Abstract
Myocarditis is a rare complication of the COVID-19 mRNA vaccine. We previously reported a case series of 15 adolescents with vaccine-associated myocarditis, 87% of whom had abnormalities on initial cardiac magnetic resonance (CMR), including late gadolinium enhancement (LGE) in 80%. We performed follow-up CMRs to determine the trajectory of myocardial recovery and better understand the natural history of vaccine-associated myocarditis. Case series of patients age < 19 years admitted to Boston Children’s Hospital with acute vaccine-associated myocarditis following the BNT162b2 vaccine who had abnormal CMR at the time of initial presentation, and underwent follow-up testing. CMR assessment included left ventricular (LV) ejection fraction, T2-weighted myocardial imaging, LV global native T1, LV global T2, extracellular volume (ECV), and late gadolinium enhancement (LGE). Ten patients (9 male, median age 15 years) with vaccine-associated myocarditis underwent follow-up CMR at a median of 92 days (range 76–119) after hospital discharge. LGE was persistent in 80% of patients, though improved from prior in all cases. Two patients (20%) had abnormal LV global T1 at presentation, which normalized on follow-up. ECV decreased between acute presentation and follow-up in 6/10 patients; it remained elevated at follow-up in 1 patient and borderline in 3 patients.
Conclusion: CMR performed ~3 months after admission for COVID-19 vaccine-associated myocarditis showed improvement of LGE in all patients, but persistent in the majority. Follow-up CMR 6–12 months after acute episode should be considered to better understand the long-term cardiac risks.
|
What is Known: • Myocarditis is a rare side effect of COVID-19 mRNA vaccine. •Late gadolinium enhancement is present on most cardiac magnetic resonance at the time of acute presentation. | |
|
What is New: •Late gadolinium enhancement improved on all repeat cardiac magnetic resonance at 3-month follow-up. •Most patients still had a small amount of late gadolinium enhancement, the clinical significance of which is yet to be determined. |
Supplementary information
The online version contains supplementary material available at 10.1007/s00431-022-04482-z.
Keywords: COVID-19 mRNA vaccine, Vaccine-associated myocarditis follow-up, Cardiac magnetic resonance imaging, Late gadolinium enhancement
Introduction
Myocarditis is a rare complication following mRNA vaccination for COVID-19. We previously reported a case series of 15 patients with vaccine-associated myocarditis, of whom 13 (87%) had cardiac magnetic resonance (CMR) abnormalities at the time of acute presentation, including late gadolinium enhancement (LGE) in 12 (80%) and myocardial edema in 2 (13%) [1]. While acute courses were overall relatively benign, LGE has been associated with adverse cardiac events (sudden cardiac death, resuscitated cardiac arrest, heart failure hospitalization) in non-vaccine-associated myocarditis [2–4]. Therefore, the natural history of LGE is of public health importance in determining longer-term risk stratification. We report the results of follow-up CMRs to provide early data on the trajectory of recovery of myocardial injury.
Methods
Case series of patients age < 19 years admitted to Boston Children’s Hospital with acute vaccine-associated myocarditis following the BNT162b2 vaccine between June and July 2021. The Center for Disease Control’s (CDC) definition for post-vaccine myocarditis was used, and cases were classified as “confirmed” or “probable” [5, 6]. From the 15 patients in our initial series, 10 underwent follow-up CMR as part of routine clinical care at a median of 3.0 (range 2.5–4.9) months after diagnosis. Three patients had normal CMR at presentation and no clinical indication for repeat testing, and 2 patients are still awaiting scheduling. The study was approved by the institutional review board and was deemed exempt from consent owing to the use of deidentified data and the requirements of 45 CFR § 46.
CMR assessment was performed using a Phillips 1.5 T Achieva-dStream (Supplemental Methods) and included left ventricular (LV) ejection fraction, T2-weighted myocardial imaging, LV global native T1, LV global T2, ECV, and LGE. Ventricular systolic dysfunction was defined as LV ejection fraction equal to or less than 55%. Based on local reference values, elevated LV global native T1 was defined as > 1100 ms, LV global T2 as > 60 ms, and LV global ECV > 30% (borderline 28–30%). All CMRs were qualitatively reviewed (AP) for the presence and extent of LGE. Quantitative variables were summarized as median and range, and categorical variables as frequencies and percentages.
Results
Ten patients (9 male, median age 15 [range 12–18] years) with vaccine-associated myocarditis (3 confirmed, 7 probable) underwent follow-up CMR at a median of 92 days (range 76–119) after hospital discharge (Table 1).
Table 1.
Clinical findings in children with vaccine-associated myocarditis at presentation and follow-up
| Patient characteristics | Presentation | Follow-up |
|---|---|---|
| Age | 15 [12, 18] | |
| Sex, no. (%) | ||
| Male | 9 (90) | |
| Female | 1 (10) | |
| Race/ethnicity, no. (%) | ||
| Non-Hispanic White | 4 (40) | |
| Hispanic White | 2 (20) | |
| Non-Hispanic other | 1 (10) | |
| Unknown | 3 (30) | |
| Time from onset of symptoms, days | 4 [2,7] | 93 [71, 145] |
| Cardiac symptoms, no. (%) | ||
| Chest pain, no. (%) | 10 (100) | 2 (20) |
| Fever, no. (%) | 6 (60) | 0 (0) |
| Myalgia, no. (%) | 6 (60) | 0 (0) |
| Headache, no. (%) | 5 (50) | 0 (0) |
| Fatigue, no. (%) | 3 (30) | 1 (10) |
| Duration of hospitalization, days | 3 [1,5] | |
| Intensive care unit admission, no. (%) | 0 (0) | |
| Immunomodulatory treatment, no. (%) | 6 (60) | |
| Laboratory values | ||
| Elevated troponin level, (normal > 0.01 ng/mL) | 10 (100) | 3 (30) |
| BNP, pg/mL (normal < 10) | 25.5 [10, 118] | < 10 [< 10, 64] |
| CRP, mg/dL (normal < 0.50) | 3.0 [0.7, 7.7] | 0.2 [0.0, 1.5] |
| ECG | ||
| ST-T wave segment changes, no. (%) | 7 (70) | 2 (20) |
| Echocardiogram | ||
| Ejection fraction (%) | 55 [44, 61] | 61 [57, 67] |
| Abnormal strain (circumferential and/or longitudinal), no. (%) | 5 (50) | 0 (0) |
| Cardiac MRI | ||
| Regional hyperintensity on T2-weighted imaging, no. (%) | 2 (20) | 0 (0) |
| Extracellular volume fraction (%) | 27 [22, 37] | 25 [23, 31] |
| LV global T2, ms | 50 [47, 55] | 49 [46, 53] |
| LV global native T1, ms | 1016 [963, 1121] | 1013 [982, 1065] |
| Late gadolinium enhancement, No. (%) | 10 (100) | 8 (80) |
| Met original or revised Lake Louise criteria, no. (%) | 2 (20) | 0 (0) |
Since hospital discharge, the majority of patients have been asymptomatic (Table 1). Two patients subsequently presented to the emergency room with chest pain in the setting of acute COVID-19 infection; in both cases, cardiac evaluation with laboratory data and electrocardiogram (ECG) was unremarkable and neither patient required hospital admission. One patient continues to endorse fatigue, and the other endorses chest pain though to be musculoskeletal in origin. Two patients continue to have non-specific ST-T segment changes on follow-up ECG; all other ECG and echocardiograms were normal at most recent follow-up.
All patients had normal ventricular systolic function on follow-up CMR. LGE was persistent in 8 patients (80%), although improved from prior in all cases. Persistent LGE was subepicardial in 5 patients, mid-wall in 3, sub-endocardial in 1; and localized to the basal infero-lateral region in 6 patients, basal inferior region in 1 patient, and basal LV free wall in 1 patient (Fig. 1). The two patients with complete resolution had initial mid-wall LGE in the mid LV, at the junction of the anterolateral and infero-lateral segments; none had basal LGE. Due to the small amount of LGE on acute and follow-up CMR, no quantification of LGE could be performed.
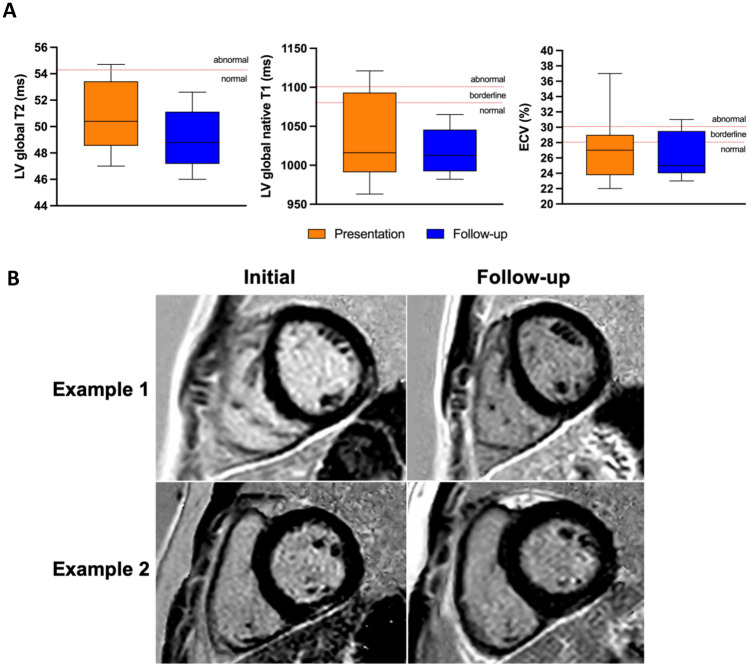
Fig. 1.
A Left ventricular (LV) global T1, LV global T2, and extracellular volume (ECV) on CMR at presentation and follow-up in children with vaccine-associated myocarditis. B Cardiac magnetic resonance short-axis views at presentation and follow-up. Example 1: LGE in the mid-wall of the mid left ventricle at the junction of the anterolateral and inferolateral segments, which completely resolved on follow-up. Example 2: late gadolinium enhancement (LGE) in the inferior basal segment, with significant improvement but slight persistent findings on follow-up
LV global T2 was within normal limit in all patients, both at presentation and on follow-up. Two patients (20%) had abnormal LV global T1 at presentation, which normalized on follow-up. ECV decreased between acute presentation and follow-up in 6/10 patients; it remained elevated at follow-up in 1 patient and borderline in 3 patients (Supplemental Fig. 1); ECV was unable to be measured for 1 patient.
Discussion
In this follow-up case series of ten patients previously admitted for COVID-19 vaccine-associated myocarditis, we report improvement in CMR findings 2.5–4 months after hospital discharge, though most patients still had subtle LGE on follow-up CMR.
LGE was the most frequent finding on CMR following vaccine-associated myocarditis. Large, adult studies investigating CMR changes following COVID-19 infection did not find evidence of myocardial LGE in their healthy controls, suggesting that these were not just incidental findings that can be found in the general population [7]. In addition, all patients included in this series met the CDC definition for post-vaccine myocarditis, with elevated troponin level and frequently abnormal ECG [1, 5, 6].
A study of 52 children with viral or idiopathic myocarditis evaluated CMR findings acutely and at median 6 months (range 0.2–69) follow-up [8]. At presentation, ventricular dysfunction was found in 45% patients on echocardiogram and LGE in 81% on CMR. On follow-up, all patients were alive and 5 underwent cardiac transplantation. Ventricular function improved in 81% of patients with dysfunction and 5% developed new ventricular dysfunction (mean EF on CMR 60% vs. 59% at presentation, p = 0.016). LGE was found in 83% patients on follow-up (persistent in 75%, new in 8%). Our cohort of patients with post-vaccine-associated myocarditis had a more benign course compared to children with viral or idiopathic myocarditis. Ventricular dysfunction was much less common at time of presentation, and all normalized on follow-up. This was also the case in larger, multicenter series of children with vaccine-associated myocarditis [9]. However, the findings on follow-up CMR in regard to LGE are similar to our cohort of patients, with LGE in 80% on follow-up.
The presence of LGE in the acute phase of myocarditis indicates cellular damage and can represent edema or necrosis [10]. Persistence of LGE on CMR may represent the formation of fibrosis and myocardial scar, which has been shown to predispose to long-term cardiac risk in non-vaccine-associated myocarditis [2–4]. However, the process of resolution may still be underway in our patient cohort at this time, and follow-up at a later duration (6–12 months) may be more useful as it relates to long-term prognosis. Data on the use of CMR as prognostic tool in myocarditis is limited to LGE and does not include mapping imaging values (T1, T2, ECV) which have laboratory specific normative values [9, 11].
Children with myocarditis following COVID-19 infection, most often seen in multisystem inflammatory syndrome in children (MIS-C), have shown rapid recovery of ventricular function on echocardiogram in the COVID-19 cohort [12]. However, patients with post-vaccine myocarditis have much more frequent CMR abnormalities than those with COVID-19 infection and MIS-C [13]. The well documented association between LGE on CMR and long-term cardiac risk in acute myocarditis raises concern for patients with vaccine-associated myocarditis, despite their relatively benign course acutely [2–4].
Conclusion
In adolescents with vaccine-associated myocarditis and LGE on CMR at time of presentation, follow-up CMR 2.5–4 months after discharge showed markedly improved LGE in all patients but persistence in the majority. The clinical significance of this persistence is yet to be determined. The finding of myocardial fibrosis beyond the acute phase emphasizes the importance of longer-term follow-up of these patients.
Supplementary information
Below is the link to the electronic supplementary material.
Abbreviations
- CMR
Cardiac magnetic resonance
- ECV
Extracellular volume
- LGE
Late gadolinium enhancement
- LV
Left ventricular
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AD, KF, and SH. Cardiac magnetic resonance were independently reviewed by AP. The first draft of the manuscript was written by SH and all authors critically reviewed the manuscript. All authors read and approved of the final manuscript.
Availability of data and material
Data can be made available upon request.
Declarations
Ethics approval
The study was approved by the institutional review board.
Consent to participate
This study was deemed exempt from consent owing to the use of deidentified data and the requirements of 45 CFR § 46.
Consent for publication
No identifiable data, not applicable.
Competing interests
JN—consultant for Pfizer for vaccine-associated myocarditis (pending).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stephanie M. Hadley, Email: Stephanie.hadley@childrens.harvard.edu
Ashwin Prakash, Email: Ashwin.prakash@cardio.chboston.org.
Annette L. Baker, Email: Annette.baker@cardio.chboston.org
Sarah D. de Ferranti, Email: Sarah.deferranti@cardio.chboston.org
Jane W. Newburger, Email: Jane.newburger@cardio.chboston.org
Kevin G. Friedman, Email: Kevin.friedman@cardio.chboston.org
Audrey Dionne, Email: Audrey.dionne@cardio.chboston.org.
References
- 1.Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, Castellanos DA, Saleeb SF, de Ferranti SD, Newburger JW, Friedman KG. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA cardiology. 2021;6(12):1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang F, Wang J, Li W, Xu Y, Wan K, Zeng R, Chen Y. The prognostic value of late gadolinium enhancement in myocarditis and clinically suspected myocarditis: systematic review and meta-analysis. Eur Radiol. 2020;30(5):2616–2626. doi: 10.1007/s00330-019-06643-5. [DOI] [PubMed] [Google Scholar]
- 3.Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo MM. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74(20):2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 4.Sachdeva S, Song X, Dham N, Heath DM, DeBiasi RL. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am J Cardiol. 2015;115(4):499–504. doi: 10.1016/j.amjcard.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. Morb Mortal Wkly Rep. 2021;70(27):977. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassani NS, Talakoob H, Karim H, Bazargany MHM, Rastad H. Cardiac magnetic resonance imaging findings in 2954 COVID-19 adult survivors: a comprehensive systematic review. J Magn Reson Imaging. 2022;55(3):866–880. doi: 10.1002/jmri.27852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, Richmond ME, Fratz S, Jain S, Johnson TR, Maskatia SA. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: a multicenter retrospective study. J Cardiovasc Magn Reson. 2015;17(1):1–8. doi: 10.1186/s12968-015-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LM, Thacker D, Elias MD, Li JS, Toro-Salazar OH, Anderson BR. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 10.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 11.Grigoratos C, Di Bella G, Aquaro GD. Diagnostic and prognostic role of cardiac magnetic resonance in acute myocarditis. Heart Fail Rev. 2019;24(1):81–90. doi: 10.1007/s10741-018-9724-x. [DOI] [PubMed] [Google Scholar]
- 12.Felstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared to severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SA, Buddhe S, Misra N, Ramachandran P, Gaur L, Eshtehardi P (2021) COVID-19 vaccination–associated myocarditis in adolescents. Pediatrics 148(5) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available upon request.