Abstract
Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative disease accompanied by mutations in CSF3R. Here, we present a patient with CNL who developed to acute myeloid leukemia (AML) at the same time that a t(4;12)(q12;p13) translocation appeared. The uniqueness of this cytogenetic abnormality led us to delineate the molecular aberrations relevant for clonal evolution. While the CSF3R mutation was present throughout the course of the disease, the SETBP1 mutation was newly acquired at the AML transformation. The present case suggests that careful monitoring of t(4;12)(q12;p13) and SETBP1 is crucial to predict AML evolution in CNL patients.
Keywords: Chronic neutrophilic leukemia, Acute myeloid leukemia, t(4 ; 12)(q12 ; p13), SETBP1
1. Introduction
Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative neoplasm accompanied by hallmark mutations in CSF3R. Approximately 200 cases with CNL have accumulated over the past several decades [1]. Disease progression occurs by acquisition of additional cytogenetic and/or molecular abnormalities, and 10–21% of cases develop into acute myeloid leukemia (AML) [1]. However, the t(4;12)(q12;p13) translocation observed in our case has not previously been associated with AML transformation.
We report herein, for the first time, a patient with CSF3R-mutated CNL developing into AML simultaneously with the appearance of t(4;12)(q12;p13).
2. Case report
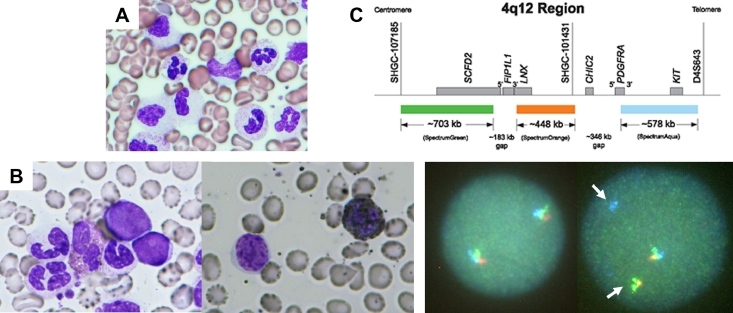
A 55-year-old man was found out to have leukocytosis on his health check-up and was referred to Saiseikai Central Hospital in July 2013. At the onset, he had systemic symptoms such as flushing and night sweats. The findings of physical examination were unremarkable, and his spleen was not palpable. The complete blood count revealed a white blood cell (WBC) count of 26.7 × 109/L with 85.5% neutrophils, 1.0% metamyelocytes and myelocytes, 12.0% lymphocytes and 1.5% eosinophils, along with a hemoglobin of 12.7 g/dl and a platelet count of 216 × 109/L. Neutrophils were characterized by toxic granulation and Döhle bodies. Bone marrow aspiration showed maturing granulopoiesis without an increase in blasts (Fig. 1A). Karyotype analysis showed no clonal abnormalities. The results of blood chemistry were all within normal limits. The serum level of granulocyte-colony stimulating factor was also within normal range (< 19.5 pg/mL). 18F-fluorodeoxy-glucose positron emission tomography/computed tomography demonstrated splenomegaly (19 cm in length) and diffuse 18F-fluorodeoxy-glucose uptake in the spleen and bone marrow (maximum standardized uptake value: 7.67 and 13.2, respectively) was observed.
Fig 1.
Clonal evolution in a patient with CSF3R-T618I CNL to AML with t(4;12)(q12;p13). (A) Bone marrow aspiration smear showing maturing granulopoiesis without an increase in blasts at diagnosis (Wright-Giemsa stain, 1,000x original magnification). (B) Bone marrow aspiration smear showing blasts with small- to medium-size, round nuclei and a blocky chromatin pattern in agranular cytoplasm at AML transformation (left, Wright-Giemsa stain, 1,000x original magnification; right, peroxidase stain, x1,000 original magnification). (C) FISH analysis was performed using break-apart probes for the 4q12 region on cultured peripheral blood cells. The tri-color break-apart probe (5′FIP1L1 in green, 3′FIP1L1→5′PDGFRA in orange, and 3′PDGFRA in aqua) showed 1 triple fusion, 1 green-orange fusion (arrow), and 1 separate aqua signal (arrow), which confirmed CHIC2 or PDGFRA gene rearrangement (presumably translocation to derivative chromosome 12) in 58% of the nuclei.
The patient was tentatively diagnosed as myeloproliferative neoplasm, unclassifiable and was followed conservatively; however, both WBC and platelet counts continued to rise, together with progressing anemia. In March 2014, nine months after the initial exam, the spleen showed a remarkable increase in size (to 24 cm in length), and the complete blood count showed a WBC of 40.5 × 109/L with 87% neutrophils but no blasts, together with a hemoglobin of 8.5 g/dl, and a platelet count of 114 × 109/L. Genomic sequencing for CSF3R gene from exons 14 and 17 confirmed the presence of heterozygous CSF3R-T618I. He was diagnosed as having CNL, and was placed on hydroxyurea.
In November 2014, he started complaining of splenic pain and early satiety due to the continuing enlargement of his spleen (now 27 cm in length). Splenic irradiation also failed to reduce the spleen size; total dose given was 10 Gy. On follow-up 1 year after initiation of hydroxyurea therapy, in March 2015, results of bone marrow biopsy showed slight reticulin fibrosis and secondary myelofibrosis was diagnosed. He was placed on ruxolitinib at the dose of 5 mg twice daily, which subsequently increased to 20 mg twice daily, and hydroxyurea was discontinued. Ruxolitinib was well tolerated, and all clinical symptoms including cachexia were improved. WBC counts were stabilized within 1 month; however, these beneficial responses were not durable.
One year later, in March 2016, the WBC count showed another rapid increase, to 214.5 × 109/L, and this time 21% of WBCs were small to medium sized blasts bone marrow aspiration showed hypercellular marrow with increased blasts complicated with multilineage dysplasia. Blasts possessed a blocky chromatin pattern but negative for myeloperoxidase activity (Fig. 1B). Some myeloid precursors showed hypogranulation. Erythroid cells were markedly reduced in number. Megakaryocytes were adequate in number, but they were dysplastic, with micromegakaryocytes and small megakaryocytes. The immunophenotype was positive for CD13, CD33, CD34, CD117, HLA-DR. and CD7. Genetic analysis revealed no FLT3/ITD mutations. Cytogenetic examination revealed a t(4;12)(q12;p13) translocation in 19 out of 20 metaphases. Thus, the diagnosis of AML with t(4;12)(q12;p13) was made. Fluorescent in situ hybridization (FISH) analysis using break-apart probes for the 4q12 region on cultured peripheral blood cells confirmed CHIC2 or PDGFRA gene rearrangement (presumably translocated to derivative chromosome 12) in 58% of nuclei (Fig. 1C).
The patient was placed on chemotherapy with cytarabine and daunorubicin. Fourteen days after the induction therapy, the peripheral blood contained as much as 60% blasts. FISH for the 4q12 split signal also showed an increase, up to 85%. Bone marrow biopsy showed a reticulin fibrosis (grade 2) without hematopoiesis. The patient underwent two cycles of azacytidine with reduction in the peripheral blood blasts to 7%. In July 2016, the patient underwent haploidentical hematopoietic cell transplantation after conditioning with fludarabine and total body irradiation (12 Gy). Although he achieved full donor chimerism, he died of hepatic veno-occlusive disease 51 days after the transplantation. The autopsy showed hypocellular marrow without blasts and moderate myelofibrosis; both the liver and spleen were free of leukemic cell infiltration.
To assess the sequential molecular events, we studied the mutations of CSF3R, SETBP1, ASXL1, SRSF2 and CBL using genomic sequencing with the stored samples from the peripheral blood both in CNL (March 2014) and AML (April 2016) phases. At the time of molecular studies in CNL, the patient did not receive any therapy. The patient signed informed consent for sample collection in accordance with the Declaration of Helsinki. The samples were polymerase-chain-reaction amplified using the primers described previously [2] and subject to mutational analysis by Sanger sequencing on an Applied Biosystems 3730xl DNA Analyzer with an Applied Biosystems BigDye Terminator v3.1 Cycle Sequencing Kit. In the chronic phase, the evaluation of mutational status identified heterozygous CSF3R-T315I as was noted previously. This heterozygous mutation remained the same at the AML-transformation phase. Additional SETBP1-gene mutation (I871T) with variant allele frequency of 69% was found at AML transformation, after being negative in the chronic phase. Hence, both t(4;12)(q12;p13) translocation as well as SETBP1-gene mutation were observed during the leukemic transformation.
3. Discussion
To the best of our knowledge, we have described, for the first time, a unique episode of transformation to AML with t(4;12)(q12;p13) in a patient previously diagnosed as CNL. The t(4;12)(q12;p13) translocation is a very rare rearrangement, reported in less than 1% of AML or myelodysplastic syndrome cases and mainly occurring in de novo AML [3]. To date, only 7 patients have been described in the blast phase of myeloproliferative neoplasm [3, 4]. In our case, several previously described features of t(4;12)(q12;p13), such as CD7 expression, absence of myeloperoxidase activity, unique blast morphology with multilineage dysplasia and failure of the first induction chemotherapy were present [3, 4].
In the t(4;12)(q12;p13) translocation, ETV6 for 12p13 has been reported to be variably fused to a genomic region which frequently involves CHIC2 and PDGFRA for 4q12 [3]. However, the impact of the CHIC2 or PDGFRA/ETV6 fusion protein on AML pathogenesis is not well understood. Intriguingly, the additional molecular events that have recently been reported in patients with t(4;12)(q12;p13)-positive AML are in line with those found in CNL. The most commonly mutated genes in the t(4;12)(q12;p13) AML were ASXL1 (50%) and SRSF2 (31%), but SETBP1 and CBL were also mutated in 6% of the cases [3]. The ASXL1, SRSF2, SETBP1 and CBL mutations are found in 30–81%, 21–44%, 14–56% and 25% of patients with CNL, respectively [1]. Of note, we detected an SETBP1 T871I mutation at the time of AML transformation, which is hypothesized to facilitate the acquisition of t(4;12)(q12;p13) translocation that can drive AML.
Clonal evolution detected by cytogenetic analyses is seen in 12.5% of patients with CNL [1]. The common chromosomal alterations observed during clonal evolution of CNL are deletion 11q, deletion 12p, deletion 20q and trisomy 21 [1, 5]. There are a total of 5 cases of CNL in which both cytogenetic and molecular clonal evolutions have been recorded. Table 1 shows the clinical features of these 5 cases including ours [[5], [6], [8]]. All cases used ruxolitinib in the clinical management of the disease. Cases 3, 4 and 5 had a chromosomal aberration at AML transformation, involving either chromosome 12 aberration or trisomy 19. Notably, the concurrent molecular parameter in all three cases was the SETBP1 mutation, which may act as a driver of the progression from CNL to AML. Zhang et al. proposed SETBP1 mutations to be early events in the clinical course of CNL [9]. In our case, while retrospective analysis of peripheral blood from CNL diagnosis was negative for SETBP1 mutations, the SETBP1 variant allele frequency of 69% at the time of AML diagnosis is consistent with an early mutation. Unfortunately, peripheral blood samples for molecular examinations were not stored at loss of hydroxyurea or ruxolitinib responsiveness in CNL phase, so the exact time for acquisition of this aberration remains speculative. While Sanger sequencing was performed it is conceivable that higher sensitivity methodologies such as comprehensive next-generation sequencing could have detected the SETBP1 variant earlier or revealed other hidden pathogenic mutations. The sensitivity of the mutational analysis we used was recognized as being approximately 25% to 30% mutant allele frequency. Banfi et al. recently showed that the SETBP1 mutation leads to aberrant proliferation, oncogene deregulation, DNA damage, and resistance to apoptosis [10]. Even though it is still controversial whether SETBP1 mutations can be prognostic markers in CNL, it is logical to believe that they may be associated with blastic transformation [1].
Table 1.
Cases of CNL in which both cytogenetic and molecular clonal evolutions were evaluated.
| Case | Reporter | Age (years)/Sex | Stage | CSF3R | SETBP1 | ASXL1 | SRSF2 | CBL | Other mutated genes | Cytogenetics | Survival (months) |
| 1 | Fathi et al. [6] | 86/F | Dx | T618I (28%) | D868N (40%) | H633fs* (24%) | WT | WT | TET2 D143fs* (40%), TP53 R273H (6%) | 46,XX | 17 |
| CLL | T618I in the CD19− cells | D868N in the CD19− cells | H633fs* in the CD19− cells | WT | WT | TET2 in the CD19− cells, TP53 in the CD19+ cells | der(6;17)(p10;q10) | ||||
| 2 | Nooruddin et al. [8] | 69/M | Dx | T618I (36%) | G870S (45%) | WT | WT | WT | RUNX1 337_341del (5%) | 46,Y,t(X;7)(p22.1;q36) [20/20] | 9 |
| progression | T618I (7%) | G870S (6%) | WT | WT | WT | RUNX1 337_341del (39%), GATA2 K392fs (37%),KIT D816V (34%) | 46,Y,t(X;7)(p22.1;q36) [20/20] | ||||
| 3 | Langabeer et al. [5] | 60/F | Dx | T618I (38%) | D868N (42%) | WT | P95H (41%) | G415S (15%) | 46,XX [17] | 25 | |
| AML | T618I (51%) | D868N (48%) | WT | P95H (51%) | WT | 47,XX,+19 [2]/46,XX [5] | |||||
| 4 | Langabeer et al. [5] | 66/M | Dx | T618I (38%) | D868N (36%) | WT | P95H (37%) | WT | 46,XY [20] | 36 | |
| AML | T618I (77%) | D868N (39%) | WT | P95H (46%) | WT | NRAS G13C (30%) | 46,XY,add(12)(p11.2) [9]/46,XY [1] | ||||
| 5 | Present case | 55/M | Dx | T618I (50%) | WT | WT | WT | WT | 46,XY [20/20] | 37 | |
| AML | T618I (50%) | I871T (69%) | WT | WT | WT | t(4;12)(q12; p13) [19/20]/46,XY [1/20] |
M male, F female, Dx diagnosis, WT wild type, CLL chronic lymphocytic leukemia, AML acute myeloid leukemia.
The resistance of ruxolitinib in CNL is also a matter for debate [11]. Parenti et al. recently reported a primary myelofibrosis patient in whom ruxolitinib seemed to increase PD-L1 expression on preexisting leukemic CD34-positive cells, along with reduced T-cell activation during disease progression to AML [12]. However, the mechanism by which ruxolitinib contributes to the process of CNL-associated transformation of AML remains to be elucidated.
Taken together, our case and the literature suggest that careful monitoring of t(4;12)(q12;p13) and SETBP1 is crucial to predict AML evolution in CNL patients.
Authors’ contributions
MH, KW and SO analyzed clinical, cytogenetic and molecular data, and wrote the manuscript. YT, HK, MO, KY, RD and TK examined and treated the patient, collected samples, provided feedback and approved the manuscript.
Declaration of Competing Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We thank Yutaka Hattori, MD, PhD, Hiroki Sugimori, MD, PhD, MMedSci, Satoru Kuriyama, MD, PhD and Otoya Miho, MD, PhD for their critical reading of the manuscript.
References
- 1.Szuber N., Elliott M., Tefferi A. Chronic neutrophilic leukemia: 2020 update on diagnosis, molecular genetics, prognosis, and management. Am. J. Hematol. 2020;95(2):212–224. doi: 10.1002/ajh.25688. [DOI] [PubMed] [Google Scholar]
- 2.Meggendorfer M., Haferlach T., Alpermann T., Jeromin S., Haferlach C., Kern W., Schnittger S. Specific molecular mutation patterns delineate chronic neutrophilic leukemia, atypical chronic myeloid leukemia, and chronic myelomonocytic leukemia. Haematologica. 2014 Dec;99(12):e244–e246. doi: 10.3324/haematol.2014.113159. Epub 2014 Sep 19.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parinet V., Chapiro E., Bidet A., Gaillard B., Maarek O., Simon L., Lefebvre C., Defasque S., Mozziconacci M.J., Quinquenel A., Decamp M., Lifermann F., Ali-Ammar N., Maillon A., Baron M., Martin M., Struski S., Penther D., Micol J.B., Auger N., Bilhou-Nabera C., Martignoles J.A., Tondeur S., Nguyen-Khac F., Hirsch P., Roos-Weil D. G.g. on behalf the Filo, Myeloid malignancies with translocation t(4;12)(q11-13;p13): molecular landscape, clonal hierarchy and clinical outcomes. J. Cell Mol. Med. 2021;25(20):9557–9566. doi: 10.1111/jcmm.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., Xu J., Abruzzo L.V., Tang G., Li S., You M.J., Lu G., Jabbour E.J., Deng Q., Bueso-Ramos C.E., Medeiros L.J., Yin C.C. Acute myeloid leukemia with t(4;12)(q12;p13): an aggressive disease with frequent involvement of PDGFRA and ETV6. Oncotarget. 2018;9(13):10987–10994. doi: 10.18632/oncotarget.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langabeer S.E., Haslam K., Kelly J., Quinn J., Morrell R., Conneally E. Targeted next-generation sequencing identifies clinically relevant mutations in patients with chronic neutrophilic leukemia at diagnosis and blast crisis. Clin. Transl. Oncol. 2018;20(3):420–423. doi: 10.1007/s12094-017-1722-2. [DOI] [PubMed] [Google Scholar]
- 6.Fathi A.T, Graubert T.A, Kulkarni N.M, Kuo F.C, Hasserjian R.P. Case 37-2016. An 86-year-old woman with leukocytosis and splenomegaly. N. Engl. J. Med. 2016;375(23):2273–2282. doi: 10.1056/NEJMcpc1509539. [DOI] [PubMed] [Google Scholar]
- 8.Nooruddin Z., Miltgen N., Wei Q., Schowinsky J., Pan Z., Tobin J., Purev E., Gutman J.A., Robinson W., Pollyea D.A. Changes in allele frequencies of CSF3R and SETBP1 mutations and evidence of clonal evolution in a chronic neutrophilic leukemia patient treated with ruxolitinib. Haematologica. 2017;102(5):e207–e209. doi: 10.3324/haematol.2016.163089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Wilmot B., Bottomly D., Dao K.-H.T., Stevens E., Eide C.A., Khanna V., Rofelty A., Savage S., Reister Schultz A., Long N., White L., Carlos A., Henson R., Lin C., Searles R., Collins R.H., DeAngelo D.J., Deininger M.W., Dunn T., Hein T., Luskin M.R., Medeiros B.C., Oh S.T., Pollyea D.A., Steensma D.P., Stone R.M., Druker B.J., McWeeney S.K., Maxson J.E., Gotlib J.R., Tyner J.W. Genomic landscape of neutrophilic leukemias of ambiguous diagnosis. Blood. 2019;134(11):867–879. doi: 10.1182/blood.2019000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banfi F., Rubio A., Zaghi M., Massimino L., Fagnocchi G., Bellini E., Luoni M., Cancellieri C., Bagliani A., Di Resta C., Maffezzini C., Ianielli A., Ferrari M., Piazza R., Mologni L., Broccoli V., Sessa A. SETBP1 accumulation induces P53 inhibition and genotoxic stress in neural progenitors underlying neurodegeneration in Schinzel-Giedion syndrome. Nat. Commun. 2021;12(1):4050. doi: 10.1038/s41467-021-24391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoner R.C., Press R.D., Maxson J.E., Tyner J.W., Dao K.-H.T. Insights on mechanisms of clonal evolution in chronic neutrophilic leukemia on ruxolitinib therapy. Leukemia. 2020;34(6):1684–1688. doi: 10.1038/s41375-019-0688-1. [DOI] [PubMed] [Google Scholar]
- 12.Parenti S., Rontauroli S., Carretta C., Mallia S., Genovese E., Chiereghin C., Peano C., Tavernari L., Bianchi E., Fantini S., Sartini S., Romano O., Bicciato S., Tagliafico E., Porta M.Della, Manfredini R. Mutated clones driving leukemic transformation are already detectable at the single-cell level in CD34-positive cells in the chronic phase of primary myelofibrosis. NPJ Precis. Oncol. 2021;5(1):4. doi: 10.1038/s41698-021-00144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 7.Gunawan A.S., McLornan D.P., Wilkins B., Waghorn K., Hoade Y., Cross N.C.P., Harrison C.N. Ruxolitinib, a potent JAK1/JAK2 inhibitor, induces temporary reductions in the allelic burden of concurrent CSF3R mutations in chronic neutrophilic leukemia. Haematologica. 2017;102(6):e238–e240. doi: 10.3324/haematol.2017.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]