Abstract
Shared decision making is important when deciding the appropriateness of dialysis for any individual, particularly for older patients with advanced chronic kidney disease who have high mortality. Emerging evidence suggests that patients with advanced age, high comorbidity burden, and poor functional status may not have any survival advantage on dialysis compared with those on a conservative kidney management pathway. The purpose of this narrative review is to summarize the existing studies on the survival of older patients with stage 4 or 5 chronic kidney disease managed with or without dialysis and to evaluate the factors that may influence mortality in an effort to assist clinicians with shared decision making. Median survival estimates of conservative kidney management patients are widely varied, ranging from 1-45 months with 1-year survival rates of 29%-82%, making it challenging to provide consistent advice to patients. In existing cohort studies, the selected group of patients on dialysis generally survives longer than the conservative kidney management cohort. However, in patients with advanced age (aged ≥80 years), high comorbidity burden, and poor functional status, the survival benefit conferred by dialysis is no longer present. There is an overall paucity of data, and the variability in outcomes reflect the heterogeneity of the existing studies; further prospective studies are urgently needed.
Index Words: Conservative kidney management, dialysis, elderly, kidney failure, renal failure, shared decision making, survival
Background
Medical advancement opens the doors to new treatment opportunities but may come with its own detriments. Dialysis is one such medical technology that has revolutionized the field of nephrology. In the 1970s, when the Medicare End-Stage Renal Disease Program was funded in the United States, the cohort of patients offered dialysis represented the youngest, fittest, most educated, and highly motivated subset of the kidney failure population.1 Although this pattern may still be true in many parts of the developing world, the demographics of the dialysis population in high-income countries has dramatically shifted over the last few decades since the reach of dialysis expanded to an older and more comorbid chronic kidney disease (CKD) population. Currently, the elderly comprises the fastest-growing group of patients on dialysis in the developed world. In Australia, the highest prevalent population of patients receiving dialysis in 2019 was the group with ages 75-84 years, with close to 2,500 patients per million population.2 This experience is not unique to Australia. Globally, the number of elderly patients initiated on dialysis has continued to rise3 and the overall number of patients on maintenance dialysis has increased, with the elderly sector demonstrating the most rapid rate of growth.4 In the United States, patients aged 65 years or older constituted half of the US dialysis population by 2007, and the numbers have only continued to rise.5 This trend is also reflected in other areas of the industrialized world,5 including Canada,6 the United Kingdom,7 other parts of Europe,8 and Asia.9
Reasons for the rising incidence of elderly patients on dialysis include more relaxed criteria for acceptance onto dialysis and increased life expectancy from other comorbidities such as obesity, diabetes, hypertension, and vascular disease in an aging population.10 However, the mortality rate of patients on dialysis is high, and self-reported quality of life and satisfaction often decreases significantly after dialysis initiation.11,12 The elderly have an additional increased morbidity and mortality risk on dialysis,13 are frailer, and may have differing medical care needs and goals in comparison to their younger counterparts. Moreover, patients with advanced kidney disease generally have a high burden of symptoms impacting their quality of life, many of which are not necessarily alleviated by renal replacement therapy.14,15
The annual mortality rates of patients on dialysis exceed 10%, and withdrawal from dialysis is a common cause of death globally,10 reflecting the poor health-related quality of life of patients on dialysis.
In 2000, the Renal Physician’s Association of the United States released guidelines that endorsed shared decision making and encouraged clinicians to discuss the various treatment options for kidney failure with patients and their families to reach a joint decision regarding the appropriateness of dialysis.16 Although studies have shown that patients more engaged in the shared decision-making process have higher treatment satisfaction, it remains underutilized in practice.17
Conservative kidney management (CKM) is a nondialysis treatment pathway for patients with kidney failure that focuses on improving quality of life, addressing symptom burden and advanced care planning.18 It also includes active management of kidney disease and its associated symptoms without the use of dialysis, with a goal to slow the progression of kidney failure and to medically treat complications that may arise. CKM is an alternative treatment pathway to dialysis for the patients who choose not to be dialyzed or for those who are unable to because of resource constraints.19
Discussions around choosing CKM are challenging because there is limited literature regarding illness trajectories, prognosis, symptom burden, and quality of life of patients with kidney failure managed without dialysis. The aim of this review is to examine the existing evidence on the survival of older patients with advanced CKD managed without dialysis with comment on several significant papers in the hope that this may provide greater information to help with these discussions. At the same time, we sought to highlight the variability among studies that may preclude overall confidence in imparting this information to patients and their families.
Literature Search
Studies were sequentially screened from the MEDLINE, Embase, PubMed, University of Sydney Library, and Cochrane Library databases with the inclusion criteria being the studies that included patients aged ≥65 years, with CKD stage 4 or 5, managed on a CKM pathway, with or without a dialysis comparator cohort (Item S1). The primary outcome of interest was survival. The studies that included acute kidney injury, kidney transplantation recipients, or pediatric patients were excluded from the review. All study designs including secondary literature were screened. A formal systematic review and meta-analysis were not undertaken because of the known heterogeneous nature of these studies.
Survival with CKM
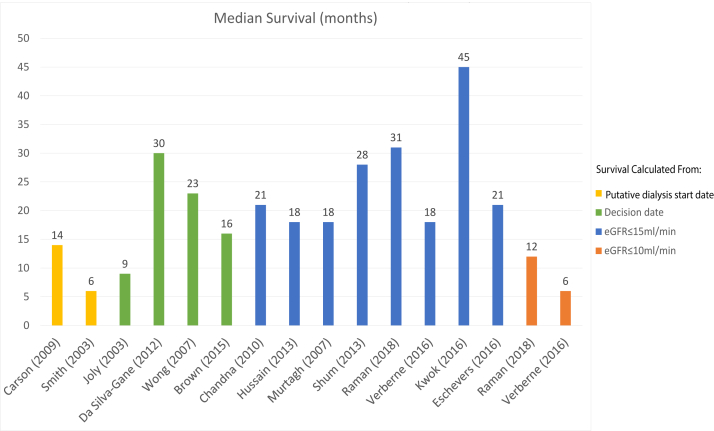
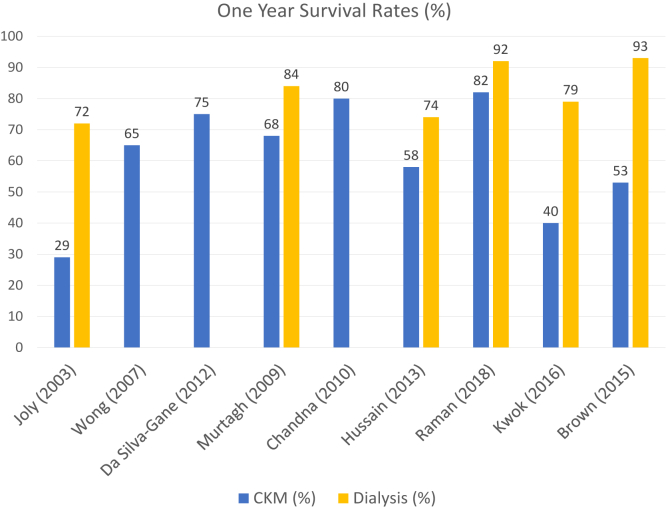
The median survival of older CKM patients ranged from 1-45 months and 1-year survival from 29%-82% (Tables 1 and 2, Figs 1 and 2). There was a high degree of heterogeneity in the existing studies, which was evident from the disparity in age and comorbidity inclusion criteria and the variability in the chosen start dates from which survival was calculated.
Table 1.
Characteristics of Included Studies
| Author | Year of Publication | Total Patient Number | No. of CKM Patients | No. of Dialysis Patients | Country | Age Inclusion (y) | Median Age CKM (y) | Median Age Dialysis (y) | Study Design | Comorbidity Scale | Measure of Overall Comorbidity | SDM | KSC Input |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Joly20 | 2003 | 144 | 37 | 107 | France | ≥80 | 84.1a | 83a | Retrospective | Own | 32.4% ≥3 comorbidities | Yes | Unclear |
| Smith30 | 2003 | 321 | 34 | 196 | England | All | 71 | 59 | Retrospective | Own | Mean comorbidity scoreb 4.7 (SD 3) | Yes | Yes |
| Murtagh21 | 2007 | 129 | 77 | 52 | England | ≥75 | 83 | Retrospective | Davies | 18.2% Davies grade 2 score | Yes | Unclear | |
| Carson29 | 2009 | 202 | 29 | 173 | England | ≥70 | 83 | 82 | Prospective | CCI | Mean CCI 13.7 | Yes | Unclear |
| Ellam48 | 2009 | 69 | 69 | 0 | England | 80 | N/A | Retrospective | SCG | Stoke's comorbidity score 2 = 24%-28% | Yes | Unclear | |
| Wong23 | 2007 | 73 | 73 | 0 | England | All | 79 | Prospective | SCG | Stoke's comorbidity grade=1 | Yes | Yes | |
| Chandna24 | 2011 | 844 | 106 | 689 | England | All | 81 | Retrospective | Own | Comorbidity scoreb > 4 in 50.9% | Yes | Yes | |
| Da Silva-Gane11 | 2012 | 170 | 30 | 124 | England | All | 77.5a | 83 (HD), 78 (PD) | Prospective | Own | Comorbidity score >3 in 74% | Yes | Yes |
| Hussain33 | 2013 | 441 | 172 | 269 | England | NR, enrolled >70 years | NR | Prospective | NR | NR | Yes | Yes | |
| Seow26 | 2013 | 101 | 63 | 38 | Singapore | ≥75 | 78 | 60 | Prospective | CCI | Mean CCI 5 | Unclear | Unclear |
| Shum25 | 2013 | 199 | 42 | 157 | Hong Kong | ≥65 | 75.3a | 73a | Retrospective | CCI | Mean CCI 4.6 | Yes | Yes |
| Brown35 | 2015 | 467 | 122 | 345 | Australia | All | 82 | 67 | Prospective | CCI | 57% had ≥2 comorbidities | Yes | Yes |
| Kwok27 | 2016 | 558 | 432 | 126 | Hong Kong | ≥65 | 80a | 78a | Retrospective | CCI | CCI 9 | Unclear | Unclear |
| Echevers22 | 2016 | 314 | 93 | 69 | Spain | ≥70 | 78 | 76 | Retrospective | CCI | CCI 8 | Unclear | Unclear |
| Verberne31 | 2016 | 311 | 107 | 204 | Netherlands | >70 | 83a | 76a | Retrospective | Davies | Davies grade ≥3 | Yes | Yes |
| Reindl-Schwaighofer42 | 2017 | 1018 | 174 | 844 | Austria | ≥65 | 81 | 74 | Retrospective | NR | |||
| Raman32 | 2018 | 204 | 81 | 123 | United Kingdom | ≥75 | 84 | 79 | Prospective | NR | N/A | Unclear | |
| Tam-Tham34 | 2018 | 838 | 338 | 500 | Canada | ≥65 | 83a | 76a | Retrospective | NR | NR | Unclear | Unclear |
Note: Measures of overall comorbidity –CCI, Charlson Comorbidity Index; Davies, Davies Score; None, comorbidities not reported; Own: Author utilized own nonvalidated method of reporting; SCG, Stoke's Comorbidity Grade.
Abbreviations: CKM, Conservative kidney management; HD, haemodialysis; KSC, kidney supportive care; N/A, not applicable; NR, not reported; PD, peritoneal dialysis; SDM, shared decision making.
Mean age
Comorbidity score: cardiovascular disease, peripheral vascular disease, respiratory disease.
Table 2.
Survival Analyses for Included Studies
| Author | Starting Point of Survival Analysis | Median Survival CKM | IQR | CKM 1-Year Survival (%) | Dialysis 1-Year Survival (%) | CKM 2-Year Survival (%) | Dialysis 2-Year Survival (%) | Median Survival Dialysis | IQR | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Joly20 | Decision date | 9 | 95% CI, 4-10 | 29 | 74 | 15 | 60 | 29 | 95% CI, 24-38 | High number of late presentations in CKM group that may have worsened survival outcomes Long follow-up time of 12 years |
| Smith30 | Putative dialysis start date | 6 | - | - | - | - | - | 8 | - | |
| Murtagh21 | eGFR < 15 | 18 | 0.1-73.1 | 68 | 84 | 47 | 76 | N/A | - | Excluded late presentations 30% of patients in dialysis group did not progress to needing dialysis |
| Carson29 | Threshold eGFR for dialysis initiation based on dialysis cohort (10.8 mL/min/1.73 m2) | 14 | 2-44 | NR | NR | NR | NR | 38 | - | Small numbers of CKM Large numbers of late presentations and emergency-start dialysis patients |
| Ellam48 | eGFR <15 mL/min/1.73 m2 | 21 | 1-100 | - | No dialysis cohort for comparison | |||||
| Wong23 | Decision date | 23 | 65 | N/A | N/A | N/A | N/A | - | No dialysis patients were included in study | |
| Chandna24 | Date of first eGFR 10-15 mL/min/1.73 m2 and subsequently eGFR <15 mL/min/1.73 m2 | 21 | - | 80.2 | - | - | - | 67 | - | No data on functional status |
| Da Silva-Gane11 | Study enrollment, late stage 4/5 CKD attending clinic | 30 | - | 75 | - | - | - | - | - | |
| Hussain33 | eGFR <20, eGFR <15, eGFR <12 | 18 | - | 58 | 72 | 20 | 46 | 38 | - | Complete outcome reporting and no loss to follow-up |
| Seow26 | Study enrollment, eGFR 8-12 | NR | - | NR | - | 62 | - | - | - | Mainly a study dedicated for health-related quality of life outcomes |
| Shum25 | eGFR < 15 | 28 | 14-45 | 80.7 | - | - | - | 45 | 30-63 | Comparison of CKM and Peritoneal dialysis patients |
| Brown35 | Decision date | 16 | (7-39) | 53 | 93 | - | - | 33a | 95% CI, 32-34 | |
| Kwok27 | eGFR <15 mL/min/1.73 m2 | 45 | 95% CI 37.3-51.9 | 40 | 79 | 13 | 54 | 10 | 95% CI, 8.3-11.7 | Limited to patients who were referred specifically for advanced care planning |
| Echevers22 | eGFR <15 mL/min/1.73 m2 | 21 | (7-42) | - | - | - | - | 46 | 27-62 | Patient decision was not documented and was not used to distinguish comparator cohorts. There may have been patients analyzed in the “CKM” group who had simply not progressed to needing dialysis |
| Verberne31 | Decision date | 18 | 8.4-3.6 | - | - | - | - | 37 | 18-82.8 | No data on functional and nutritional status |
| Verberne31 | eGFR <20 mL/min/1.73 m2 | 29 | - | - | - | - | - | 54 | - | - |
| Verberne31 | eGFR <15 mL/min/1.73 m2 | 18 | - | - | - | - | - | 37 | - | - |
| Reindl-Schwaighofer42 | eGFR <10 mL/min/1.73 m2 | 6 | - | - | - | - | - | 33 | - | - |
| Raman32 | eGFR <10 mL/min/1.73 m2 | 1 | 95% CI 0.4-10.8 | - | - | - | - | 27 | 95% CI, 26-28 | Very high mortality in CKM group compared to other studies. Likely related to indication bias and lead time bias |
| Raman32 | eGFR <15 mL/min/1.73 m2 | 31 | 21-41 | 82 | 92 | N/A | N/A | 42 | 33-50 | Excluded comorbidities: NYHA3/4 heart failure, previous cardiac arrest, Solid organ malignancy, Karnofsky performance score <60, dementia High uptake of CKM (48%) |
| Brown35 | eGFR <15 mL/min/1.73 m2 | 13a | 95% CI 9-16 | - | - | - | - | 20a | 95% CI, 19-20 | - |
| Tam-Tham34 | eGFR <10 mL/min/1.73 m2 | NR | - | - | - | - | - | - | - | Registry trial. Dialysis was associated with lower mortality in the first 3 years, HR 0.59 [95% CI 0.46-0.77]. Median survival and 1-year survival rates not reported |
Note: Measures of overall comorbidity –CCI, Charlson Comorbidity Index; Davies, Davies Score; None, Comorbidities not reported; Own: Author utilized own nonvalidated method of reporting; SCG, Stoke's Comorbidity Grade.
Abbreviations: CKM, conservative kidney management; KSC, kidney supportive care; N/A, not applicable; NR, not reported; NYHA, New York Heart Association Classification; SDM, shared decision making.
a Mean survival (months).
Figure 1.
Median survival of conservative kidney management patients.
Figure 2.
Reported 1-year survival rates for conservative kidney management (CKM) patients.
Joly et al20 reported the survival outcomes of 144 patients, including 37 CKM patients, aged 80 years or older in a retrospective analysis that spanned 12 years and found that the median survival of CKM patients from the decision of treatment pathway was 9 months (95% confidence interval [CI], 4-10) compared with 29 (95% CI, 24-38) months in a dialysis cohort. Although there was a multidisciplinary shared decision-making team, the decision for dialysis was more “asymmetrical than shared”,21 whereby the decision was mainly made by the treating physician and the patient was given the option to either refuse or accept the proposed pathway. Six out of the 107 patients who were offered dialysis declined. CKM patients were found to have a lower Karnofsky score (55 ± 18 vs 63 ± 20, P = 0.03), were more often socially isolated (P = 0.03), and had a higher proportion of late referrals (P = 0.01) and diabetes (P = 0.008). Strengths of this study included its complete outcome reporting and long follow-up time.
Murtagh et al21 conducted a retrospective observational cohort study examining the survival of the patients older than 75 years with kidney failure who received multidisciplinary predialysis care and had chosen a treatment pathway with shared decision making. CKM patients had a median survival of 18 months (interquartile range [IQR], 0.1-73.1). Strengths of this study include the balanced representation of both the CKM (n = 77) and dialysis (n = 52) cohorts who had similar baseline comorbidity scores. One-year survival was 68% in the CKM group compared with 84% in the dialysis group; the dialysis cohort had 2.9-fold better survival (P < 0.001). An important finding in this study was that the survival advantage offered by dialysis was lost in patients with a Davies comorbidity grade of 2 or higher. Age, overall comorbidity score, and ischemic heart disease alone significantly impacted survival. The presence of ischemic heart disease had a stronger impact on mortality than overall comorbidity grade. However, not all the studies evaluating ischemic heart disease have found it to be a significant predictor of mortality.22
Wong et al23 similarly found that comorbidity was an independent prognostic factor for survival, as defined by the Stoke’s Comorbidity Grade (SCG) in a prospective analysis of 73 CKM patients conducted in England over a period of 3 years. With increment in the SCG, the hazard ratio for mortality was 2.53 (95% CI, 1.32-4.83; P = 0.005). The 1-year survival rates were 83% for SCG 0, 70% for SCG 1, and 56% for SCG 2. The overall median survival was 23 months with overall 1-year survival being 65%.
In a retrospective study of 106 CKM and 844 elderly patients on dialysis conducted in England, Chandna et al24 found that median survival from study enrollment was 21 months in the CKM group versus 67 months in the dialysis cohort (P < 0.001). However, in an adjusted Cox proportional hazards model, whereby patients aged >75 years in both the groups were adjusted in terms of age, comorbidities, and presence of diabetes, the survival advantage conferred by dialysis was less than 4 months and was not statistically significant (P = 0.83).
In a prospective cohort study including 124 CKM patients, Da Silva et al11 reported median survival from the time of decision of modality choice when mean estimated glomerular filtration rate (eGFR) was 14 mL/min/1.73 m2 as 30 months in the CKM group and 44 months in their dialysis cohort (P < 0.001). The mortality risk was almost halved with dialysis in the Cox models adjusted for comorbidity, Karnofsky performance score, age, physical health component (SF-36), and propensity scores (hazard ratio, 0.47; 95% CI, 0.20-1.10; P = 0.08). CKM patients were older and had higher levels of comorbidity and dependence. This study utilized propensity scores to adjust for selection bias, albeit confounders likely remained given the notable demographic differences in the baseline characteristics of the patients in the comparator groups.
Studies From Asia
The initial studies on CKM patients were predominantly conducted in England. Around 2013, the first published studies conducted in Asia revealed similar trends. Shum et al25 studied a retrospective cohort of 42 CKM patients and 157 patients on peritoneal dialysis in Hong Kong over a period of 7 years. The peritoneal dialysis group had longer median survival (45 months [IQR, 30-63] vs 28 months [IQR, 14-45]; P = 0.03). Independent predictors of mortality included age, comorbidity burden, functional impairment, and requirement of emergency dialysis. The survival advantage of peritoneal dialysis was no longer present if there was a high comorbidity score or functional impairment.
In a prospective observational study of 63 CKM patients, aged ≥75 years or with age-adjusted Charlson Comorbidity Index ≥8, with a median eGFR of 10 mL/min/1.73 m2, 2-year survival was estimated to be 62%.26 Following this, Kwok et al27 conducted a retrospective registry trial in Hong Kong on 558 (including 432 CKM) patients aged ≥65 years with kidney failure who were referred for advanced care planning. Survival was calculated from the time of advanced care planning. CKM patients had higher comorbidity burden and poorer functional status than the dialysis cohort, and these were independent predictive factors of mortality. Patients on dialysis survived a median of 45 months (95% CI, 37-52) versus 10 months (95% CI, 8-12) in the CKM cohort (P < 0.001). In subgroup analyses, the survival advantage with dialysis was lost in patients ≥85 years and those with high Charlson Comorbidity Index (>11) and poor functional status (defined in this study as being chair or bed-bound). Notably, this study’s generalizability is limited given that these were a specific group of patients from a single center who were referred for the purposes of advanced care planning and had already been identified as a group who would likely have a higher risk of mortality.
Studies With Multiple Start Points for Survival Analyses
A major criticism of observational survival studies is that of lead time bias, whereby the time point from which survival is calculated may be variable, leading to heterogeneity of the results and affecting outcomes. Lead time bias provides the illusion of longer survival when the diagnosis is identified earlier.28 Commonly utilized time points for survival calculations are eGFR <15 mL/min/1.73 m2, indicating entry into end-stage kidney disease or time of decision regarding treatment modality.
The challenge of addressing lead time bias is difficult, and completely removing it from an observational study may prove impossible; nonetheless, some studies have used differing techniques in an effort to limit its impact. Carson et al,29 in their prospective analysis of 202 (including 29 CKM) patients, utilized a “putative dialysis start date” in the CKM cohort by performing a Cox regression analysis of eGFR in the dialysis cohort to calculate a “threshold eGFR” for the date of dialysis commencement, which was found to be 10.8 mL/min/1.73 m2. There was a high number of late referrals (17%) and emergent-start dialysis (30%) in the dialysis cohort.29 Median survival was 38 versus 14 months for dialysis versus CKM patients (P < 0.01), and subgroup analyses dividing the dialysis cohort into emergency referrals still demonstrated that dialysis overall conferred a survival benefit.
Smith et al30 similarly used a putative dialysis start date in a retrospective study of 321 patients, of whom 34 chose a CKM pathway. CKM patients were older and had impaired function and diabetes. In this study, comorbidity score was not found to be a significant predictor of mortality. This study did not report the overall median survival but focused on comparing a subgroup of 10 patients who were initially recommended for conservative management by their physicians but opted for dialysis and were initiated on dialysis during the study. These patients had a median survival of 8.3 months compared with 6.3 months in the CKM cohort, and the difference was not statistically different.
Other methods employed to address lead time bias include using time-varying analyses and multiple differing start points for survival analyses. Verberne et al,31 in their retrospective observational study of 107 CKM patients and 204 dialysis patients aged >70 years, used multiple time points to estimate survival at different stages of the disease. It was found that from all time points, patients on dialysis survived longer the CKM patients (P < 0.001), but this survival advantage was lost in patients ≥80 years of age. Independent factors that were significant predictors of survival were age and Davies comorbidity score.
Raman et al,32 in their trial of 204 (including 81 CKM) patients aged ≥75 years, which deliberately excluded patients with comorbidities likely to reduce life expectancy in order to minimize selection bias, likewise found that dialysis conferred a survival advantage from all time points, albeit this benefit was no longer present in those aged 85 years or older. Median survival of CKM patients compared with patients on dialysis were 31 (IQR, 21-41) versus 42 (IQR, 33-50) months and 12 (IQR, 0-5) versus 36 (IQR, 25-47) months when eGFR reached ≤15 and 10 mL/min/1.73 m2, respectively. The adjusted hazard ratio for death in the dialysis group compared with CKM when eGFR reached 10 mL/min/1.73 m2 was 0.36 (95% CI 0.21-0.62, P < 0.001). Despite this study design, there may still have been an element of hidden selection bias as the CKM cohort were older, more likely to live alone, and had a higher prevalence of peripheral vascular disease.
Hussain et al33 studied 441 (including 172 CKM) patients and found median survival from eGFR <20 mL/min/1.73 m2 was 2.2 years in CKM versus 4.6 years in patients on dialysis (P < 0.001). Dialysis also conferred a survival advantage from subsequent time points of eGFR <15 mL/min/1.73 m2 and <12 mL/min/1.73 m2 (P < 0.001). However, there was no difference in survival in very elderly patients aged >80 years or in patients aged >70 years combined with poor functional status (defined as World Health Organization performance score of ≥3), and the survival benefit was substantially reduced in patients aged >70 years with high Charlson Comorbidity Index scores.
A retrospective cohort study performed in Canada utilized time-varying analyses on the registry data of 838 patients aged ≥65 years, which included 338 CKM patients.34 Patients were enrolled in the study if they had a series of ≥2 consecutive eGFR measurements <10 mL/min/1.73 m2 spanning at least 90 days. Survival was calculated from the first date, and those who had initiated dialysis prior to this were excluded. These authors found that dialysis was associated with lower mortality risk in the first 3 years (hazard ratio, 0.59; 95% CI, 0.46-0.77), regardless of age or comorbidity burden. Strengths of this registry trial include a robust design in its sensitivity analyses, whereby late referrals were excluded and methods to limit lead time and immortal time biases were employed. However, there may still have been an element of lead time bias given that the point in which eGFR first fell <10 mL/min/1.73 m2 may have potentially not been recorded.
Brown et al35 studied 345 patients on dialysis and 122 CKM patients who were supported with a multidisciplinary kidney supportive care program, and shared decision making was actively practiced. Survival was calculated from the decision date and from eGFR <15 mL/min/1.73 m2. Mean survival was longer in the dialysis cohort from both time of decision (33 [95% CI, 32-34] vs 20 months [95% CI, 17-23; P < 0.001) and eGFR <15 mL/min/1.7 3 m2 (20 [95% CI, 19-21] vs 13 months [95% CI, 9-16]; P < 0.001). It was found that the survival benefit conferred by dialysis was no longer present in patients aged ≥75 years with at least 2 comorbidities.
Factors Associated With Loss of Survival Advantage
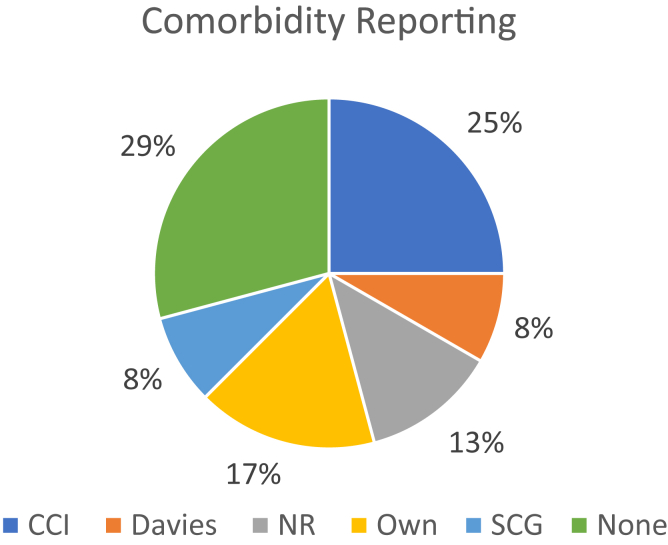
Several studies examined the effects of different predictive factors on survival, albeit there was again a high degree of heterogeneity, which makes direct comparisons difficult, and not all studies performed subgroup analyses to test the effect of these variables on survival outcomes. Studies reported on comorbidity using various scales (Fig 3). Ten out of 18 studies (56%) reported on the effect of comorbidity, and 4 of these demonstrated a loss of survival advantage offered by dialysis in the presence of high comorbidity burden.21,24,25,27 Some studies showed that high comorbidity reduced survival substantially but found that patients still lived longer on dialysis compared to CKM.20,33
Figure 3.
Comorbidity reporting. Comorbidity scales utilized: CCI, Charlson Comorbidity Index; Davies, Davies Score; NR, comorbidities not reported; Own, Author utilized own nonvalidated method of reporting; SCG, Stoke’s Comorbidity Grade.
Advanced age was found to be associated with the loss of a survival benefit in the dialysis cohort in 5 studies (29%).22,27,31, 32, 33 Only 3 of 18 studies (17%) found that dialysis still showed a statistically significant survival advantage compared with CKM in the population of patients with advanced age (for example, ≥80 or 85 years).20,22,31,35 A few studies demonstrated that in a subcohort of patients with both advanced age and high comorbidity, there was no longer a survival benefit conferred by dialysis.27,31,35 Kwok et al27 and Hussain et al33 also showed that this was true when combining advanced age and poor functional status.
Functional status is another important factor that impacts a patient’s quality of life and is a surrogate marker of frailty in the elderly population.36 Only 5 of the 18 (28%) studies reported on the effect of functional status on survival outcomes. Three of these studies found that patients with poor functional status (defined by either World Health Organization Score >3 or other nonvalidated grading systems) no longer had significant survival benefit from dialysis treatment.25,27,33
Nursing home patients initiated on dialysis continued to have a continued decline in their functional status despite treatment with kidney replacement therapy.37,38 Kurella et al37 conducted a registry trial (n = 7,054) on patients aged ≥80 years and confirmed that advanced age, nonambulatory status, and high comorbidity were associated with higher risk of mortality. One-year mortality for octogenarians was 46% and median survival was 25 months (IQR, 8-52) after dialysis initiation.37 Following this, a further registry trial was conducted, which included 3,702 nursing home residents in the United States, which showed that predialysis functional status, as defined by the Minimum Data Set-Activities of Daily Living scale, was only maintained in 13% of patients after 1 year of dialysis treatment. Initiation of dialysis was associated with a sharp decline in function, independent of age.38
Limitations in Current Knowledge
There is paucity of data on the survival of CKM patients, and the existing body of studies is heterogeneous, with large survival estimates ranging between 1 month and 45 months without dialysis. However, emerging themes are that survival advantage with dialysis may be lost in the very elderly and in highly comorbid patients.33,39
An inherent limitation of the existing observational data is selection bias, whereby those who choose a conservative nondialysis pathway usually have a higher burden of comorbidity, frailty, or other factors that impact survival. The only definitive method to overcome these inherent biases would be to conduct a randomized controlled trial, which is underway.40
Lead time bias is a major limitation in these survival observational studies in the measurement of outcomes (Table 3).41 Many of the studies chose the decision date as the starting point for calculating survival. However, we know from clinical practice that this decision date may be vastly variable in a patient’s illness trajectory and some patients may never deteriorate to the point of requiring dialysis. This was the case for 16 of 107 patients in a study by Murtagh et al,21 in which the patients decided on a dialysis pathway but remained clinically stable. These patients will likely have a longer survival and have important implications on the overall results. Conversely, if there are a high number of late presentations, such as in the analysis by Joly et al,20 survival outcomes will appear worse. Clinical variability in eGFR may pose additional bias in the studies that calculate survival from eGFR time points; for instance, frail elderly patients who lose muscle mass may appear to have preserved kidney function because of lower serum creatinine levels despite their actual function deteriorating over time. Information regarding the rate of progression of CKD was not available in the majority of studies, apart from the retrospective analysis by Echevers et al.22 Moreover, in a number of retrospective trials, the decision for dialysis or CKM may not have been clearly documented, and patients may have been included in the CKM treatment arm if their kidney function simply did not deteriorate to the point of requiring dialysis, resulting in bias in classification of intervention.22,34,42 Furthermore, there may be clinician-driven indication bias whereby the way in which shared decision-making discussions are conducted, and the availability of kidney supportive care support may strongly influence a patient’s modality choice. Once again, there is large heterogeneity between studies with no standardized shared decision-making approach and variability in kidney supportive care availability.
Table 3.
Study Bias of Included Studies
| Author | Year of Publication | Biasa |
||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection | Classification of Intervention | Deviation from Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Result | ||
| Joly20 | 2003 | High | High | Low | Low | Low | High | Low |
| Smith30 | 2003 | High | High | Low | Moderate | Low | High | Moderate |
| Murtagh21 | 2007 | High | High | Low | Low | Low | High | Low |
| Carson29 | 2009 | High | High | Low | Moderate | Low | Moderate | Low |
| Ellam | 2009 | High | High | Low | Low | Low | High | Low |
| Wong23 | 2009 | High | High | Low | Low | NR | High | Low |
| Chandna24 | 2011 | High | High | Low | Low | Low | High | Low |
| Da Silva-Gane11 | 2012 | High | High | Low | Low | Low | High | Low |
| Hussain33 | 2013 | High | High | Low | Moderate | Low | Moderate | Low |
| Seow26 | 2013 | High | High | Low | Low | Low | High | Low |
| Shum25 | 2013 | High | High | Low | Low | Low | High | Low |
| Brown35 | 2015 | High | High | Low | Low | Low | Moderate | Low |
| Verberne31 | 2016 | High | High | Low | Low | Low | Moderate | Low |
| Kwok27 | 2016 | High | High | Low | Moderate | Low | High | Low |
| Echevers22 | 2016 | High | High | Moderate | Low | NR | High | Low |
| Verberne31 | 2016 | High | High | Low | Low | Low | Moderate | Low |
| Reindl-Schwaighofer42 | 2017 | High | High | Moderate | Low | NR | High | Low |
| Raman32 | 2018 | Moderate | High | Low | Low | Moderate | High | Low |
| Tam-Tham34 | 2018 | High | High | Moderate | Low | Low | Moderate | Low |
Note: Definitions of Domains of Bias according to ROBINS-I44
Confounding of intervention effects occurs when one or more prognostic factors (factors that predict the outcome of interest) also predict whether an individual receives one or the other intervention of interest.
Selection: When exclusion of some eligible participants, or the initial follow-up time of some participants, or some outcome events, is related to both intervention and outcome, there will be an association between interventions and outcome even if the effects of the interventions are identical.
Classification of intervention: Bias introduced by either differential or non-differential misclassification of intervention status.
Deviation from intervention: Bias that arises when there are systematic differences between experimental intervention and comparator groups in the care provided, which represent a deviation from the intended intervention(s).
Missing data: Bias that arises when later follow-up is missing for individuals initially included and followed (eg. differential loss to follow-up that is affected by prognostic factors); bias due to exclusion of individuals with missing information about intervention status or other variables such as confounders.
Measurement of outcomes: Bias introduced by either differential or non-differential errors in measurement of outcome data. Such bias can arise when outcome assessors are aware of intervention status, if different methods are used to assess outcomes in different intervention groups, or if measurement errors are related to intervention status or effects.
Selection of reported result: Selective reporting of results in a way that depends on the findings.
ROBINS-I Tool Risk of Bias Assessment (2016)
The studies included were conducted in high-income nations, where the patients who chose CKM were presumably not limited by choice because of financial or dialysis-access constraints, albeit this has not been explicitly stated within the articles.
Prediction Tools
There are a number of existing predictive tools for the estimation of mortality on dialysis, such as the Cohen,43 Ivory,44 and Schmidt45 models, which may also be useful in identifying the patients who may benefit from early palliative care input. However, the clinical utility of these tools may be limited in the elderly CKD population who have not yet decided on a treatment pathway.
Patient Preferences
An issue separate from survival but important to patients and to shared decision-making discussions is that patients on dialysis also have a higher number of days spent in hospital, including intensive care stays,23,29, or have a progressive decline in functional status after dialysis initiation.38 Qualitative studies suggest that many patients may choose quality of life over quantity.46 A recent systematic review found that CKM may be associated with improved quality of life and lower symptom burden and hospitalization.47 Of note, the intended purpose of this review is not so much to make a direct head-to-head comparison of dialysis versus CKM but rather to illustrate the potential expected life trajectory of an older individual with advanced CKD managed either conservatively or with a dialysis pathway. It is clear that not all patients are suitable for or will benefit from kidney replacement therapy, particularly in those who are frail or highly comorbid. In our Australian experience, we have found that these selected patients can achieve a reasonable quality of life and survive a number of months to years on a supportive care pathway while being managed through an integrated multidisciplinary kidney supportive care program.35 In essence, patients trade off the way one stays alive by one treatment compared with another. Regardless of the choice, one assumes the patients and families start with a desire for the longest life that can be offered at that particular standard of living. Our hope is that this review will assist clinicians in shared decision-making discussions to provide more clarity regarding the factors that reduce survival and prompt more prospective studies to increase our understanding in this area.
Conclusion
International guidelines advocate for shared decision making regarding the appropriateness of dialysis for an individual. In this process, patients and their families desire knowledge of their expected prognosis and illness trajectory if managed by dialysis or conservative nondialysis management. There is emerging evidence from the literature described here that very elderly patients, such as those aged ≥80 years or those with high comorbidity and poor functional status, no longer have survival advantage with dialysis treatment. The older patients who choose not to be dialyzed can still expect to live a number of months to years from the time of their decision and upon reaching kidney failure.
The range of survival estimates for CKM is wide, largely because of such heterogeneity in studies, and it is clear that there is an urgent need for more prospective studies on the survival of older patients with advanced CKD managed without dialysis to assist physicians with shared decision making. In the meantime, we suggest that clinicians seek to match their patients as closely as possible to those in other specific studies (Table 1) when providing prognostic information.
Article Information
Authors’ Full Names and Academic Degrees
Angela Chou, MBBS, Kelly Chenlei Li, MBBS, MD, and Mark Ashley Brown, MBBS, MD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received December 6, 2021, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form January 15, 2022.
Footnotes
Complete author and article information provided before references.
Item S1. Search Terminology.
Supplementary Material
Item S1.
References
- 1.Eggers P.W. Medicare’s end stage renal disease program. Health Care Financ Rev. 2000;22(1):55–60. [PMC free article] [PubMed] [Google Scholar]
- 2.ANZDATA Registry 43rd Annual Report, Chapter 2: Prevalence of Renal Replacement Therapy for End Stage Kidney Disease. Australia and New Zealand Dialysis and Transplant Registry. https://www.anzdata.org.au/wpcontent/uploads/2019/09/c02_prevalence_2018_ar_2019_v1.0_20191202.pdf Available at:
- 3.Chan C.T., Blankestijn P.J., Dember L.M., et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;96(1):37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Thomas B., Wulf S., Bikbov B., et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26(11):2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens L.A., Viswanathan G., Weiner D.E. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17(4):293–301. doi: 10.1053/j.ackd.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan M., Lok C.E., Jassal S.V. Epidemiology and demographic aspects of treated endsStage renal disease in the elderly. Semin Dial. 2002;15(2):79–83. doi: 10.1046/j.1525-139x.2002.00028.x. [DOI] [PubMed] [Google Scholar]
- 7.Pyart R., Evans K.M., Steenkamp R., et al. The 21st UK Renal Registry annual report: a summary of analyses of adult data in 2017. Nephron. 2020;144(2):59–66. doi: 10.1159/000504851. [DOI] [PubMed] [Google Scholar]
- 8.Berthoux F., Gellert R., Jones E., et al. Epidemiology and demography of treated end-stage renal failure in the elderly: from the European Renal Association (ERA-EDTA) Registry. Nephrol Dial Transplant. 1998;13:65–68. doi: 10.1093/ndt/13.suppl_7.65. [DOI] [PubMed] [Google Scholar]
- 9.Ho Y.W., Chau K.F., Choy B.Y., et al. Hong Kong Renal Registry report 2012. Hong Kong J Nephrol. 2013;15(1):28–43. [Google Scholar]
- 10.Davison S.N., Levin A., Moss A.H., et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 11.Da Silva-Gane M., Wellsted D., Greenshields H., Norton S., Chandna S.M., Farrington K., et al. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7(12):2002–2009. doi: 10.2215/CJN.01130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini T., Murtagh F.E., Dupont P.J., McKinnon P.M., Hatfield P., Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631–636. doi: 10.1177/0269216306070236. [DOI] [PubMed] [Google Scholar]
- 13.Lamping D.L., Constantinovici N., Roderick P., et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356(9241):1543–1550. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
- 14.Kalender B., Ozdemir A.C., Dervisoglu E., Ozdemir O. Quality of life in chronic kidney disease: effects of treatment modality, depression, malnutrition and inflammation. Int J Clin Pract. 2007;61(4):569–576. doi: 10.1111/j.1742-1241.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 15.Yong D.S.P., Kwok A.O.L., Wong D.M.L., Suen M.H.P., Chen W.T., Tse D.M.W. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23(2):111–119. doi: 10.1177/0269216308101099. [DOI] [PubMed] [Google Scholar]
- 16.Rockville M. 2nd ed. Renal Physicians Association, United States of America; 2010. Shared Decision Making in the Appropriate Initiation and Withdrawal from Dialysis; pp. 1–12. [Google Scholar]
- 17.Ladin K., Lin N., Hahn E., Zhang G., Koch-Weser S., Weiner D.E. Engagement in decision-making and patient satisfaction: a qualitative study of older patients’ perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant. 2017;32(8):1394–1401. doi: 10.1093/ndt/gfw307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davison S.N., Tupala B., Wasylynuk B.A., Siu V., Sinnarajah A., Triscott J. Recommendations for the care of patients receiving conservative kidney management: Focus on management of CKD and symptoms. Clin J Am Soc Nephrol. 2019;14(4):626–634. doi: 10.2215/CJN.10510917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunney M., Bello A.K., Levin A., et al. Availability, accessibility, and quality of conservative kidney management worldwide. Clin J Am Soc Nephrol. 2020;16(1):79–87. doi: 10.2215/CJN.09070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joly D., Anglicheau D., Alberti C., et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14(4):1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 21.Murtagh F.E.M., Marsh J.E., Donohoe P., Ekbal N.J., Sheerin N.S., Harris F.E. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(7):1955–1962. doi: 10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 22.Echevers Y.M., Gaibor N.G.T., Pérez N.N., Martin F.B., Delgado R.M., Riscos M.Á.G. Survival of patients ≥70 years with advanced chronic kidney disease: dialysis vs. conservative care. Nefrología. 2016;36(3):283–291. doi: 10.1016/j.nefro.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Wong C.F., McCarthy M., Howse M.L.P., Williams P.S. Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail. 2007;29(6):653–659. doi: 10.1080/08860220701459634. [DOI] [PubMed] [Google Scholar]
- 24.Chandna S.M., Da Silva-Gane M., Marshall C., Warwicker P., Greenwood R.N., Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608–1614. doi: 10.1093/ndt/gfq630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shum C.K., Tam K.F., Chak W.L., Chan T.C., Mak Y.F., Chau K.F. Outcomes in older adults with stage 5 chronic kidney disease: comparison of peritoneal dialysis and conservative management. J Gerontol A Biol Sci Med Sci. 2014;69(3):308–314. doi: 10.1093/gerona/glt098. [DOI] [PubMed] [Google Scholar]
- 26.Seow Y.Y., Cheung Y., Qu L.M., Yee A.C.P. Trajectory of quality of life for poor prognosis stage 5D chronic kidney disease with and without dialysis. Am J Nephrol. 2013;37(3):231–238. doi: 10.1159/000347220. [DOI] [PubMed] [Google Scholar]
- 27.Kwok W.H., Yong S.P., Kwok O.L. Outcomes in elderly patients with end-stage renal disease: comparison of renal replacement therapy and conservative management. Hong Kong J Nephrol. 2016;19:42–56. [Google Scholar]
- 28.Celentano D.D., Szklo M. 6th ed. Epidemiology. Elsevier; 2019. Gordis epidemiology. [Google Scholar]
- 29.Carson R.C., Juszczak M., Davenport A., Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–1619. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith C., Da Silva-Gane M., Chandna S., Warwicker P., Greenwood R., Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95(2):c40–c46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 31.Verberne W.R., Geers A.B.M.T., Jellema W.T., Vincent H.H., van Delden J.J.M., Bos W.J.W. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol. 2016;11(4):633–640. doi: 10.2215/CJN.07510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raman M., Middleton R.J., Kalra P.A., Green D. Outcomes in dialysis versus conservative care for older patients: a prospective cohort analysis of stage 5 chronic kidney disease. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0206469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain J.A., Mooney A., Russon L. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med. 2013;27(9):829–839. doi: 10.1177/0269216313484380. [DOI] [PubMed] [Google Scholar]
- 34.Tam-Tham H., Quinn R.R., Weaver R.G., et al. Survival among older adults with kidney failure is better in the first three years with chronic dialysis treatment than not. Kidney Int. 2018;94(3):582–588. doi: 10.1016/j.kint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Brown M.A., Collet G.K., Josland E.A., Foote C., Li Q., Brennan F.P. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol. 2015;10(2):260–268. doi: 10.2215/CJN.03330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nixon A.C., Bampouras T.M., Pendleton N., Woywodt A., Mitra S., Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11(2):236–245. doi: 10.1093/ckj/sfx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurella M., Covinsky K.E., Collins A.J., Chertow G.M. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kurella Tamura M., Covinsky K.E., Chertow G.M., Yaffe K., Landefeld C.S., McCulloch C.E. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foote C., Kotwal S., Gallagher M., Cass A., Brown M.A., Jardine M. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 2016;21(3):241–253. doi: 10.1111/nep.12586. [DOI] [PubMed] [Google Scholar]
- 40.Murphy E., Burns A., Murtagh F.E.M., Rooshenas L., Caskey F.J. The Prepare for Kidney Care Study: prepare for renal dialysis versus responsive management in advanced chronic kidney disease. Nephrol Dial Transplant. 2021;36(6):975–982. doi: 10.1093/ndt/gfaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterne J.A., Hernan M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 (8080):i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reindl-Schwaighofer R., Kainz A., Kammer M., Dumfarth A., Oberbauer R. Survival analysis of conservative vs. dialysis treatment of elderly patients with CKD stage 5. PloS One. 2017;12(7) doi: 10.1371/journal.pone.0181345. e0181345-e0181345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen C. Ethical and legal considerations in the care of the infant with end-stage renal disease whose parents elect conservative therapy. An American perspective. Pediatr Nephrol. 1987;1(2):166–171. doi: 10.1007/BF00849289. [DOI] [PubMed] [Google Scholar]
- 44.Ivory S.E., Polkinghorne K.R., Khandakar Y., et al. Predicting 6-month mortality risk of patients commencing dialysis treatment for end-stage kidney disease. Nephrol Dial Transplant. 2017;32(9):1558–1565. doi: 10.1093/ndt/gfw383. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt R.J., Landry D.L., Cohen L., et al. Derivation and validation of a prognostic model to predict mortality in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2019;34(9):1517–1525. doi: 10.1093/ndt/gfy305. [DOI] [PubMed] [Google Scholar]
- 46.Morton R.L., Snelling P., Webster A.C., et al. Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ. 2012;184(5):E277–E283. doi: 10.1503/cmaj.111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelbrecht B.L., Kristian M.J., Inge E., et al. Does conservative kidney management offer a quantity or quality of life benefit compared to dialysis? A systematic review. BMC Nephrol. 2021;22(1):307. doi: 10.1186/s12882-021-02516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellam T., El-Kossi M., Prasanth K.C., El-Nahas M., Khwaja A. Conservatively managed patients with stage 5 chronic kidney disease—outcomes from a single center experience. QJM. 2009;102(8):547–554. doi: 10.1093/qjmed/hcp068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1.