der Goot A., Boom R. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015;45:327–335. doi: 10.1016/j.tifs.2015.04.013. http://linkinghub.elsevier.com/retrieve/pii/S092422441500120X URL: [DOI] [Google Scholar]
- Shrinivas P., Kasapis S. Morphology and mechanical properties of bicontinuous gels of agarose and gelatin and the effect of added lipid phase. Langmuir. 2009;25:8763–8773. doi: 10.1021/la9002127. [DOI] [PubMed] [Google Scholar]
- van der Sman R., Meinders M. Prediction of the state diagram of starch water mixtures using the Flory-Huggins free volume theory. Soft Matter. 2011;7:429–442. doi: 10.1039/c0sm00280a. [DOI] [Google Scholar]
- Tananuwong K., Reid D.S. DSC and NMR relaxation studies of starch – water interactions during gelatinization. Carbohydr. Polym. 2004;58:345–358. doi: 10.1016/j.carbpol.2004.08.003. [DOI] [Google Scholar]
- Tolstoguzov V. Thermodynamic aspects of dough formation and functionality. Food Hydrocolloids. 1997;11:181–193. doi: 10.1016/S0268-005X(97)80025-2. [DOI] [Google Scholar]
- Tolstoguzov V. Texturising by phase separation. Biotechnol. Adv. 2006;24:626–628. doi: 10.1016/j.biotechadv.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vogelsang-O’Dwyer M., Bez J., Petersen I.L., Joehnke M.S., Sørensen J.C., Detzel A., Busch M., Krueger M., O'Mahony J.A., Arendt E.K., Zannini E. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: techno-functional, nutritional and environmental performance. Foods. 2020;9:1–25. doi: 10.3390/foods9030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M., Nam S., Pettee B., Carpenter C. Characterization of texturized whey protein produced at varying protein concentrations. J. Food Process. Preserv. 2008;32:503–516. doi: 10.1111/j.1745-4549.2008.00194.x. [DOI] [Google Scholar]
- Wootton M., Bamunuarachchi A. Application of differential scanning calorimetry to starch gelatinization. III. Effect of sucrose and sodium chloride. Starch Staerke. 1980;32:126–129. doi: 10.1002/star.19800320408. [DOI] [Google Scholar]
- Xu M., Jin Z., Simsek S., Hall C., Rao J., Chen B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019;295:579–587. doi: 10.1016/j.foodchem.2019.05.167. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li S., Zhang B., Drago S., Zhang J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016;174:29–36. doi: 10.1016/j.jfoodeng.2015.11.011. URL: [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.