Abstract
Background
Lumbar puncture (LP) may be challenging for patients with scoliosis and other conditions following previous posterior fusion and instrumentation from thoracic to sacral levels. Interventional radiologists have described CT approaches to transforaminal LP. We hypothesized that combined C-arm fluoroscopy and ultrasound could be a feasible approach to transforaminal LP for interventional pain physicians and regional anesthesiologists.
Methods
With institutional review board approval, we reviewed medical records and imaging of six patients with spinal muscular atrophy and prior spine fusion. Non-cutting needles of 24 or 25 gage were advanced through 20-gage introducers. Prior imaging guided selection of a preferred side and spinal level. Initial procedures were performed in the interventional radiology suite. Subsequent procedures were performed in an operating room (OR). We report on technical success and complications and describe a case using this approach for spinal anesthesia.
Results
Six patients underwent a total of 54 transforaminal LPs, including 51 for administration of the antisense oligonucleotide nusinersen, 2 for myelography, and 1 for spinal anesthesia; 45 of these procedures were performed using OR C-arm fluoroscopy and ultrasound. Transient paresthesias and short-term headaches occurred; none required intervention. No other complications were noted.
Conclusions
Transforaminal LP appears technically feasible for patients with full-spine fusions using a straight-needle approach with combined fluoroscopy and ultrasound guidance. Larger case series and prospective studies may better define the success rates, risks, and benefits of this approach relative to alternative approaches to intrathecal access for patients with previous long-segment posterior spine fusions.
Keywords: diagnostic techniques and procedures; injections, spinal; multimodal imaging; pediatrics
Introduction
Lumbar puncture (LP) is traditionally performed via an interlaminar approach using either midline or paramedian approaches. Many patients with idiopathic scoliosis undergo posterior spine fusion and instrumentation with sparing of the lumbosacral junction, permitting interlaminar LP at lower lumbar levels. In contrast to approaches for idiopathic scoliosis, a traditional surgical approach for patients with neuromuscular scoliosis has been to extend posterior spine fusion and instrumentation from thoracic levels to the sacrum or sacrum and ilium, leaving no feasible interlaminar opening for subsequent spinal needle entry. Practical techniques for LP in these situations could facilitate spinal anesthesia, spinal medication delivery, diagnostic LP, or myelography.
In recent years, the requirement for spinal medication delivery in patients with previous extensive posterior spine fusion for neuromuscular scoliosis has become especially relevant due to use of the antisense oligonucleotide nusinersen as a disease-modifying therapy for patients with spinal muscular atrophy.1 Nusinersen is administered via LP in a series of four loading doses over 2 months followed by a maintenance regimen requiring three LPs per year long term.
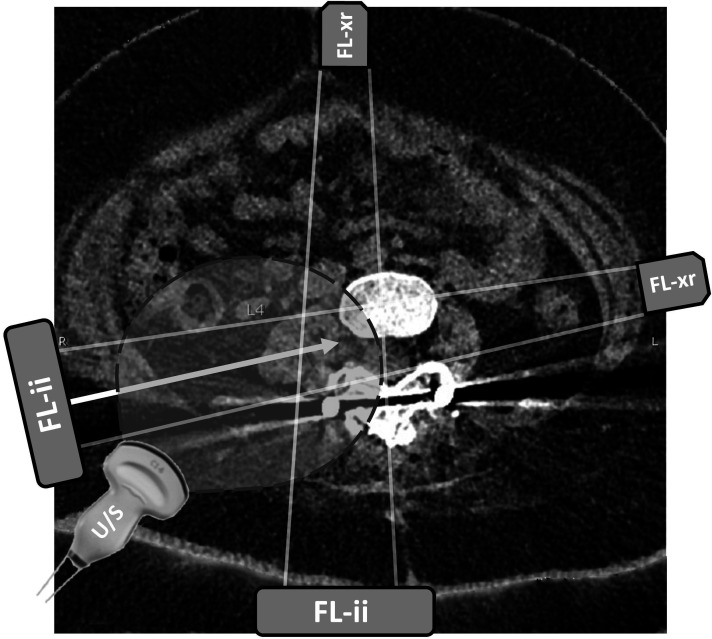
Previous approaches for transforaminal LP for delivery of nusinersen have been reported by interventional radiologists, primarily using cross-sectional CT imaging and navigation software.2–4 In our view, transforaminal LP is facilitated by a more lateral skin entry than is typically used for transforaminal epidural injections5 6 (figure 1). A more lateral approach could potentially route the needle near the bowel, kidney, or lung, especially in patients with severe scoliosis and atypical locations of viscera and lung.
Figure 1.
Axial CT view at the level of lumbar vertebra 4. Axial CT image shows locations of the lumbar spine, with planned needle trajectory shown with the solid arrow. Projected needle path crosses the posterior aspect of the peritoneum but avoids the bowel and other viscera. Atrophic psoas muscle is seen immediately lateral to the spine. Fluoroscopy beam positions for transforaminal lumbar puncture are shown with source (FL-xr) and image intensifier (FL-ii) in tunnel view and anterior–posterior view. Curvilinear ultrasound (U/S) probe position and field of view are drawn over the image. Image included with patient assent and parental consent. LP, lumbar puncture.
We describe here a modified straight-needle approach to image-guided transforaminal LP using combined C-arm fluoroscopy and ultrasound guidance, with application for spinal anesthesia, myelography, intrathecal dosing of nusinersen or other novel medications, cancer chemotherapy, or trial dosing of baclofen or morphine prior to intrathecal pump or port placement.7 8 Since the current approach to transforaminal LP does not require cone beam CT or Dyna CT guidance, it may be especially practical for interventional pain physicians and anesthesiologists specializing in regional anesthesia, either in a pain clinic or in the operating room (OR). We hypothesized that combined use of fluoroscopy and ultrasound for transforaminal LP would show preliminary evidence for technical success and safety.
Methods
With institutional review board approval, we reviewed the medical records of six patients who had a total of 54 transforaminal LPs at Boston Children’s Hospital from June 2019 to July 2021. Patients/parents also consented to inclusion of images and clinical description in this paper.
Technique
Previous lumbar spine CT images were reviewed to identify a preferred foramen for a needle path that could avoid iliac crest, bowel, kidney, and pleura (figure 1). For each of these patients, a first procedure was performed in the interventional radiology (IR) suite under collaboration with an interventional radiologist and an anesthesiologist–pain physician (CB, AKo, and BLR). Subsequent procedures were performed by anesthesiologist–pain physicians in an OR procedure suite.
Standard interlaminar approaches to LP and spinal anesthesia in adults commonly use 8.9 cm (3.5-inch) needles. Occasional patients with large body mass indices require 12.7 cm (5-inch) needles or, very rarely, 17.8 cm (7-inch) needles for interlaminar LP. Early in the development of this approach, one procedure was attempted using a 17.8 cm needle, the longest spinal needle available at our hospital at that time. The needle length was insufficient to reach the dura, so the procedure was aborted.
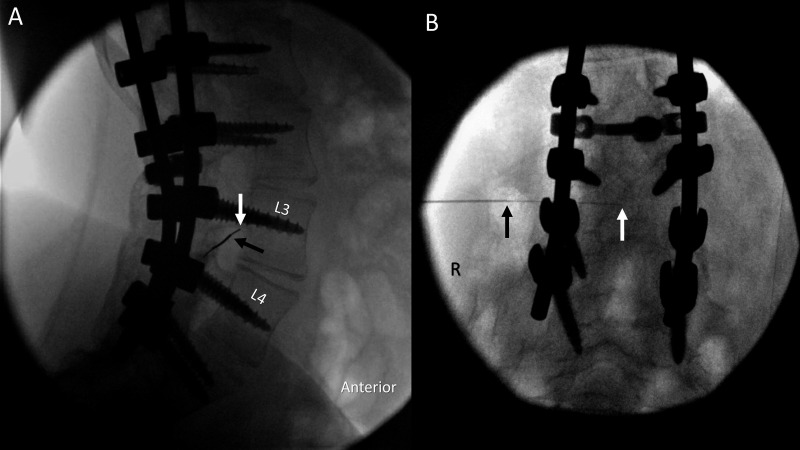
We subsequently identified 25-gage non-cutting spinal needles (Gertie Marx, International Medical Development, Huntsville, Utah, USA) available in lengths of 19.0 or 21.5 cm. Because these needles are so long and thin, it became apparent that standard 20-gage 3.2–3.8 cm introducer needles commonly used for routine spinal anesthesia were insufficient to prevent needle deflection from the intended path. Depending on the projected distance from skin to dura and the length of the spinal needle required, 24-gage Sprotte 12 cm needles were placed through 20-gage 7.6 cm (3-inch) introducer needles, and 25-gage Gertie Marx 19.5 or 21.5 cm needles were placed through either 7.6 or 15.2 cm 20-gage introducer needles (figure 2). In general, the length of the introducer should be chosen to permit 4–5 cm of the smaller gage spinal needle to extend past the introducer tip. Needle advancement is guided by fluoroscopy in lateral tunnel view and anterior–posterior views (figure 2), with repeated checks using ultrasound in an axial in plane view to reconfirm that the needle trajectory avoids the bowel, kidney, or lung (figure 3).
Figure 2.
Needle advancement using fluoroscopic guidance. A thin spinal needle enters the spinal canal via transforaminal approach through an introducer needle in lateral tunnel (A) and anterior–posterior (B) views. Arrows show positions of the introducer needle tip (black) and the thin spinal needle tip (white). Image included with patient assent and parental consent. R, right.
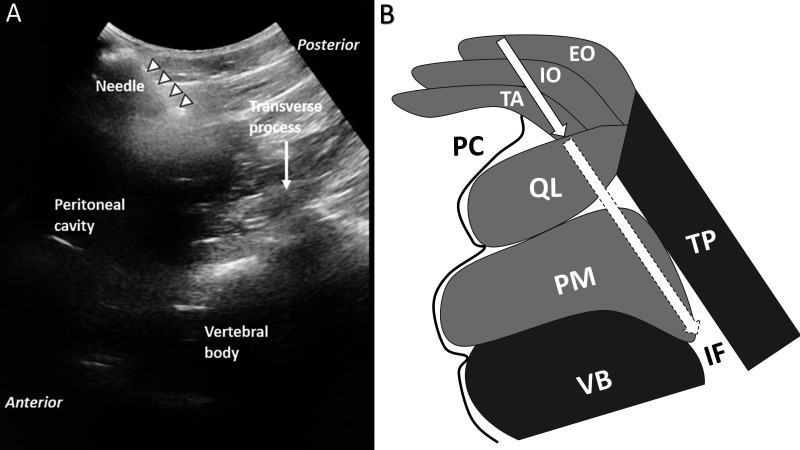
Figure 3.
Ultrasound view. (A) Ultrasound view of needle trajectory. Curvilinear robe is oriented in an axial in-plane view to observe the introducer needle and its anticipated trajectory toward the spine. In this example, the needle trajectory extends just along the posterior border of the peritoneum toward the foramen. (B) Drawing of structures shown in A. Solid border on arrow shows the needle advanced to the depth shown in A. Dashed border on arrow shows the anticipated trajectory of the needle into the foramen. Image included with patient consent. EO, external oblique; IF, intervertebral foramen; IO, internal oblique; PC, peritoneal cavity; PM, psoas major; QL, quadratus lumborum; TA, transversus abdominis; TP, transverse process; VB, vertebral body.
Procedure steps are outlined in box 1.
Box 1. Summary of procedural steps.
Select preferred spinal level, side, and needle trajectory from prior CT axial views and/or from prior use of Dyna-CT in interventional radiology suite.
-
Operating room fluoroscopy–ultrasound technique.
i. Position in lateral decubitus with preferred non-dependent side. Apply monitors and administer sedation and analgesia as indicated.
ii. Confirm that C-arm range of angles permits anatomical lateral and AP spine views or adjust the patient’s position to permit these views. Relative positions of rods, pedicle screws, and vertebral bodies are helpful guides to correct for obliquity.
iii. Prepare and drape the field.
iv. Identify the foramen in lateral (tunnel) fluoroscopy view and infiltrate local anesthetic.
v. Advance 20-gage introducer needle of appropriate length (see the Methods section and figure 2) initially toward the center of the foramen.
vi. Use ultrasound in an axial in-plane view to identify retroperitoneal structures (especially kidney) and peritoneal structures (especially bowel) near the projected needle path (figure 3). If trajectory passes too close to the viscera, remove the introducer; select a more posterior skin entry point; and repeat the fourth to sixth steps.
vii. Alternate between lateral tunnel and anterior posterior fluoroscopy views and continue advancing the introducer.
viii. Repeat the fifth to seventh steps until introducer tip is 3–4 cm from the spine on the AP view. Pass the non-cutting needle of 24 or 25 gage through the introducer and tilt very slightly to advance the thin spinal needle into the foramen (either subpedicular or infraneural approach) using combined lateral and AP fluoroscopic guidance.
ix. A pop on dural entry is often, but not always, appreciated. If the needle tip appears near mid-canal on the AP view, remove the stylette and gently aspirate for CSF.
x. The spinal needle may fall inward if unattended. Paresthesias referred to the dependent side should raise concern for excessive needle advancement.
xi. Sample CSF and inject the therapeutic substance (antisense oligonucleotide, myelography, and spinal anesthesia) as appropriate.
Results
Six patients each received between 8 and 10 procedures over the study time period, for a total of 54 procedures. Among these 54 procedures, 9 were performed in the IR suite and 45 were performed in an OR procedure room using the C-arm fluoroscopy and ultrasound approach described here. All had the diagnosis of spinal muscular atrophy, and all had undergone prior posterior fusion from T2 to sacrum.
All patients received at least seven doses of nusinersen. Five of the six patients had a first transforaminal LP for dosing of nusinersen in the IR suite and all subsequent procedures in the OR using fluoroscopy and ultrasound. The sixth patient, described in the following case, had a first LP as a myelogram via transforaminal approach in the IR suite and all subsequent procedures, including seven doses of nusinersen and one spinal anesthetic, in the OR.
Ages ranged from 11 years to 52 years at the start, 14–53 years at present. Weights ranged from 22 kg to 107 kg. Needle lengths ranged from 5 cm (25-gage Pencan) to 21.5 cm (25-gage Gertie Marx).
For the cases done with OR C-arm and ultrasound, median estimated radiation exposure was 4.52 mGy, (IQR 2.37–13.7). For the cases done in the IR suite with Dyna CT, median estimated radiation exposure was 509 mGy (IQR 500–527).
Complications
Prior to refinement of the aforementioned approach, there was one attempted procedure that was aborted due to insufficient spinal needle length. At that time, the longest spinal needle available in our hospital was 17.8 cm (7 inches). After we obtained 19.5 and 21.5 cm needles, as described in the Methods section, we have had sufficient needle length to reach the neuraxis for all subsequent procedures. For one additional patient, prior CT imaging showed no feasible transforaminal needle path that could avoid viscera, so we did not attempt transforaminal LPs but instead have used a bone biopsy drill-based interlaminar approach similar to an approach we described previously for intrathecal pump and port placements.7 8
There were no infections, no cases of cerebrospinal fluid leak requiring epidural blood patch, and no neurological injuries. Two patients had lumbar radicular pain postprocedure. In one case, pain referral was ipsilateral to the side of needle entry and persisted for 1 week. In the other case, pain referral was to the contralateral side and persisted for 3 weeks. In both cases, radicular symptoms resolved without specific treatment. Two additional patients had transient headaches that resolved within 3 days without specific treatment.
Case description: spinal anesthesia
An adult in his late 30s with SMA II and severe weakness and cachexia underwent transforaminal LPs for three indications: myelography, nusinersen administration, and spinal anesthesia. His posterior fusion had been performed without correction of severe scoliosis, so that his thoracic and lumbar spine were displaced to the far left side of his thorax and abdomen (figure 4).
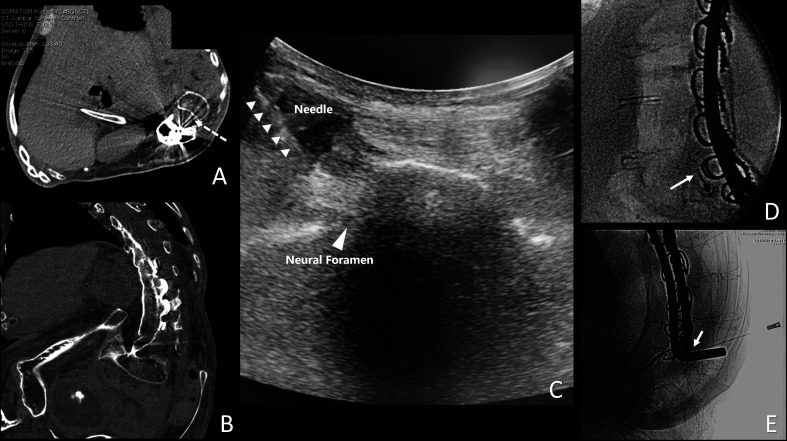
Figure 4.
Multiple views of the spine in a patient undergoing transforaminal spinal anesthesia. (A) Planned transforaminal needle trajectory (dashed arrow) from an axial view from a prior CT scan. (B) Far left: lateral position of the spine in a coronal view from a prior CT scan. (C) Ultrasound view of the needle trajectory. (D) Lateral tunnel fluoroscopic view. (E) Anterior–posterior fluoroscopic view of a 25-gage 5 cm non-cutting needle used for spinal anesthesia. Image included with patient consent.
Prior to initiation of nusinersen, he had sensory symptoms suggestive of cervical radiculopathy. Plain radiographs showing progressive cervical spine kyphotic deformity above the upper level of his long-standing T2-sacrum fusion and instrumentation. MRI was non-diagnostic due to metal artifact at the upper end of his spinal fusion. He underwent myelography via lumbar transforaminal approach in the IR suite, with subsequent CT images. Extensive degenerative changes were identified, with some canal and foraminal stenosis, but surgical intervention was not recommended. Based on defining a safe needle trajectory from this initial CT myelogram, he subsequently underwent five transforaminal LPs in an OR for administration of nusinersen using C-arm fluoroscopy and ultrasound. Because the spine was so close to the skin on the left side of the torso (figure 4), 25-gage 5 cm pediatric non-cutting needles were used without an introducer. These injections were tolerated well using local anesthetic infiltration and no systemic sedation or analgesia.
Ten months after the initial LP, he was hospitalized with a large bladder stone, with intermittent urinary tract obstruction. Per discussion with the attending urologist, cystoscopy and laser fracture of the stone was recommended on an urgent basis. Along with severe weakness, restrictive lung disease, cachexia, and the aforementioned cervical spine deformity, assessment predicted extreme difficulty for tracheal intubation, either by video or flexible fiberoptic approaches. Previous office nasal fiberoptic exam by an otolaryngologist could not visualize the laryngeal inlet. Based on review with otolaryngology colleagues and the Boston Children’s Hospital Difficult Airway team, we made a plan to conduct spinal anesthesia for this procedure. A full difficult airway set-up was prepared, including a tracheostomy set, with an otolaryngologist and a second attending anesthesiologist in attendance. Dural puncture was performed in lateral decubitus position using the same technique as for the six previous LPs. The patient’s weight was 22 kg. Bupivacaine 6 mg hyperbaric solution was administered. He was positioned sitting upright for 5 min and then in a 30° upright position for the cystoscopy. Exam confirmed dense sensory block to L3 and partial sensory block to T11. Cystoscopy and laser fracturing of the stone proceeded over the next 2 hours. After approximately 90 min, he began reporting the sensation of a full bladder during bladder irrigation. This was managed by the urologists using intermittent bladder emptying. He tolerated completion of the procedure and remained alert, conversant, and stable throughout from respiratory and hemodynamic standpoints. He subsequently received two additional transforaminal LPs for dosing of nusinersen.
Discussion
We describe here an approach to transforaminal LP that may be applicable for patients with previous posterior fusion of the entire lumbosacral spine. This approach may have application for spinal delivery of a range of medications or diagnostic LP. Examples of spinal medication delivery using this approach could include (1) local anesthetics for spinal anesthesia; (2) baclofen or morphine for trial dosing prior to intrathecal catheter and pump implantation7; (3) myelography; (4) cancer chemotherapy; or (5) novel medications that insert genes or modify gene expression, including antisense oligonucleotides, viral vectors for gene therapy, or CRISPR-based constructs. In our view, combined use of fluoroscopy and ultrasound is a practical approach for this procedure in the OR or pain clinic procedure suite. Fluoroscopy in a lateral/tunnel view guides the introducer needle and thin needle toward the foramen. Fluoroscopy in an anterior–posterior view confirms the proximity of the introducer and thin needle to the spine, and subsequently the central location of the thin needle tip within the neuraxis. Ultrasound permits real-time viewing of the introducer needle and its anticipated trajectory relative to nearby bowel, kidney, lung, peritoneum, or pleura, lowering the odds of unintended visceral puncture.
This approach does have potential limitations. It requires that the operator have combined skills in fluoroscopy (historically more common among chronic pain physicians) and in ultrasound (historically more common among regional anesthesiologists/acute pain physicians). If prolonged postdural puncture headache were to occur, epidural blood patch could be approached using a transforaminal approach.9 10 The requirement for fluoroscopy may preclude consideration of spinal anesthesia via this approach for cesarian delivery in patients with previous full-spine fusions. Transient paresthesias occurred, though no patient in this series had a persistent nerve root injury.
Conclusions
An approach described here may facilitate transforaminal LP for patients with previous posterior spine fusion and instrumentation that precludes use of lumbar interlaminar approaches. This approach may be especially applicable for interventional pain physicians and regional anesthesiologists. Larger case series and/or prospective studies may better define the success rates and frequencies of nerve root injuries or other complications of this technique.
Footnotes
Presented at: Portions were presented in preliminary form as a poster at the Society for Pediatric Pain Medicine Annual Meeting in February 2020.
Contributors: CB conceived and designed the study, wrote the text, and collaborated with HP in the adaptation of previous techniques. HP supervised all procedures in the interventional radiology suite, directed planning of approaches based on CT imaging, collaborated in editing of the manuscript. AF, AKh, and CRC were involved in review of medical records and imaging, preparation of figures, and editing of the manuscript. BLR and AKo were involved in the supervision of some of the procedures and in the review and editing of the manuscript.
Funding: Supported by the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment.
Competing interests: Research activities in the division of pain medicine are supported by philanthropy from the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment. This provides general support for research costs. No direct financial support to any of the authors. Aside from this support, none of the authors had any conflicts of interest relevant to the subject of this manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Boston Children’s Hospital Committee on Clinical Investigation (reference number IRB-P00027628). This was a retrospective review of medical records and images, and per the institutional review board, individual informed consent was not required.
References
- 1. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 2016;388:3017–26. 10.1016/S0140-6736(16)31408-8 [DOI] [PubMed] [Google Scholar]
- 2. Towbin R, Schaefer C, Kaye R, et al. The complex spine in children with spinal muscular atrophy: the Transforaminal Approach-A transformative technique. AJNR Am J Neuroradiol 2019;40:1422–6. 10.3174/ajnr.A6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weaver JJ, Natarajan N, Shaw DWW, et al. Transforaminal intrathecal delivery of nusinersen using cone-beam computed tomography for children with spinal muscular atrophy and extensive surgical instrumentation: early results of technical success and safety. Pediatr Radiol 2018;48:392–7. 10.1007/s00247-017-4031-6 [DOI] [PubMed] [Google Scholar]
- 4. Nascene DR, Ozutemiz C, Estby H, et al. Transforaminal lumbar puncture: an alternative technique in patients with challenging access. AJNR Am J Neuroradiol 2018;39:986–91. 10.3174/ajnr.A5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vad VB, Bhat AL, Lutz GE, et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine 2002;27:11–16. 10.1097/00007632-200201010-00005 [DOI] [PubMed] [Google Scholar]
- 6. Helm Ii S, Harmon PC, Noe C, et al. Transforaminal epidural steroid injections: a systematic review and meta-analysis of efficacy and safety. Pain Physician 2021;24:S209–32. [PubMed] [Google Scholar]
- 7. Robinson S, Robertson FC, Dasenbrock HH, et al. Image-guided intrathecal baclofen pump catheter implantation: a technical note and case series. J Neurosurg Spine 2017;26:621–7. 10.3171/2016.8.SPINE16263 [DOI] [PubMed] [Google Scholar]
- 8. Shashi KK, Stone SSD, Berde CB, et al. Intrathecal catheter and port placement for nusinersen infusion in children with spinal muscular atrophy and spinal fusion. Pediatr Radiol. In Press 2021;51:2588–95. 10.1007/s00247-021-05126-4 [DOI] [PubMed] [Google Scholar]
- 9. Fujiwara A, Watanabe K, Hashizume K, et al. Transforaminal epidural blood patch for intractable spontaneous cerebrospinal fluid leak: a case report. JA Clin Rep 2017;3:2. 10.1186/s40981-016-0073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dʼsouza G, Seidel FG, Krane EJ. Management of a ventral cerebrospinal fluid leak with a lumbar Transforaminal epidural blood patch in a child with persistent postdural puncture headache: a case report. Reg Anesth Pain Med 2017;42:263–6. 10.1097/AAP.0000000000000562 [DOI] [PubMed] [Google Scholar]