Abstract
Immunotherapy has altered the treatment paradigm for soft tissue sarcomas (STSs). Considering the limited information regarding the clinical significance of immunohistochemical markers in STS, the purpose of this study was to determine the clinical significance of programmed cell death-1 (PD-1), PD ligand-1(PD-L1), New York esophageal squamous cell carcinoma-1 (NY-ESO-1), and melanoma-associated antigen-A4 (MAGE-A4) expression in STSs. Twenty-two patients (median age, 72.5 years) with STSs treated at our hospital were included in this study. The specimens obtained at the time of biopsy were used to perform immunostaining for PD-1, PD-L1, NY-ESO, and MAGE-A4. The rates of PD-1-, PD-L1-, NY-ESO-, and MAGE-A4-positive cells and cases were calculated. The correlations among the positive cell rates of the immunohistochemical markers as well as their correlations with the histological grade, tumor size, or maximum standardized uptake (SUVmax) value were also determined. The average rates of PD-1-, PD-L1-, NY-ESO-, and MAGE-A4-positive cells were 4.39%, 28.0%, 18.2%, and 39.4%, respectively. PD-1-, PD-L1-, NY-ESO-1-, and MAGE-A4- positive cell rates showed weak to strong correlations with the SUVmax value. Thus, PD-1, PD-L1, NY-ESO, and MAGE-A4 expressions might be involved in the aggressive elements of STSs.
Key words: soft tissue sarcomas, immunohistochemical markers, programmed cell death-1, programmed cell death ligand-1, New York esophageal squamous cell carcinoma-1, melanoma-associated antigen-A4
Introduction
Soft tissue sarcomas (STSs) are a diverse group of neoplasms that arise in connective tissues throughout the body, accounting for approximately 1% of malignant tumors in adults and 7% to 15% of malignant tumors in children.1 Extremity STSs account for approximately 50-60 % of all STSs.2
The primary treatment for STSs is extensive resection with adequate surgical margins.3 Survival rates have also been shown to improve after the establishment of postoperative adjuvant chemotherapy with doxorubicin, cyclophosphamide, and highdose methotrexate.4 Although the timing of adjuvant radiotherapy is controversial, systematic reviews have shown that it reduces local recurrence.5,6 Recently, systemic chemotherapeutic regimens using agents such as eribulin and trabectedin have been developed for advanced STSs, but their indications and efficacy vary depending on the histological type, and they are not definitive treatments. 7,8 Despite these multidisciplinary treatments, more than 40% of patients eventually experience tumor recurrence, resulting in an overall survival of less than 12 months.9 When conventional therapies are unsuccessful, the remaining treatment options are limited; therefore, novel and effective STS therapies are urgently needed for patients with metastasis or unresectable malignancies.
Immune checkpoint inhibitors have been recently used to alleviate the immunosuppressive state of the tumor microenvironment in solid malignancies by restoring the immune function of T cells and killing tumor cells.10 Cancer/testis antigens (CTAs) belong to a group of tumor antigens whose normal expression is restricted to male germ cells in the testis and are not found in adult somatic tissues. 11 In addition to their tissue-specific expression profile, the common features of CTAs include their presence as a multigene family, frequent mapping to the X chromosome, induction of expression by hypomethylation and histone acetylation, immunogenicity in cancer patients, heterogeneous protein expression in various tumor types, and potential association with tumor progression. 11 Therefore, over the past decade, CTAs have emerged as a therapeutic target for the treatment of malignant diseases.12 New York esophageal squamous cell carcinoma-1 (NY-ESO-1) is an immunogenic CTA associated with innate and vaccine-induced immunity that may lead to clinical cancer responses.13 Melanomaassociated antigen A4 (MAGE-A4) is another CTA that has been reported to be expressed in many cancers.14 Patients with MAGEA4- expressing tumors exhibit specific cellular and humoral immune responses to MAGE-A4 targeting.15 Although PD-1/PDL1 and NY-ESO-1/MAGE-A4 have been reported to be involved in the pathogenesis of STSs, the details regarding their involvement remain unclear.16-19 Thus, the purpose of the current study was to clarify the clinical significance of PD-1/PD-L1 and NYESO- 1/MAGE-A4 in highly aggressive STSs.
Materials and Methods
The summary data of patients enrolled in the current study are presented in Table 1. Twenty-two patients (13 men, 9 women) with STSs were included in the current study. The study protocol was approved by the Kindai University Ethics Committee (approval number: R03-021; approved on April 27, 2021). Written informed consent was obtained from patients who could sign the consent form, while comprehensive consent was obtained from those who were unable to sign. The median age of the patients was 72.5 years (range: 34-101 years). The tumor was located in the upper extremities in 4 cases, the lower extremities in 13 cases, and the trunk in 5 cases. The histological grades20 were grade 1, grade 2, and grade 3 in 2, 8, and 12 patients, respectively.
The median tumor diameter was 5.8 cm (range, 1.5-15.1 cm). The median maximum standardized uptake (SUVmax) value was 7.38 (range, 3.13-24.1). The treatment consisted of wide resection with a flap in 2 cases, wide resection and skin graft in 2 cases, additional wide resection with a flap in 1 case, wide resection and postoperative radiotherapy in 3 cases, wide resection in 11 cases, marginal resection in 2 cases, and postoperative radiotherapy only in 1 case. Eight cases showed recurrence while 14 cases did not show recurrence. Five patients reported metastasis while 17 did not report metastasis. The final clinical outcomes were continuous disease free (CDF) in 11 patients, no evidence of disease (NED) in 6, alive with disease (AWD) in 3, and dead of disease (DOD) in 2.
Immunohistochemical staining
Immunostaining for PD-1, PD-L1, NY-ESO-1, and MAGE-A4 was performed on pathological specimens harvested at the time of biopsy from patients with STS, including undifferentiated pleomorphic sarcoma (UPS) (n=10), myxofibrosarcoma (MFS) (n=9), and malignant peripheral nerve sheath tumors (MPNSTs) (n=3) who were treated at our institution between January 2006 and December 2019. Tissue sections were formalin-fixed and paraffinembedded. Sections of 4-μm thickness were cut and mounted onto slides. The tissues were deparaffinized, rehydrated, and subjected to endogenous peroxidase inhibition using 3% hydrogen peroxide. Antigen activation was performed using antigen-specific heat treatments at pH 9 as follows: PD-1 at 95°C for 64 min; PD-L1 at 95°C for 64 min, NY-ESO at 100°C for 64 min; and MAGE-A4 at 95°C for 36 min. After heat activation, the tissue sections were incubated with the following primary antibodies: PD-1 antibody (mouse monoclonal, ab52587; Abcam, Cambridge, UK) for 30 min at 37°C after 30 min at low pH (6.0); PD-L1 antibody (rabbit monoclonal, ab205921; Abcam) for 32 min at 37°C after 60 min at high pH (9.0); NY-ESO-1 antibody (mouse monoclonal, E978; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 32 min at 37°C; MAGE-A4 antibody (rabbit monoclonal, ab229011; Abcam) for 16 min at 37°C; and p53 antibody (mouse monoclonal, 7902912; Roche Diagnostics, Risch-Rotkrcuz, Switzerland) for 16 min at 37°C. The reaction was visualized using 3,3-diaminobenzidine (DAB Substrate Chromogen System; DAKO, Kyoto, Japan), and the sections were counterstained with hematoxylin. Tissues from the tonsils were used as positive controls for PD-1 and PD-L1. Testis specimens were also used as positive controls for NY-ESO, MAGE-A4, and uterine serous carcinoma. Primary IgG was used as a negative control for antibodies for PD-1, PD-L1, NY-ESO, and MAGE-A4. The negative control was established using the same tissue that was used for the positive control during immunostaining. The slides were observed under a microscope (BIOREVO BZ-9000; Keyence, Osaka, Japan), and brown granules in the cytoplasm or nuclei indicated positive staining. Immune marker staining within the tumor was quantified in 4 representative high-power fields (40× magnification). The positivity rate for each immune component was calculated. The positivity rate was defined as the number of positive cells/total cell number and was quantified using BIOREVO BZ-9000 software (Keyence).21
Correlations among positive cell rates of immunohistochemical markers
The correlations between the positive cell rates for PD-1 and NY-ESO-1, PD-L1 and NY-ESO-1, PD-1 and MAGE-A4, and PDL1 and MAGE-A4 were examined. In addition, the positive cell rates for each immune component was compared with the average positive cell rate for each of the other components by one-way analysis of variance (ANOVA).
Correlations of the positive cell rates of immunohistochemical markers with histological grade, tumor size, or SUVmax value
The correlations of the positive cell rates for PD-1, PD-L1, NY-ESO-1, or MAGE-A4 with histological grade,22 tumor size, and SUVmax were evaluated.
Statistcal analysis
The positivity rate of each marker was plotted, and correlation diagrams were drawn.21 The coefficient of determination (r) was calculated by drawing an approximation line to examine the correlations among each set of markers. Pearson’s method was used to test the correlations. The correlation between the clinical parameters and the positive rate of each molecule was also investigated. We categorized 1.0 ≥ |r| > 0.7 as very strong, 0.7 ≥ |r| > 0.5 as strong, 0.5 ≥ |r| > 0.4 as moderate, 0.4 ≥ |r| > 0.3 as medium, 0.3 ≥ |r| > 0.2 as weak, and 0.2 ≥ |r| ≥ 0.0 as no correlation.22 The chi-square test was used to compare the cases in remission with the non-remission cases. Statistical significance was set at p < 0.05. Analyses were performed using Stat Mate 5.05 (ATMS, Tokyo, Japan).
Results
The positive cell rate for each immunohistochemical marker
Representative images showing hematoxylin and eosin (HE) staining for UPS, MFS, and MPNST are provided in Figure 1 a-c. Representative images for PD-1, PD-L1, NY-ESO, and MAGE-A4 staining are shown in Figure 1 d-o. PD-1 and PD-L1 positivity was observed in tumor-infiltrated lymphocytes and tumor cells (Figure 1 d-i). NY-ESO and MAGE-A4 were stained in the cytoplasm (Figure 1 j-o). Data for the PD-1-, PD-L1-, NY-ESO-, and MAGEA4- positive cell rates are shown in Table 2. The average PD-1-, PD-L1-, NY-ESO-, and MAGE-A4-positive cell rates were 4.39%, 28.0%, 18.2%, and 39.4%, respectively. The PD-L1-positive cell rate was significantly higher than the PD-1-positive cell rate (p<0.01). The MAGE-A4-positive cell rate was also significantly higher than the PD-1-positive cell rate (p<0.01). Furthermore, the MAGE-A4-positive cell rate was significantly higher than the NYESO- 1-positive cell rate (p<0.05). The rates of patients showing positivity for PD-1, PD-L1, NY-ESO, and MAGE-A4 were 18.1% (4/22), 96.3% (19/22), 90.9% (20/22), and 72.7% (16/22), respectively.
Correlations among the positive cell rates of immunohistochemical markers
No significant correlation was observed between the PD-1- and NY-ESO-1/MAGE-A4 positivity rates (r=0.1 and 0.19, p=0.23 and 0.19, respectively; data not shown). Also, no significant correlation was observed between the PD-L1 and NY-ESO-1 positivity rates (r=0.59, p=0.21, Figure 2a), and between the PD-L1 and MAGE-A4 positivity rates (r=0.57, p=0.42, Figure 2b).
Correlation of the PD-1/PD-L1-positive cell rates with the histological grade
The correlation between the PD-1 positivity rate and histological grade was not significant (r=0.28, p=0.05, Figure 3a). No significant correlation was observed between the PD-L1 positivity rate and histological grade (r=0.68, p=0.40, Figure 3b).
Correlation between the PD-1/PD-L1-positive cell rates and tumor size
No significant correlation between the PD-1 positivity rate and tumor size was observed (r=0.35, p=0.23, Figure 3c). No significant correlation was also observed between the PD-L1 positivity rate and tumor size (r=0.59, p=0.44, Figure 3d).
Table 1.
Characteristics of the study population.
| Factor | Patients’ number | |
|---|---|---|
| Age (years) | > 70 | 9 |
| ≤ 70 | 13 | |
| Sex | Male | 13 |
| Female | 9 | |
| Tumor site | Arms | 4 |
| Legs | 9 | |
| Trunk | 9 | |
| Histological type | MFS | 9 |
| UPS | 10 | |
| MPNST | 3 | |
| Histological grade | Grade 1 | 2 |
| Grade 2 | 8 | |
| Grade 3 | 12 | |
| Tumor size (cm | < 5 | 8 |
| 5-10 | 11 | |
| > 10 | 3 | |
| SUV-max | < 5 | 4 |
| 5-10 | 4 | |
| > 10 | 5 | |
| Treatment | Wide resection, flap | 4 |
| Wide resection, post op radiation | 3 | |
| Wide resection | 10 | |
| Wide resection, skin graft | 2 | |
| Marginal resection | 2 | |
| Radiation | 1 | |
| Recurrence | (+) | 8 |
| (-) | 14 | |
| Metastasis | (+) | 5 |
| (-) | 17 | |
| Outcome | CDF | 11 |
| NED | 6 | |
| AWD | 3 | |
| DOD | 2 | |
| Follow-up periods (years) | > 3 | 8 |
| ≥ 3 | 14 |
MFS, myxofibrosarcoma; UPS, undifferentiated pleomorphic sarcoma; MPNST, malignant peripheral nerve sheath tumor; SUV-max, maximum standardized uptake value-max; op, operation; CDF. continuous disease free; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease.
Table 2.
The summary of the positive cell rates.
| Positive cell rate (%) | |
|---|---|
| PD-1 | |
| 0-10 | 18 |
| 10-50 | 4 |
| >50 | 0 |
| PD-L1 | |
| 0-10 | 9 |
| 10-50 | 7 |
| >50 | 6 |
| NY-ESO-1 | |
| 0-10 | 12 |
| 10-50 | 7 |
| >50 | 3 |
| MAGE-A4 | |
| 0-10 | 8 |
| 10-50 | 4 |
| >50 | 10 |
PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; NY-ESO-1, New York esophageal squamous cell carcinoma-1; MAGE-A4, melanoma-associated antigen A4.
Correlation between the PD-1/PD-L1-positive cell rates and the SUVmax value
The PD-1-positive cell rate and SUVmax value showed a significant weak correlation (r=0.28, p<0.001, Figure 3e), whereas the correlation between PD-L1-positivity rate and SUVmax value was significantly strong (r=0.55, p<0.001, Figure 3f).
Correlation between the NY-ESO-1/MAGE-A4-positive cell rates and histological grade
No significant correlation between the NY-ESO-1 positivity rate and histological grade was strong (r=0.53, p=0.23, Figure 4a). Also, no significant correlation between the MAGE-A4 positivity rate and histological grade was observed (r=0.72, p=0.47, Figure 4b).
Correlation between the NY-ESO-1/MAGE-A4-positive cell rates and tumor size
No significant correlation between the NY-ESO-1 positivity rate and tumor size was observed (r=0.46, p=0.25, Figure 4c). Also, no significant correlation between the MAGE-A4 positivity rate and tumor size was observed (r=0.61, p=0.32, Figure 4d).
Correlation between the NY-ESO-1/MAGE-A4-positive cell rates and SUVmax value
The NY-ESO-1-positive cell rate and SUVmax value showed significantly weak correlation (r=0.26, p=0.01, Figure 4e), whereas the correlation between the MAGE-A4 positivity rate and SUVmax value was significantly strong (r=0.53, p=0.01, Figure 4f).
Figure 1.
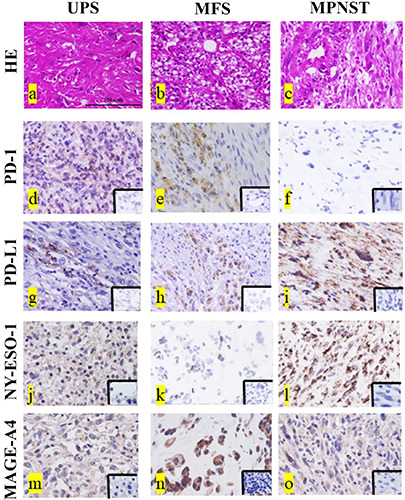
Representative histopathological findings for undifferentiated pleomorphic sarcoma (UPS) (a), myxofibrosarcoma (MYF) (b), and malignant peripheral sheath tumor (MPNST) (c). Hematoxylin and eosin (HE) staining (a-c). PD-1-positive histological findings in UPS (d) and MFS (e), and negative findings in MPNST (f ). PD-L1-positive histological findings in UPS (g), MFS (h), and MPNST (i). NY-ESO- 1-positive histological findings in UPS (j), MFS (k), and MPNST (l). MAGE-A4-positive histological findings in UPS (m), MFS (n), and MPNST (o). The lower right inset images are the negative control for each immunostaining image (d-o). Scale bars: 100 μm.
Figure 2.
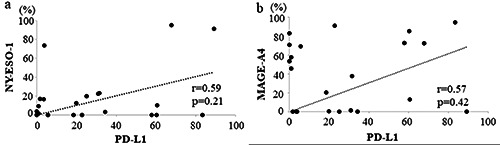
Graphs showing no significant correlation between the PD-L1 and NY-ESO-1 positivity rates (r = 0.59, p=0.21) (a) and between the PD-L1 and MAGE-A4 positivity rates (r=0.57, p=0.42) (b) in STSs.
Figure 3.
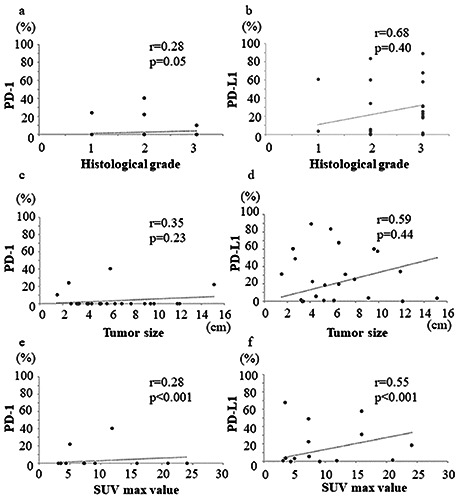
Graphs showing no significant correlation between the histological grade and PD-1 positivity rate (r=0.28, p=0.05) (a); no significant correlation between the histological grade and PD-L1 positivity rate (r=0.68, p=0.40) (b); no significant correlation between the tumor size and PD-1 positivity rate (r=0.35, p=0.23) (c); no significant correlation between the tumor size and PD-L1 positivity rate (r=0.59, p=0.44) (d); medium positive correlation between the SUVmax value and PD-1 positivity rate (r=0.28, p<0.001) (e); and strong positive correlation between the SUVmax value and PD-L1 positivity rate (r=0.55, p<0.001) (f ) in STSs.
Discussion
Various expression rates of PD-1, PD-L1, NY-ESO-A1, and MAGE-A4 have been reported in STSs.19,23-25 In the current study, we confirmed the expression of PD-1, PD-L1, NY-ESO-1, and MAGE-A4 and showed that the expression of these markers was correlated with the clinical features of highly aggressive STSs. The expression rates of PD-1 and PD-L1 in UPS, MFS, and MPNST have been reported to be 34%-80%, 0%-35%, and 13%-17%, respectively.23,26-29 The expression rate of NY-ESO-1 has been reported to be 0-11.1% in UPS, 0-50% in MFS, and 0-12% in MPNST, 18,25,29-31 while the MAGE-A4-positive rate has been reported to be 0-18.2% in UPS, 0% in MFS, and 0%-24.6% in MPNST.18,25 The PD-1-, PD-L1-, NY-ESO-1-, and MAGE-A4- positive rates in this study were similar to or higher than those reported in previous studies.
Our findings also showed a positive correlation between PD- 1/PD-L1- and MAGE-A4-positive cell rates. In the current study, the PD-L1-positive cell rate was higher than the PD-1-positive cell rate, and the MAGE-A4-positive cell rate was higher than the PD- 1-positive cell rate. These findings suggest that PD-1, PD-L1, NYESO- 1, and MAGE-A4 immune molecules are involved in the pathogenesis of UPS, MFS, and MPNST. In particular, the expression of PD-L1 and MAGE-A4 plays important roles in the pathogenesis of UPA, MFS, and MPNST. Also, the expression of PD-1, PD-L1, NY-ESO, and MAGE-A4 may be useful biomarkers for the diagnosis of UPS, MFS, and MPNST.
Recent studies have reported major advances in the characterization of the tumor microenvironment of STS, with the description of “hot tumors” massively infiltrated by immune cells and “cold tumors” with no significant immune infiltration.31 Moreover, Petitprez et al. established an immune-based classification relying on the composition of the tumor microenvironment and identified 5 distinct phenotypes: immune-low, immune-high, and highly vascularized. 32 Interestingly, many immune-related cells in the tumor microenvironment, as well as tumor cells, express genetic mutations that are essential for prognosis and treatment of different subtypes of STS.33 Recently, HLA-DQA1 has also been reported as a specific immune biomarker in high-grade UPS and MFS.34 Moreover, evaluation of the positivity rate has been reported to be an indicator of whether anti-immune molecular inhibitors are effective.23 In the current study, we observed infiltration of immune cells into the UPS, MFS, and MPNST. Thus, these findings suggest that PD-1, PD-L1, NY-ESO-1, and MAGE-A4 might be predictors of response to immune molecular inhibitors in UPS, MFS, and MPNST.
Figure 4.
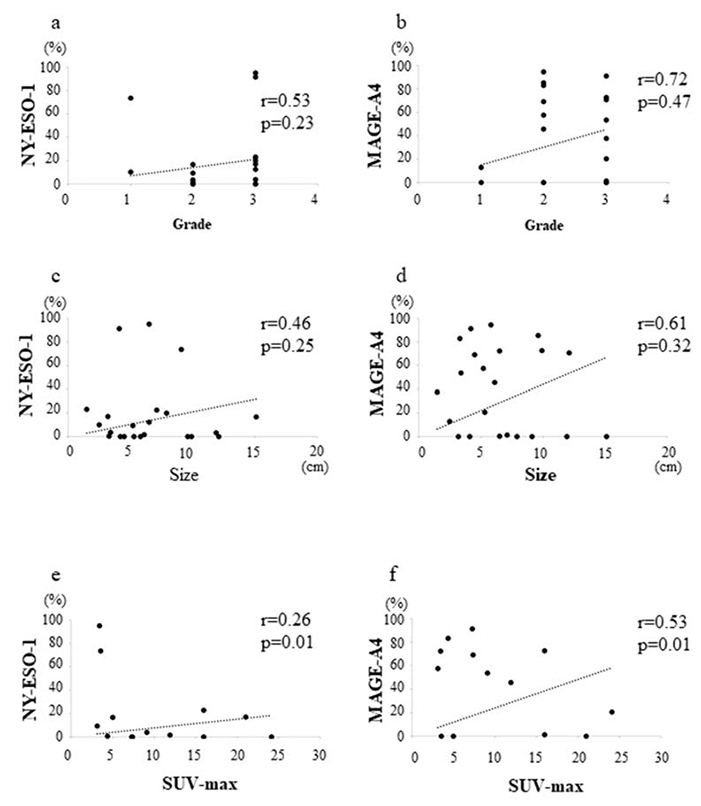
Graphs showing no significant correlation between the histological grade and NY-ESO-1 positivity rate (r=0.53, p=0.23) (a); no significant correlation between the histological grade and MAGE-A4 positivity rate (=0.72, p=0.47) (b); no significant correlation between the tumor size and NY-ESO-1 positivity rate (r=0.46, p=0.25) (c); no significant correlation between the tumor size and MAGE-A4 positivity rate (r=0.61, p=0.32) (d); weak positive correlation between the SUVmax value and NY-ESO-1 positivity rate (r=0.26, p=0.01) (e); and strong positive correlation between the SUVmax value and MAGE-A4 positivity rate (r=0.53, p=0.01) (f ) in STSs.
In general, larger size, higher histological grade, and higher SUVmax values have been reported as poor prognostic factors for STSs.35-37 Previous studies have reported that tumor size, histological grade, and SUVmax value are adverse prognostic factors in UPS,38-40 MFS, 35,40,41 and MPNST. 40,42-44 SUVmax has also been reported to be associated with tumor activity such as tumor necrosis and mitosis.45 In the current study, SUVmax were correlated with PD-1, PD-L1, NY-ESO-1, and MAGE-A4 expression. These findings suggest that PD-1, PD-L1, NY-ESO-1, and MAGE-A4 expression might indicate a poor prognosis. PD-1, PD-L1, NYESO- 1, and MAGE-A4 were also suggested to be potentially involved in the tumor activity of UPS, MFS, and MPNST.
This study had some limitations that need to be addressed. First, the small sample size may have affected the significance of the findings and the potency of the statistical results in this study. Secondly, the confirmation of diagnosis of UPS, MFS, and MPNST was not performed. Thirdly, since only immunostaining studies were used in this study, PD-1, PD-L1, NY-ESO-1, and MAGE-A4 expression at the genetic level was not confirmed. Fourthly, we could not investigate the downstream pathways or other immune molecules such as the PI3K/Akt/mTOR signaling pathway for PD-1/PD-L1, 46 or p53-MDM2 or p16/Rb pathways for NY-ESO/MAGE-A4.47 Finally, the direct effects of PD-1, PDL1, NY-ESO-1, and MAGE-A4 on the pathogenesis of STSs have not been elucidated. Despite these limitations, the current study provides evidence for the involvement of PD-1, PD-L1, NY-ESO- 1, and MAGE-A4 in STS pathogenesis and prognosis. However, further studies with larger sample sizes are required to validate these findings.
Our results suggest that PD-1, PD-L1, NY-ESO-1, and MAGE-A4 may be involved in UPS, MFS, and MPNST with aggressive clinical behavior and could be used as diagnostic and prognostic markers for highly aggressive STSs.
Acknowledgements
The authors would like to thank Chikoto Tanaka for providing technical assistance.
References
- 1.Morrison BA. Soft tissue sarcomas of the extremities. Proc (Bayl Univ Med Cent) 2003;16:285-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan M, Alektiar KM, Maki RG. Soft tissue sarcoma. In: DeVita VT, Hellmann S, Rosenberg SA, editors: Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins; 2001-1891. pp 1841. [Google Scholar]
- 3.Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol 2005;23:96-104. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Tepper J, Glatstein E, Costa J, Young R, Baker A, et al. Prospective randomized evaluation of adjuvant chemotherapy in adults with soft tissue sarcomas of the extremities. Cancer 1983;52:424-34. [DOI] [PubMed] [Google Scholar]
- 5.Hoefkens F, Dehandschutter C, Somville J, Meijnders P, Van Gestel D. Soft tissue sarcoma of the extremities: pending questions on surgery and radiotherapy. Radiat Oncol 2016;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strander H, Turesson I, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol 2003;42:516-31. [DOI] [PubMed] [Google Scholar]
- 7.Pang A, Carbini M, Maki RG. Contemporary therapy for advanced soft-tissue sarcomas in adults: a review. JAMA Oncol 2016;2:941-7. [DOI] [PubMed] [Google Scholar]
- 8.Kawai A, Yonemori K, Takahashi S, Araki N, Ueda T. Systemic therapy for soft tissue sarcoma: proposals for the optimal use of pazopanib, trabectedin, and eribulin. Adv Ther 2017;34:1556-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15:415-23. [DOI] [PubMed] [Google Scholar]
- 10.Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell 2019;178:346-60.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002;188:22-32. [DOI] [PubMed] [Google Scholar]
- 12.Burgess M, Tawbi H. Immunotherapeutic approaches to sarcoma. Curr Treat Options Oncol 2015;16:26. [DOI] [PubMed] [Google Scholar]
- 13.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015;21:914-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weon JL, Potts PR. The MAGE protein family and cancer. Curr Opin Cell Biol 2015;37:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, et al. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine 2014;32:5901-7. [DOI] [PubMed] [Google Scholar]
- 16.Smolle MA, Herbsthofer L, Granegger B, Goda M, Brcic I, Bergovec M, et al. T-regulatory cells predict clinical outcome in soft tissue sarcoma patients: a clinico-pathological study. Br J Cancer 2021;125:717-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orth MF, Buecklein VL, Kampmann E, Subklewe M, Noessner E, Cidre-Aranaz F, et al. A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol Immunother 2020;69:1353-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakimoto T, Matsumine A, Kageyama S, Asanuma K, Matsubara T, Nakamura T, et al. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol Lett 2019;17:3937-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iura K, Maekawa A, Kohashi K, Ishii T, Bekki H, Otsuka H, et al. Cancer-testis antigen expression in synovial sarcoma: NYESO- 1, PRAME, MAGEA4, and MAGEA1. Hum Pathol 2017;61:130-9. [DOI] [PubMed] [Google Scholar]
- 20.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37-42. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem. 2021;65:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 1996;14:869-77. [DOI] [PubMed] [Google Scholar]
- 23.Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in softtissue sarcoma. Oncoimmunology 2017;6:e1279777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ, et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology 2018;7:e1442168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iura K, Kohashi K, Ishii T, Maekawa A, Bekki H, Otsuka H, et al. MAGEA4 expression in bone and soft tissue tumors: its utility as a target for immunotherapy and diagnostic marker combined with NY-ESO-1. Virchows Arch 2017;471:383-92. [DOI] [PubMed] [Google Scholar]
- 26.Boxberg M, Steiger K, Lenze U, Rechl H, von Eisenhart-Rothe R, Wörtler K, et al. PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue – prognostic implications and rationale for immunotherapy. Oncoimmunology 2018;7:e1389366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017;123:3291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol 2015;46:357-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shurell E, Singh AS, Crompton JG, Jensen S, Li Y, Dry S, Nelson S, et al. Characterizing the immune microenvironment of malignant peripheral nerve sheath tumor by PD-L1 expression and presence of CD8+ tumor infiltrating lymphocytes. Oncotarget 2016;7:64300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemminger JA, Iwenofu OH. NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms. Mod Pathol 2013;26:1204-10. [DOI] [PubMed] [Google Scholar]
- 31.Endo M, de Graaff MA, Ingram DR, Lim S, Lev DC, Briairede Bruijn, et al. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol 2015;28:587-95. [DOI] [PubMed] [Google Scholar]
- 32.Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556-60. [DOI] [PubMed] [Google Scholar]
- 33.Raj S, Miller LD, Triozzi PL. Addressing the adult soft tissue sarcoma microenvironment with intratumoral immunotherapy. Sarcoma 2018;2018:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae JY, Choi KU, Kim A, Lee SJ, Kim K, Kim J, et al. Evaluation of immune-biomarker expression in high-grade soft-tissue sarcoma: HLA-DQA1 expression as a prognostic marker. Exp Ther Med 2020;20:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuglø HM, Jørgensen SM, Loft A, Hovgaard D, Petersen MM. The diagnostic and prognostic value of ¹⁸F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol Imaging 2012;39:1416-24. [DOI] [PubMed] [Google Scholar]
- 36.Koea JB, Leung D, Lewis JJ, Brennan MF. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol 2003;10:432-40. [DOI] [PubMed] [Google Scholar]
- 37.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol 2003;21:2719-25. [DOI] [PubMed] [Google Scholar]
- 38.Lou Y, Wan W, Wu Z, Yang J, Xu K, Huang Q, et al. Prognostic factors for patients with undifferentiated high grade pleomorphic sarcoma of the spine. Spine (Phila Pa 1976) 2019;44:E539-48. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Huang W, Luo P, Cai W, Yang L, Sun Z, et al. Undifferentiated pleomorphic sarcoma: long-term follow-up from a large institution. Cancer Manag Res 2019;11:10001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chodyla M, Demircioglu A, Schaarschmidt BM, Bertram S, Morawitz J, Bauer S, et al. Evaluation of the predictive potential of 18F-FDG PET and DWI data sets for relevant prognostic parameters of primary soft-tissue sarcomas. Cancers 2021;13:2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewan V, Darbyshire A, Sumathi V, Jeys L, Grimer R. Prognostic and survival factors in myxofibrosarcomas. Sarcoma 2012;2012:830879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998;42:351-60. [DOI] [PubMed] [Google Scholar]
- 43.Martin E, Coert JH, Flucke UE, Slooff WM, Ho VKY, van der Graaf WT, et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur J Cancer 2020;124:77-87. [DOI] [PubMed] [Google Scholar]
- 44.Yadav D, Shamim SA, Rastogi S, Upadhyay DMR, Pandey AK, Kumar R. Role of 18F-FDG PET/computed tomography in prognostication and management of malignant peripheral nerve sheath tumors. Nucl Med Commun 2020;41:924-32. [DOI] [PubMed] [Google Scholar]
- 45.Rakheja R, Makis W, Skamene S, Nahal A, Brimo F, Azoulay L, et al. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: a retrospective review of 136 patients. AJR Am J Roentgenol 2012;198:1409-16. [DOI] [PubMed] [Google Scholar]
- 46.Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, et al. PD-1/PDL1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif 2019;52:e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forghanifard MM, Gholamin M, Farshchian M, Moaven O, Memar B, Forghani MN, et al. Cancer-testis gene expression profiling in esophageal squamous cell carcinoma: identification of specific tumor marker and potential targets for immunotherapy. Cancer Biol Ther 2011;12:191-7. [DOI] [PubMed] [Google Scholar]


