Summary
Background
While the etiology of sudden sensorineural hearing loss (SSNHL) remains unclear, viral infection has been suggested as a possible cause. Human papillomavirus (HPV) might trigger immune-mediated reaction and induce inflammatory cytokines which are injurious to the cochlea. This study aimed to investigate the association between HPV infection and the risk of developing SSNHL using a nationwide population-based data set.
Methods
In this study, we used the population-based National Health Insurance Research Database of Taiwan to enroll 49,247 individuals with HPV infection from January 1st, 2000, to December 31st, 2013, and compared with a control group of 98,494 individuals who had never been diagnosed with HPV infection (at a 1:2 ratio matched by age, sex, index year, and comorbidities) in relation to the risk of subsequent SSNHL. The primary outcome was the time from the index date to the date when the first diagnosis of SSNHL occurred, death, withdrawal from the National Health Insurance Program, or the end of the study. Cox model with frailty was conducted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), relative to comparison group. Sensitivity analyses were performed to validate our findings.
Findings
The adjusted hazard ratio (aHR) of developing SSNHL was 1.37 (95% CI, 1.07–1.74) after adjustment for demographic characteristics, comorbidities, and medications. Sensitivity analyses showed consistent positive association. In our sub-group analysis, a significantly higher effect of HPV on SSNHL was noted in the patients with a previous diagnosis of cerebrovascular disease, compared with those without cerebrovascular disease (aHR: 4.59 versus 1.27, p-value for interaction = 0.024).
Interpretation
HPV infections are associated with higher risk of subsequent SSNHL in the Taiwanese population. More research is needed to examine the causality and to determine the potential efficacy of specific precautions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Keywords: Human papillomavirus infection, Sudden sensorineural hearing loss, Cohort study
Research in context.
Evidence before this study
We searched PubMed for publications without language restriction, published before March 21th, 2022, using search terms papillomavirus infections, human papillomavirus infection (HPV), or HPV infection, and sudden hearing loss, sudden deafness, or hearing loss with search terms found in abstract, title or headings. We expanded our search by adding the term virus diseases to the above search strategy, further restricting to publications with virus in the title. Many virus species have been proposed to be associated with sudden sensorineural hearing loss (SSNHL), and varied viruses have different mechanisms for causing hearing loss, ranging from direct injury to inner ear structure to induction of host immune-mediated damage. However, no previous longitudinal studies evaluated the risk of SSNHL in the patients with HPV infection.
Added value of this study
In this propensity score–matched cohort study, with 49,247 HPV cases and 98,494 matched individuals, we found that the patients with HPV infection had a 37% increased risk of developing SSNHL versus those without HPV infection. Furthermore, we conducted a number of sensitivity analyses to increase the accuracy of the diagnosis for SSNHL and the robustness of our findings.
Implications of all the available evidence
As our findings suggested that patients with HPV infection might be at an increased risk of SSNHL, clinicians may consider to take precautions. We also call for further studies to explore the underlying mechanisms and to determine the potential efficacy of specific precautions.
Alt-text: Unlabelled box
Introduction
Sudden sensorineural hearing loss (SSNHL) is most widely defined as sensorineural hearing loss of at least 30 dB over at least three consecutive test frequencies occurring within a 72-h period.1 The sudden deafness symptoms are often considered as a medical emergency, while the causes and pathogenesis remain unknown for the vast majority of SSNHL patients. Secondary causes of SSNHL include neoplasm, cerebrovascular accident, and irradiation.2 Inner ear disorders that could cause unexpected sensorineural hearing loss should also be differentiated, such as Meniere's disease, ototoxicity, and acoustic trauma. As for idiopathic SSNHL, commonly proposed etiologies include viral infection, vascular insufficiency, immune-mediated mechanisms, and stress response.3,4 Hypertension, diabetes mellitus, cigarette smoking, and cardiovascular diseases were risk factors associated with SSNHL.5,6 Many virus species have been proposed to be associated with SSNHL,7 including mumps virus,8 rubella virus, herpes simplex virus (HSV),9 human immunodeficiency virus (HIV),10 and hepatitis virus.11
Human papillomaviruses (HPV) are double-stranded DNA viruses,12,13 and HPV infection is one of the most common sexually transmitted diseases. The specific HPV types are established risk factors associated with several cancers.14,15 It was described that the Nuclear Factor kappa B (NF-kB) pathway might play an important role in the development of cervical cancer, as the activation of NF-kB triggered by a HPV infection regulates the immune response of the host.16 Also, production of inflammatory ligands and cellular stress pathways involving NF-kB within the cochlea might contribute to the development of idiopathic SSNHL, based on the findings of temporal bone histopathology.17, 18, 19, 20 We hypothesized that HPV infection might trigger immune-mediated reaction that induces SSNHL. We conducted this matched cohort study to investigate the epidemiological relationship between HPV infection and SSNHL, using the nationwide population-based database.
Methods
Data source
In this study, the data were obtained from the National Health Insurance (NHI) Research Database of Taiwan, which consists of comprehensive health care data for more than 99% of Taiwan's population since 1995. The database contains beneficiaries’ registration files regarding demographics, medical visits of all types, laboratory tests codes, prescriptions codes, procedure codes, and diagnostic codes based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) system. We retrieved the data from the Longitudinal Health Insurance Database 2000 (LHID 2000), a subset which is composed of all the claim data of 1 million people randomly sampled from registry for beneficiaries in the year 2000. This dataset has been validated by the National Health Research Institutes (NHRI) as representative of the national population in Taiwan. Each patient's identifiable information in the LHID 2000 was encrypted by the NHRI to protect privacy. Our study did not include person-level, institutional-level, or other data linkage across two or more databases.
Study subjects and study design
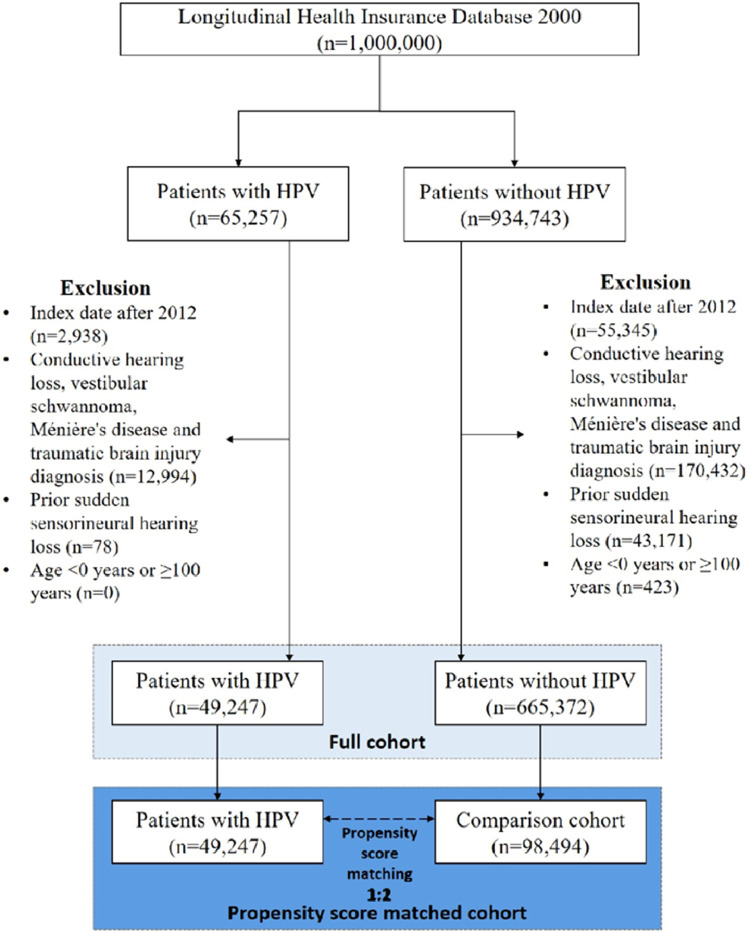
In our retrospective cohort study, we identified patients who were diagnosed with HPV infection (ICD-9 codes 079.4, 078.1, 078.10–078.12, 078.19, 795.05, 795.09, 795.15, 795.19, 796.75 and 796.79)21 between Jan. 1st, 2000, and Dec. 31st, 2013. The flowchart for patients with HPV infection and patients without HPV infection is presented in Figure 1. The index date was defined as the date on which a diagnosis of HPV infection was initially made and was assigned to the matched non-HPV individuals with the same enrollment criteria. To avoid inaccurate diagnoses, we selected only patients with at least 3 outpatient service claims or any inpatient admission with a corresponding diagnosis. The exclusion criteria for the study subjects were: (i) patients with a history of SSNHL (ICD-9-CM code 388.2) before the index date; (ii) patients diagnosed with conductive hearing loss (ICD-9-CM code 389.0x), vestibular schwannoma (ICD-9-CM code 225.1), Ménière's disease (ICD-9-CM code 386.0), or traumatic brain injury (ICD-9-CM codes 310.2, 800–804, 905.0, 850–854, 907.0, 959.01, V15.52); (iii) patients whose index dates were after 2012. Also, as there were few missing values for sex in the database (46 out of 1 million people; 0.046%), we excluded those individuals that were missing information of sex. A total of 49,247 individuals with HPV infection were identified. Moreover, to avoid possible confounding effects and biases, we applied a propensity score matching (PSM) strategy (using OneToManyMTCH macro) to obtain between-group balance.22 We matched the PSM cohort pairs in a ratio of 1:2 by age, gender, index year, income, urbanization, all comorbidities and medication. Ultimately, 98,494 individuals who had never been diagnosed with HPV infection were enrolled as a comparison cohort. Individuals in both cohorts were tracked until a SSNHL event, the date of death, withdrawal from the National Health Insurance Program, or the end of 2013. The reporting is informed by the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement for cohort studies.23
Figure 1.
Flowchart for patients with human papillomaviruses (HPV) infection and comparison cohort. Study period: January 1st, 2000, to December 31st, 2013.
Outcome and covariates
The main outcome of our study was the time from the index date to the date when the first diagnosis of SSNHL occurred, death, withdrawal from the National Health Insurance Program, or the end of the study (December 31st, 2013). Those who were free of SSNHL until the end of 2013 were considered as censoring. To ensure the accurate diagnoses of SSNHL, we selected only patients with at least 3 outpatient visits or any inpatient admission with a rigorous diagnosis (ICD-9-CM code 388.2)24 by certificated otolaryngologists. Risk of developing SSNHL was examined according to the covariates including demographic characteristics, comorbidities, various infections and potentially ototoxic drugs. Demographic characteristics and comorbid medical disorders were also retrieved from the claims data. Age in years at admission was categorized into 4 groups: 0–20, 20–40, 40–60, and >60 years old. Monthly income in New Taiwan dollars (NTD) was categorized into 3 levels: 0–20,000, 20,000–40,000, and > 40,000.
The comorbidities associated with the development of SSNHL were matched and analyzed in our study, including hypertension ((ICD-9-CM codes 401.xx–405.xx), diabetes mellitus (ICD-9-CM codes 250.xx), hyperlipidemia (ICD-9-CM codes 272.xx), coronary artery disease (ICD-9-CM codes 414.xx, 410.xx, and 429.xx), cerebrovascular diseases (ICD-9-CM codes 430–438), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, 496), chronic liver diseases (ICD-9-CM code 571.4), chronic kidney disease (ICD-9-CM codes 585.xx and 586.xx), hyperthyroidism (ICD-9-CM code 242), systemic lupus erythematosus (ICD-9-CM code 710.0), hepatitis B virus infection (ICD-9-CM codes 070.2, 070.3, V02.61), hepatitis C virus infection (ICD-9-CM codes 070.41, 070.44, 070.51, 070.54, V02.62, 070.7), human immunodeficiency virus infection (ICD-9-CM codes 042–044, 795.8, V08), Epstein-Barr virus infection (ICD-9-CM code 075), herpes simplex virus infection (ICD-9-CM code 054.9), mumps (ICD-9-CM code 072.9), streptococcal tonsillitis (ICD-9-CM code 034.0), meningitis (ICD-9-CM code 322), syphilis (ICD-9-CM code 097), and toxoplasmosis (ICD-9-CM code 130). Cervical disease (ICD-9-CM code 616: inflammatory disease of cervix vagina and vulva) was also included as a covariate. To obtain the information on comorbidities, we traced all the outpatient and inpatients records in the NHI dataset within 2 years before the index date. Since additional health data was not available in the database, such as body mass index, smoking status and alcohol consumption, we applied proxy diagnoses to control the possible effects, such as obesity (ICD-9-CM codes 278.0, 278.00, 278.01, and 278.02), smoking (V15.82, 305.1), and alcohol (291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3). The medication confounders in this study were potentially ototoxic drugs, including aminoglycosides (including gentamicin [Anatomical Therapeutic Chemical Classification System (ATC) code S01AA11] and streptomycin [ATC code J01GA01]), chemotherapeutic agents (including cisplatin [ATC code L01XA01], carboplatin [ATC code L01XA02] and cyclophosphamide [ATC code L01AA01]), non-steroidal anti-inflammatories (NSAIDs) (including ibuprofen [ATC code G02CC01] and naproxen [ATC code G02CC02]), salicylates (aspirin, ATC code B01AC06), and loop diuretics (sulfonamides, ATC code C03CA). Drug use was defined as use of a drug for 7 days or more within 1 month before index date.
Statistical analysis
Chi square (χ2) tests were performed to analyze the homogeneity of category variables, including age, sex, income level, urbanization level, comorbidities and medications between the HPV study group and non-HPV comparison group. The incidence rates of SSNHL were calculated in both groups. The adjusted Kaplan-Meier curves were plotted to describe the cumulative incidence of SSNHL among the two groups, and the differences between the two groups were evaluated using the adjusted log-rank test. To investigate the independent association of HPV infection with SSNHL, we conducted a Cox model with frailty to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) after controlling for demographic covariates, relevant medications and comorbidities (listed in Table 1). We also analyzed the risks of SSNHL stratified by different types of HPV infection (cutaneous and mucosal), and we conducted subgroup analysis stratified by age, sex and comorbidities. In further analysis, we analyzed inpatient and outpatient SSNHL as separate outcomes. We also analyzed the risks of SSNHL in the HPV cohort stratified by different consecutive years with HPV diagnoses, and we explored the risks of SSNHL in the HPV cohort in terms of different follow-up time. Individuals with missing information on the variables of interest were already excluded from the study.
Table 1.
Baseline patient characteristics.
| Non-HPV |
HPV |
||||
|---|---|---|---|---|---|
| (N = 98,494) |
(N = 49,247) |
||||
| Variables | n | % | n | % | SMD |
| Age | |||||
| 0–20 | 27,249 | 27.7 | 13,861 | 28.1 | 0.011 |
| 20–40 | 37,535 | 38.1 | 18,877 | 38.3 | 0.005 |
| 40–60 | 25,093 | 25.5 | 11,996 | 24.4 | 0.026 |
| >60 | 8617 | 8.7 | 4513 | 9.2 | 0.015 |
| mean, (SD) | 33.39 | 18.0 | 33.2 | 18.1 | 0.01 |
| Sex | 0.011 | ||||
| female | 50,954 | 51.7 | 25,206 | 51.2 | |
| male | 47,540 | 48.3 | 24,041 | 48.8 | |
| Monthly income, (NTD) | |||||
| 0–20,000 | 75,302 | 76.5 | 37,738 | 76.6 | 0.004 |
| 20,000–40,000 | 14,706 | 14.9 | 7100 | 14.4 | 0.015 |
| >40,000 | 8486 | 8.6 | 4409 | 9.0 | 0.012 |
| Urbanization | |||||
| low | 34,488 | 35.0 | 17,055 | 34.6 | 0.008 |
| medium | 28,779 | 29.2 | 14,377 | 29.2 | 0.001 |
| high | 35,227 | 35.8 | 17,815 | 36.2 | 0.001 |
| Comorbidities | |||||
| Hypertension | 14,590 | 14.8 | 7065 | 14.3 | 0.013 |
| DM | 9545 | 9.7 | 4584 | 9.3 | 0.013 |
| Hyperlipidemia | 13,266 | 13.5 | 6250 | 12.7 | 0.023 |
| CAD | 7675 | 7.8 | 3636 | 7.4 | 0.015 |
| CVD | 3699 | 3.8 | 1838 | 3.7 | 0.001 |
| COPD | 11,142 | 11.3 | 5356 | 10.9 | 0.014 |
| CLD | 14,714 | 14.9 | 6988 | 14.2 | 0.021 |
| CKD | 1364 | 1.4 | 687 | 1.4 | 0.001 |
| Hyperthyroidism | 2897 | 2.9 | 1395 | 2.8 | 0.006 |
| SLE | 113 | 0.1 | 56 | 0.1 | <0.001 |
| HBV | 3960 | 4.0 | 1870 | 3.8 | 0.012 |
| HCV | 729 | 0.7 | 385 | 0.8 | 0.005 |
| HIV | 143 | 0.1 | 85 | 0.2 | 0.007 |
| EBV | 93 | 0.1 | 49 | 0.1 | 0.002 |
| HSV | 6617 | 6.7 | 3361 | 6.8 | 0.004 |
| Mumps | 329 | 0.3 | 119 | 0.2 | 0.017 |
| Streptococcal tonsillitis | 837 | 0.8 | 338 | 0.7 | 0.019 |
| Meningitis | 342 | 0.3 | 157 | 0.3 | 0.005 |
| Syphilis | 252 | 0.3 | 124 | 0.3 | 0.001 |
| Toxoplasmosis | 61 | 0.1 | 36 | 0.1 | 0.004 |
| Obesity | 1257 | 1.3 | 651 | 1.3 | 0.004 |
| Smoking | 952 | 1.0 | 429 | 0.9 | 0.010 |
| Alcohol | 389 | 0.4 | 171 | 0.3 | 0.008 |
| Cervical disease | 16,329 | 16.6 | 9718 | 19.7 | 0.024 |
| Medication | |||||
| Aminoglycosides | 0 | 0.0 | 0 | 0.0 | – |
| Chemotherapeutic agents | 13 | 0.01 | 8 | 0.02 | 0.003 |
| Non-steroidal anti-inflammatories | 0 | 0.0 | 0 | 0.0 | – |
| Salicylates | 1665 | 1.7 | 856 | 1.74 | 0.004 |
| Loop diuretics | 265 | 0.3 | 150 | 0.3 | 0.007 |
SMD: standard mean difference. A standardized mean difference of ≤ 0.10 indicates a negligible difference between the two groups. HPV: Human Papillomavirus; DM: diabetes mellitus; CAD: coronary artery disease; CVD: cerebrovascular diseases; COPD: chronic obstructive pulmonary disease; CLD: chronic liver disease; CKD: chronic kidney disease; SLE: systemic lupus erythematosus; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; EBV: Epstein-Barr virus; HSV: herpes simplex virus; NTD: New Taiwan dollar.
We conducted a number of sensitivity analyses to validate the robustness of our findings. In the first model (our main model), we assessed the effect of HPV infection with demographic variables, medications, and comorbidities adjusted. In the second model, we did the analysis based an unmatched sample. In the third model, we selected only the SSNHL patients who were administered with systemic glucocorticoids (ATC code H02AB) or intratympanic steroid injection (procedure code 54009B). In the fourth model, we defined drug use as use of a drug for > 7 drug days within 1 month before outcome date. The sensitivity analyses were implemented to evaluate the hazard ratio (HR) of SSNHL with presence of HPV infection. The significance level was set at p-value less than 0.05 for 2-side testing. Significance of interaction will be determined by a 2-sided p-value less than 0.05 in our main model with the interaction terms. All the data analyses were performed using SAS software Version 9.4 (SAS Institute Inc.).
Ethics committee approval
This study was approved by the Institutional Review Board of China Medical University (CMUH104-REC2–115 (AR-4)).
Role of funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The corresponding authors (Wei and Hung) had full access to the full data in the study and accept responsibility to submit for publication.
Findings
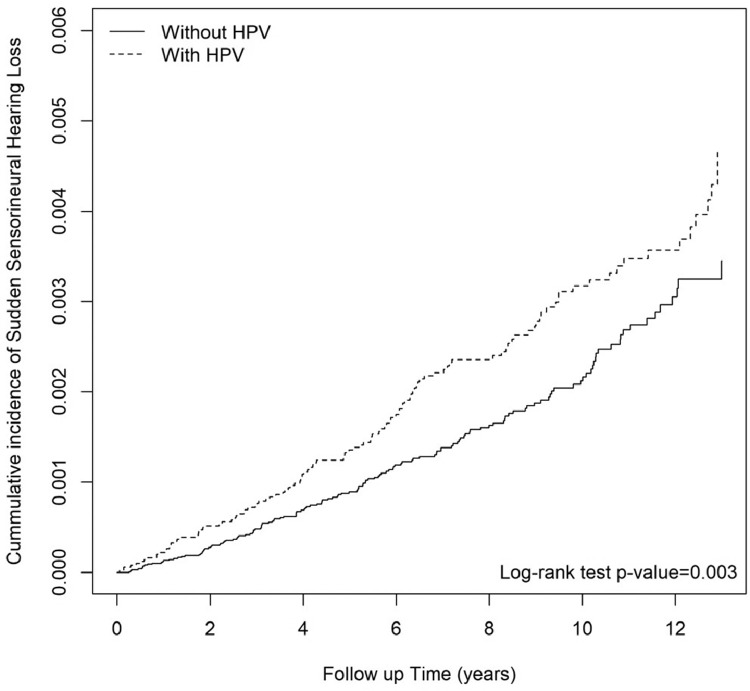
A total of 49,247 patients with HPV infection (mean age 33.2 ± 18.1 year) were enrolled from Jan. 1st, 2000, to Dec. 31st, 2013, and 98,494 propensity score-matched controls (mean age 33.39 ± 18.0 year) without HPV infection were selected as a comparison cohort. The 2 cohorts shared similar baseline characteristics (Table 1). Figure 2 shows the adjusted Kaplan-Meier curves of cumulative incidence of SSNHL in subjects with and without HPV infection. The cumulative incidence of SSNHL was increased in the HPV group compared with the non-HPV group (log-rank P = 0.003). Table 2 shows the results of Cox model with frailty. The subjects who had a history of HPV infection had a higher risk of developing SSNHL compared with the non-HPV comparison group, with an incidence rate of 3.24 per 10,000 person-years versus 2.18 per 10,000 person-years. The crude hazard ratio (cHR) of HPV infection was 1.43 (95% CI, 1.12–1.82). In the adjusted analysis, variables with fewer than ten outcome events were removed. After adjustment for demographic variables (including sex, age, urbanization and income level), medications and comorbidities at baseline mentioned in Table 1, individuals with HPV infection had a 37% increased risk of developing SSNHL versus those without HPV infection, with an adjusted hazard ratio (aHR) of 1.37 (95% CI, 1.07–1.74). The difference of the risk of having SSNHL between males and females was not significant. The risk of developing SSNHL increased with age, with an aHR of 3.45 (95% CI, 2.17–5.47) for those aged 40–60 years, and an aHR of 5.59 (95% CI, 3.27–9.55) for those aged over 60, compared with those aged below 20 as reference. Also, patients with HSV infection were shown to have an increased risk of SSNHL (aHR = 1.78; 95% CI, 1.17–2.70). Our main findings were robust in serial sensitivity analyses. In model 1 (our main model), the aHR was 1.37 (95% CI, 1.07–1.74). In model 2 (Supplemental Table 1), the cHR was 1.40 (95% CI, 1.16–1.69) and the aHR was 1.47 (95% CI, 1.22–1.77). In model 3 (Supplemental Table 2), the cHR was 1.44 (95% CI, 1.11–1.86) and the aHR was 1.37 (95% CI, 1.07–1.74). In model 4 (Supplemental Table 3), the cHR was 4.58 (95% CI, 2.60–8.09); however, the aHR was 1.78 (95% CI, 0.97–3.27). Table 3 shows the results of stratified analysis. The effect of HPV on SSNHL did not significantly differ between age groups, nor did it differ between sex. A significantly higher effect of HPV on SSNHL was noted in the patients with a previous diagnosis of cerebrovascular disease, compared with those without cerebrovascular disease (aHR: 4.59 versus 1.27, p-value for interaction = 0.024).
Figure 2.
Cumulative incidence of sudden sensorineural hearing loss (SSNHL) in subjects with and without human papillomaviruses (HPV) infection.
Table 2.
Sudden sensorineural hearing loss incidence rate and risk factors.
| Sudden Sensorineural Hearing Loss |
Cox model with frailty |
||||||
|---|---|---|---|---|---|---|---|
| Variables | N | PY | IR | cHR (95% CI) | p-value | aHR† (95% CI) | p-value |
| HPV | |||||||
| No | 149 | 684,595 | 2.18 | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 124 | 383,004 | 3.24 | 1.43 (1.12, 1.82)⁎⁎ | 0.004 | 1.37 (1.07, 1.74)⁎ | 0.011 |
| Cutaneous type | 39 | 100,224 | 3.89 | 2.07 (1.44, 2.98)⁎⁎⁎ | <0.001 | 1.96 (1.36,2.83)⁎⁎⁎ | <0.001 |
| Mucosal type | 85 | 282,992 | 3.00 | 1.24 (0.95, 1.63) | 0.120 | 1.20 (0.91,1.58) | 0.119 |
| Age | |||||||
| 0–20 | 29 | 321,806 | 0.90 | 1.00 (reference) | – | 1.00 (reference) | – |
| 20–40 | 75 | 412,262 | 1.82 | 2.03 (1.32, 3.12)⁎⁎ | 0.001 | 1.81 (1.17, 2.81)⁎⁎ | 0.008 |
| 40–60 | 108 | 257,035 | 4.20 | 4.75 (3.15, 7.16)⁎⁎⁎ | <0.001 | 3.45 (2.17, 5.47)⁎⁎⁎ | <0.001 |
| >60 | 61 | 76,495 | 7.97 | 9.34 (5.99, 14.5)⁎⁎⁎ | <0.001 | 5.59 (3.27, 9.55)⁎⁎⁎ | <0.001 |
| Sex | |||||||
| female | 131 | 556,549 | 2.35 | 1.00 (reference) | – | 1.00 (reference) | – |
| male | 142 | 511,049 | 2.78 | 1.19 (0.94, 1.51) | 0.155 | 1.12 (0.88, 1.43) | 0.365 |
| Monthly income, (NTD) | |||||||
| 0–20,000 | 162 | 807,688 | 2.01 | 1.00 (reference) | – | 1.00 (reference) | – |
| 20,000–40,000 | 67 | 160,855 | 4.17 | 2.05 (1.54, 2.73)⁎⁎⁎ | <0.001 | 1.16 (0.85, 1.57) | 0.345 |
| >40,000 | 44 | 99,055 | 4.44 | 2.17 (1.55, 3.03)⁎⁎⁎ | <0.001 | 1.12 (0.77, 1.61) | 0.559 |
| Urbanization | |||||||
| low | 75 | 368,467 | 2.04 | 1.00 (reference) | – | 1.00 (reference) | – |
| medium | 89 | 313,689 | 2.84 | 1.39 (1.02, 1.89)⁎ | 0.037 | 1.38 (1.01, 1.87)⁎ | 0.043 |
| high | 109 | 385,442 | 2.83 | 1.38 (1.03, 1.86)⁎ | 0.031 | 1.32 (0.98, 1.77) | 0.067 |
| Comorbidities | |||||||
| Hypertension | 82 | 134,027 | 6.12 | 3.10 (2.39, 4.02)⁎⁎⁎ | <0.001 | 1.14 (0.81, 1.61) | 0.446 |
| DM | 48 | 87,639 | 5.48 | 2.47 (1.81, 3.38)⁎⁎⁎ | <0.001 | 0.95 (0.66, 1.36) | 0.789 |
| Hyperlipidemia | 70 | 113,502 | 6.17 | 3.14 (2.39, 4.13)⁎⁎⁎ | <0.001 | 1.33 (0.95, 1.87) | 0.092 |
| CAD | 44 | 67,305 | 6.54 | 3.02 (2.18, 4.17)⁎⁎⁎ | <0.001 | 1.18 (0.80, 1.74) | 0.404 |
| CVD | 17 | 30,710 | 5.54 | 2.38 (1.45, 3.89)⁎⁎⁎ | 0.001 | 0.86 (0.51, 1.45) | 0.564 |
| COPD | 48 | 99,108 | 4.84 | 2.23 (1.63, 3.06)⁎⁎⁎ | <0.001 | 1.18 (0.84, 1.65) | 0.341 |
| CLD | 67 | 136,882 | 4.89 | 2.34 (1.77, 3.09)⁎⁎⁎ | <0.001 | 1.26 (0.92, 1.73) | 0.147 |
| CKD | 4 | 10,461 | 3.82 | 1.61 (0.60, 4.33) | 0.347 | ||
| Hyperthyroidism | 6 | 25,888 | 2.32 | 0.96(0.42, 2.15) | 0.914 | ||
| SLE | 1 | 1023 | 9.78 | 4.04 (0.56, 29.1) | 0.165 | ||
| HBV | 19 | 34,840 | 5.45 | 2.37 (1.48, 3.78)⁎⁎⁎ | <0.001 | 1.58 (0.97, 2.59) | 0.069 |
| HCV | 5 | 6152 | 8.13 | 3.44 (1.42, 8.37)⁎⁎ | 0.006 | ||
| HIV | 0 | 1311 | 0.00 | ||||
| EBV | 0 | 856 | 0.00 | ||||
| HSV | 25 | 56,696 | 4.41 | 1.97 (1.30, 2.99)⁎⁎ | 0.001 | 1.78 (1.17, 2.70)⁎⁎ | 0.007 |
| Mumps | 1 | 2491 | 4.01 | 1.74 (0.24, 12.4) | 0.583 | ||
| Streptococcal tonsillitis | 2 | 6534 | 3.06 | 1.32 (0.33, 5.33) | 0.696 | ||
| Meningitis | 2 | 3527 | 5.67 | 2.23 (0.55, 9.03) | 0.259 | ||
| Syphilis | 0 | 2268 | 0.00 | ||||
| Toxoplasmosis | 0 | 724 | 0.00 | ||||
| Obesity | 5 | 10,875 | 4.60 | 1.95 (0.80, 4.73) | 0.141 | ||
| Smoking | 4 | 5899 | 6.78 | 3.16 (1.17, 8.53)⁎ | 0.023 | ||
| Alcohol | 0 | 2930 | 0.00 | ||||
| Cervical disease | 63 | 172,732 | 3.65 | 2.21 (0.33,17.0) | 0.390 | 1.42 (0.98, 2.05) | 0.065 |
| Medication | |||||||
| Chemotherapeutic agents | 0 | 98 | 0.00 | ||||
| Salicylates | 15 | 13,466 | 11.14 | 4.92 (2.91, 8.32)⁎⁎⁎ | <0.001 | 1.58 (0.88, 2.84) | 0.122 |
| Loop diuretics | 1 | 1793 | 5.58 | 2.37 (0.33, 17.00) | 0.390 | ||
Abbreviations: N: number of events; PY: person-year; IR: incidence rate per 10,000 person-year; cHR: crude hazard ratio; aHR: adjusted hazard ratio; HPV: Human Papillomavirus; DM: diabetes mellitus; CAD: coronary artery disease; CVD: cerebrovascular diseases; COPD: chronic obstructive pulmonary disease; CLD: chronic liver disease; CKD: chronic kidney disease; SLE: systemic lupus erythematosus; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; EBV: Epstein-Barr virus; HSV: herpes simplex virus; NTD: New Taiwan dollar.
adjusted by age, sex, monthly income, urbanization, hypertension, DM, hyperlipidemia, CAD, CVD, COPD, CLD, HBV, HSV, cervical disease and salicylates..
P < 0.05.
P < 0.01.
P < 0.001.
Table 3.
Subgroup analysis, stratified by age, sex and comorbidities.
| Non-HPV |
HPV |
Sudden Sensorineural Hearing Loss |
p-value for interaction |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | PY | IR | N | PY | IR | cHR | (95% CI) | p-value | aHR† | (95% CI) | p-value | |
| Age | 0.594 | ||||||||||||
| 0–20 | 20 | 209,636 | 0.95 | 9 | 112,170 | 0.80 | 0.82 | (0.37, 1.80) | 0.618 | 0.79 | (0.36, 1.74) | 0.564 | |
| 20–40 | 41 | 262,715 | 1.56 | 34 | 149,547 | 2.27 | 1.34 | (0.84, 2.12) | 0.219 | 1.36 | (0.86, 2.16) | 0.191 | |
| 40–60 | 59 | 166,968 | 3.53 | 49 | 90,068 | 5.44 | 1.49 | (1.02, 2.19)⁎ | 0.039 | 1.50 | (1.03, 2.20)⁎ | 0.037 | |
| >60 | 29 | 45,276 | 6.41 | 32 | 31,219 | 10.25 | 1.58 | (0.95, 2.62) | 0.078 | 1.46 | (0.87, 2.43) | 0.150 | |
| Sex | 0.787 | ||||||||||||
| female | 71 | 359,096 | 1.98 | 60 | 197,453 | 3.04 | 1.48 | (1.04, 2.09)⁎ | 0.028 | 1.48 | (1.05, 2.09)⁎ | 0.027 | |
| male | 78 | 325,499 | 2.40 | 64 | 185,550 | 3.45 | 1.38 | (0.99, 1.93) | 0.056 | 1.25 | (0.89, 1.74) | 0.201 | |
| Monthly income, (NTD) | 0.518 | ||||||||||||
| 0–20,000 | 91 | 518,470 | 1.76 | 71 | 289,218 | 2.45 | 1.37 | (1.00, 1.87)⁎ | 0.048 | 1.30 | (0.95, 1.78) | 0.098 | |
| 20,000–40,000 | 38 | 103,719 | 3.66 | 29 | 57,136 | 5.08 | 1.24 | (0.76, 2.03) | 0.393 | 1.27 | (0.77, 2.07) | 0.349 | |
| >40,000 | 20 | 62,405 | 3.20 | 24 | 36,649 | 6.55 | 2.04 | (1.12, 3.70)⁎ | 0.019 | 1.81 | (0.99, 3.30) | 0.054 | |
| Urbanization | 0.912 | ||||||||||||
| low | 42 | 237,766 | 1.77 | 33 | 130,701 | 2.52 | 1.30 | (0.82, 2.07) | 0.261 | 1.26 | (0.79, 2.00) | 0.333 | |
| medium | 47 | 201,073 | 2.34 | 42 | 112,616 | 3.73 | 1.58 | (1.04, 2.39)⁎ | 0.033 | 1.53 | (1.01, 2.34)⁎ | 0.047 | |
| high | 60 | 245,755 | 2.44 | 49 | 139,687 | 3.51 | 1.40 | (0.96, 2.04) | 0.083 | 1.32 | (0.90, 1.93) | 0.158 | |
| Comorbidities | |||||||||||||
| Hypertension | 0.112 | ||||||||||||
| No | 111 | 599,929.2 | 1.85 | 80 | 333,642.1 | 2.40 | 1.23 | (0.92, 1.64) | 0.163 | 1.21 | (0.91, 1.62) | 0.193 | |
| Yes | 38 | 84,666 | 4.49 | 44 | 49,362 | 8.91 | 1.94 | (1.25, 3.01)⁎⁎ | 0.003 | 1.79 | (1.15, 2.78)⁎ | 0.010 | |
| DM | 0.060 | ||||||||||||
| No | 129 | 628,813 | 2.05 | 96 | 351,146 | 2.73 | 1.27 | (0.98, 1.66) | 0.074 | 1.24 | (0.95, 1.62) | 0.118 | |
| Yes | 20 | 55,782 | 3.59 | 28 | 31,857 | 8.79 | 2.36 | (1.33, 4.19)⁎⁎ | 0.003 | 2.11 | (1.18, 3.78)⁎ | 0.012 | |
| Hyperlipidemia | 0.087 | ||||||||||||
| No | 116 | 610,508.2 | 1.90 | 87 | 343,587.9 | 2.53 | 1.27 | (0.96, 1.68) | 0.091 | 1.23 | (0.93, 1.63) | 0.148 | |
| Yes | 33 | 74,087 | 4.45 | 37 | 39,416 | 9.39 | 2.02 | (1.26, 3.23)⁎⁎ | 0.003 | 1.84 | (1.14, 2.95)⁎ | 0.012 | |
| CAD | 0.264 | ||||||||||||
| No | 128 | 641,089.3 | 2.00 | 101 | 359,204.6 | 2.81 | 1.34 | (1.03, 1.74)⁎ | 0.028 | 1.31 | (1.01, 1.70)⁎ | 0.046 | |
| Yes | 21 | 43,506 | 4.83 | 23 | 23,799 | 9.66 | 1.98 | (1.09, 3.57)⁎ | 0.024 | 1.66 | (0.91, 3.03) | 0.097 | |
| CVD | 0.024 | ||||||||||||
| No | 145 | 665,757 | 2.18 | 111 | 371,131.1 | 2.99 | 1.31 | (1.02, 1.69)⁎ | 0.032 | 1.27 | (0.99, 1.63) | 0.064 | |
| Yes | 4 | 18,837.85 | 2.12 | 13 | 11,872.58 | 10.95 | 5.53 | (1.70, 18.0)⁎⁎ | 0.004 | 4.59 | (1.47, 14.3)⁎⁎ | 0.009 | |
| COPD | 0.374 | ||||||||||||
| No | 125 | 620,267.1 | 2.02 | 100 | 348,223.1 | 2.87 | 1.36 | (1.04, 1.77)⁎ | 0.024 | 1.32 | (1.01, 1.72)⁎ | 0.040 | |
| Yes | 24 | 64,328 | 3.73 | 24 | 34,781 | 6.90 | 1.82 | (1.03, 3.21)⁎ | 0.038 | 1.55 | (0.88, 2.75) | 0.132 | |
| CLD | 0.419 | ||||||||||||
| No | 114 | 594,699.8 | 1.92 | 92 | 336,017.2 | 2.74 | 1.35 | (1.02, 1.78)⁎ | 0.035 | 1.31 | (0.99, 1.73) | 0.059 | |
| Yes | 35 | 89,895 | 3.89 | 32 | 46,986 | 6.81 | 1.75 | (1.08, 2.82)⁎ | 0.023 | 1.57 | (0.97, 2.54) | 0.069 | |
| HBV | 0.617 | ||||||||||||
| No | 137 | 661,470.6 | 2.07 | 117 | 371,288.1 | 3.15 | 1.45 | (1.13, 1.86)⁎⁎ | 0.003 | 1.40 | (1.09, 1.79)⁎⁎ | 0.009 | |
| Yes | 12 | 23,124 | 5.19 | 7 | 11,716 | 5.97 | 1.16 | (0.46, 2.95) | 0.756 | 0.99 | (0.38, 2.56) | 0.985 | |
| HSV | 0.104 | ||||||||||||
| No | 139 | 647,939.5 | 2.15 | 109 | 362,962.8 | 3.00 | 1.34 | (1.04, 1.73)⁎ | 0.023 | 1.30 | (1.01, 1.68)⁎ | 0.040 | |
| Yes | 10 | 36,655 | 2.73 | 15 | 20,041 | 7.48 | 2.67 | (1.20, 5.96)⁎ | 0.016 | 2.09 | (0.92, 4.74) | 0.079 | |
| Salicylates | 0.395 | ||||||||||||
| No | 143 | 676,450.9 | 2.11 | 115 | 377,681.6 | 3.04 | 1.38 | (1.08, 1.77)⁎ | 0.010 | 1.34 | (1.04, 1.71)⁎ | 0.021 | |
| Yes | 6 | 8143.997 | 7.37 | 9 | 5322.023 | 16.91 | 2.13 | (0.75, 6.06) | 0.155 | 1.72 | (0.58, 5.07) | 0.327 | |
Abbreviations: N: number of events; PY: person-year; IR: incidence rate per 10,000 person-year; cHR: crude hazard ratio; aHR: adjusted hazard ratio; HPV: Human Papillomavirus; DM: diabetes mellitus; CAD: coronary artery disease; CVD: cerebrovascular diseases; COPD: chronic obstructive pulmonary disease; CLD: chronic liver disease; HBV: hepatitis B virus; HSV: herpes simplex virus; NTD: New Taiwan dollar
adjusted by age, sex, monthly income, urbanization, hypertension, DM, hyperlipidemia, CAD, CVD, COPD, CLD, HBV, HSV, and salicylates.
P < 0.05.
P < 0.01.
In Table 4, we analyzed inpatient and outpatient SSNHL as separate outcomes since half of the SSNHL patients were not hospitalized and there could be a difference in severity. There was a prominent risk of SSNHL with >20 outpatient visits (aHR: 2.88; 95% CI: 1.31–6.31). In Table 5, we analyzed the risks of SSNHL in the HPV cohort, stratified by different consecutive years with HPV diagnoses, defined as at least three outpatient service claims or any inpatient admission with a corresponding HPV diagnosis in the index year, in the two years following the index, and in the three years following the index. The results showed that patients with HPV diagnoses in more consecutive years tend to have a higher risk of SSNHL. There was a prominent risk of SSNHL in the patients with HPV diagnoses in the three consecutive years since the index (aHR: 2.14; 95% CI: 1.19–3.87). Table 6 shows the risks of SSNHL in the HPV cohort relative to the non-HPV cohort in terms of different follow-up time. In the follow-up period > 1 year, the aHR was 1.34 (95% CI, 1.04–1.72).
Table 4.
The risks of inpatient and outpatient SSNHL in the HPV cohort relative to the non-HPV cohort.
| Non-HPV |
HPV |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | N | PY | IR | N | PY | IR | cHR | (95% CI) | p-value | aHR† | (95% CI) | p-value |
| SSNHL | ||||||||||||
| with outpatient visits | ||||||||||||
| <10 | 109 | 684,595 | 1.59 | 77 | 383,004 | 2.01 | 1.19 | (0.89,1.60) | 0.245 | 1.15 | (0.85,1.54) | 0.369 |
| 10–20 | 28 | 684,595 | 0.41 | 28 | 383,004 | 0.73 | 1.79 | (1.06,3.02)⁎ | 0.030 | 1.65 | (0.97,2.79) | 0.062 |
| >20 | 10 | 684,595 | 0.15 | 17 | 383,004 | 0.44 | 3.07 | (1.40,6.70)⁎⁎ | 0.005 | 2.88 | (1.31,6.31)⁎⁎ | 0.008 |
| with inpatient visits | ||||||||||||
| >1 | 138 | 684,595 | 2.02 | 118 | 383,004 | 3.08 | 1.47 | (1.15,1.88)⁎⁎ | 0.002 | 1.40 | (1.09,1.79)⁎⁎ | 0.008 |
Abbreviations: N: number of events; PY: person-year; IR: incidence rate per 10,000 person-year; cHR: crude hazard ratio; aHR: adjusted hazard ratio.
adjusted by age, sex, monthly income, urbanization, hypertension, DM, hyperlipidemia, CAD, CVD, COPD, CLD, HBV, HSV, and salicylates.
P < 0.05.
P < 0.01.
Table 5.
The risks of SSNHL in the HPV cohort relative to the non-HPV cohort, stratified by different consecutive years with HPV diagnoses.
| Sudden Sensorineural Hearing Loss |
Cox model with frailty |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | N | PY | IR | cHR | (95% CI) | p-value | aHR† | (95% CI) | p-value |
| HPV | |||||||||
| No | 149 | 684,595 | 2.18 | 1.00 | (reference) | – | 1.00 | (reference) | – |
| Yes | 124 | 383,004 | 3.24 | 1.43 | (1.12, 1.82)⁎⁎ | 0.004 | 1.37 | (1.07, 1.74)⁎ | 0.011 |
| HPV diagnoses in the index year | 73 | 265,173 | 2.75 | 1.23 | (0.93,1.63) | 0.153 | 1.23 | (0.93,1.63) | 0.150 |
| HPV diagnoses in the two consecutive years since the index | 14 | 30,828 | 4.54 | 1.93 | (1.09,3.40)⁎ | 0.023 | 1.78 | (1.01,3.15)⁎ | 0.046 |
| HPV diagnoses in the three consecutive years since the index | 12 | 22,631 | 5.30 | 2.40 | (1.33,4.32)⁎⁎ | 0.004 | 2.14 | (1.19,3.87)⁎⁎ | 0.001 |
Abbreviations: N: number of events; PY: person-year; IR: incidence rate per 10,000 person-year; cHR: crude hazard ratio; aHR: adjusted hazard ratio.
adjusted by age, sex, monthly income, urbanization, hypertension, DM, hyperlipidemia, CAD, CVD, COPD, CLD, HBV, HSV, and salicylates.
P < 0.05.
P < 0.01.
Table 6.
The risks of SSNHL in the HPV cohort relative to the non-HPV cohort in terms of different follow-up time.
| Non-HPV |
HPV |
Sudden Sensorineural Hearing Loss |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up years | N | PY | IR | N | PY | IR | cHR | (95% CI) | p-value | aHR† | (95% CI) | p-value |
| 1 | 13 | 97,038 | 1.34 | 11 | 49,184 | 2.24 | 1.67 | (0.75, 3.72) | 0.212 | 1.60 | (0.72, 3.59) | 0.250 |
| >1 | 136 | 587,556 | 2.31 | 113 | 333,819 | 3.39 | 1.41 | (1.10, 1.81)⁎⁎ | 0.008 | 1.34 | (1.04, 1.72)⁎ | 0.024 |
Abbreviations: N: number of events; PY: person-year; IR: incidence rate per 10,000 person-year; cHR: crude hazard ratio; aHR: adjusted hazard ratio.
adjusted by age, sex, monthly income, urbanization, hypertension, DM, hyperlipidemia, CAD, CVD, COPD, CLD, HBV, HSV, and salicylates.
P < 0.05.
P < 0.01.
Discussion
To our knowledge, this 13-year population-based retrospective cohort study is the first to investigate the epidemiologic association between HPV and SSNHL. We found HPV infection as an independent risk factor for the development of SSNHL, after adjustment for the baseline characteristics, comorbidities and ototoxic medications.
Although the exact mechanisms contributing to the association between HPV infection and SSNHL remain uncertain, several potential mechanisms have been proposed to explain the contribution of viral infection to SSNHL.25 One hypothesis is through direct viral invasion of the inner ear, including cochlear nerve, hair cells and organ of Corti. For instance, Esaki et al. suggested that herpes simplex virus (HSV) infection induces vestibular neuritis and sudden deafness based on their HSV labyrinthitis mouse model, as HSV infection destroyed the organ of Corti and its supporting structures.26 Another hypothesis is through the reactivation of latent virus within tissues of the inner ear. Ramsay Hunt syndrome (herpes zoster oticus), for example, reflects reactivation of latent varicella-zoster virus in the geniculate ganglion, with spread of the infection to the vestibulocochlear nerve, triggering facial paralysis and hearing loss.27 The third hypothesis involves a systemic viral infection that triggers an immune-mediated reaction, or activates the stress response.28,29 It was suggested that viral peptides trigger immunologic response, with anti-phospholipids autoantibodies developed among patients with idiopathic SSNHL.30 Also, by triggering a circulating ligand, viral infection may cause pathologic activation of cellular stress pathways within the cochlea and further lead to hearing loss.31 Tumor necrosis factor-α (TNF-α) reduces cochlear microcirculation through activation of vascular sphingosine-1-phosphate signaling.32 A previous study even demonstrated that reduction of TNF-α during steroids treatment is associated with hearing recovery.33 Systemic stress and immune system dysregulation are also involved in the activation of NF-kB, IL-6, neutrophils, and natural killer cell activity. Synchronism of different types of NF-kB activation, and the pathologic activation of cellular stress pathways within the cochlea and may further lead to hearing loss.34 Furthermore, based on the fact that corticosteroids control the activation state of NF-kB,35 it could be suggested that the treating effect of steroids on hearing loss may be mediated through actions on NF-kB.36, 37, 38 Nevertheless, there is currently insufficient evidence to define the pathophysiological relationship between HPV infection and SSNHL.
SSNHL may occur at any age and most commonly affects individuals aged 43–53 years.39 Our sub-group analysis found that the effect of HPV infection is significant in the age group of 40–60 years. Another finding that deserves attention is the prominent risk of developing SSNHL noted in the patients with a previous diagnosis of cerebrovascular disease. Prior population-based studies had demonstrated the association between SSNHL and cardiocerebrovascular diseases.40,41 As the blood supply of inner ear lacks adequate collateral blood flow, the cochlea could be especially vulnerable to ischemic events and circulatory alterations.42,43 Furthermore, in our additional analysis stratified by different consecutive years with HPV diagnoses, we found a prominent risk of SSNHL in the patients with HPV diagnoses in the three consecutive years since the index, which might suggest potential effect of persistent HPV infection.
Clinicians should consider patients with HPV infection to be at an increased risk of SSNHL, and may take specific precautions. On the other hand, HPV vaccination appears to be a safe and effective measure in preventing subsequent infection.44 However, we did not bring into our study the covariate of vaccination status, since it would be inaccurate to identify the vaccination status of the patients using the NHI Research Database. Further survey to explore the protecting effect and possible risk of HPV vaccination regarding the development of SSNHL is surely needed.
A specific strength of our study was the use of nationwide population-based data, which provided sufficient sample size to explore the association between HPV infection and new-onset SSNHL risk.45 Many previous studies have confirmed the associations of important exposures and outcome, using this data set.46,47 To avoid inexact diagnoses, our main outcome was defined as rigorous diagnoses only by certificated otolaryngologists. In addition, we included into our multivariate modeling the confounding factors such as demographic characteristics, comorbidities, various infections and potentially ototoxic drugs. Furthermore, our main findings were validated through a series of sensitivity analyses, with robust results.
We recognized several limitations in this study. First, the definition of HPV and SSNHL diagnoses relied on ICD-9-CM codes reported by physicians, and may be less accurate than those made on a clinical basis. Second, there was a potential problem of unmeasured confounding since additional health data was not available in the database, including body mass index, ethnic groups, and information of behavioral risk factors such as smoking status and alcohol consumption. Third, the severity of HPV infection could not be determined in our database. Fourth, the generalisability of our findings may be limited in other countries since our study was based on the population in our nationwide database. Last but not least, we did not bring into our analysis the vaccination status of the patients. Further study is needed to clarify the protecting effect and possible risk of HPV vaccination regarding the development of SSNHL.
In conclusion, this 13-year nationwide population-based cohort study found that HPV infection was associated with a higher risk of SSNHL. Also, we call for further studies to explore the underlying mechanisms and to determine the potential efficacy of specific precautions.
Declaration of interests
We declared no conflict of interest.
Acknowledgments
Contributors
Chen and Chang with assistance by Hung and Wei conceptualised the idea. Chen and Chang designed the study and received assistance from Hung and Wei. Yip had the primary responsibility for data curation, and was assisted by Chen, Chang and Hung. Hung administered the project. Yip and Chang accessed and were responsible for the raw data associated with the study. Yip performed most data analysis and was assisted by Chen and Chang. Chen and Chang wrote the original draft; Hung and Wei edited subsequent versions of the draft until finalization of the manuscript. Hung took the decision to submit the manuscript for publication.
Data sharing statement
Data is available from the Taiwan National Health Insurance (NHI) Bureau's National Health Insurance Research Database (NHIRD). Data cannot be made publicly available due to legal constraints placed by the Taiwanese government in regard to the “Personal Information Protection Act.” Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).
Acknowledgements
This study was supported in part by the Ministry of Health and Welfare in Taiwan (MOHW109-TDU-B-212–114004), and China Medical University Hospital (DMR-111–105). These agencies did not influence the study design, data collection and analysis, decision to publish or preparation of the article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101402.
Appendix. Supplementary materials
References
- 1.Chandrasekhar S.S., Tsai Do B.S., Schwartz S.R., et al. Clinical practice guideline: sudden hearing loss (update) executive summary. Otolaryngol Head Neck Surg. 2019;161(2):195–210. doi: 10.1177/0194599819859883. [DOI] [PubMed] [Google Scholar]
- 2.Young Y.H. Contemporary review of the causes and differential diagnosis of sudden sensorineural hearing loss. Int J Audiol. 2020;59(4):243–253. doi: 10.1080/14992027.2019.1689432. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber B.E., Agrup C., Haskard D.O., Luxon L.M. Sudden sensorineural hearing loss. Lancet. 2010;375(9721):1203–1211. doi: 10.1016/S0140-6736(09)62071-7. [DOI] [PubMed] [Google Scholar]
- 4.Lazarini P.R., Camargo A.C. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol. 2006;72(4):554–561. doi: 10.1016/s1808-8694(15)31004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin R.J., Krall R., Westerberg B.D., Chadha N.K., Chau J.K. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. 2012;122(3):624–635. doi: 10.1002/lary.22480. [DOI] [PubMed] [Google Scholar]
- 6.Capaccio P., Ottaviani F., Cuccarini V., et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117(3):547–551. doi: 10.1097/MLG.0b013e31802f3c6a. [DOI] [PubMed] [Google Scholar]
- 7.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18 doi: 10.1177/2331216514541361. Published 2014 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto H., Fujioka M., Kinumaki H., Kinki Ambulatory Pediatrics Study Group An office-based prospective study of deafness in mumps. Pediatr Infect Dis J. 2009;28(3):173–175. doi: 10.1097/INF.0b013e31818a8ca8. [DOI] [PubMed] [Google Scholar]
- 9.Koide J., Yanagita N., Hondo R., Kurata T. Serological and clinical study of herpes simplex virus infection in patients with sudden deafness. Acta Otolaryngol Suppl. 1988;456:21–26. doi: 10.3109/00016488809125072. [DOI] [PubMed] [Google Scholar]
- 10.Lin C., Lin S.W., Weng S.F., Lin Y.S. Increased risk of sudden sensorineural hearing loss in patients with human immunodeficiency virus aged 18 to 35 years: a population-based cohort study. JAMA Otolaryngol Head Neck Surg. 2013;139(3):251–255. doi: 10.1001/jamaoto.2013.1709. [DOI] [PubMed] [Google Scholar]
- 11.Chen H.C., Chung C.H., Wang C.H., et al. Increased risk of sudden sensorineural hearing loss in patients with hepatitis virus infection. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175266. Published 2017 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Krogh G., Lacey C.J., Gross G., Barrasso R., Schneider A. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex Transm Infect. 2000;76(3):162–168. doi: 10.1136/sti.76.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutner K.R. Nongenital human papillomavirus infections. Clin Lab Med. 2000;20(2):423–430. [PubMed] [Google Scholar]
- 14.Franco E.L., Duarte-Franco E., Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164(7):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 15.Gillison M.L., Castellsagué X., Chaturvedi A., et al. Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134(3):497–507. doi: 10.1002/ijc.28201. [DOI] [PubMed] [Google Scholar]
- 16.Tilborghs S., Corthouts J., Verhoeven Y., et al. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol. 2017;120:141–150. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Adams J.C. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23(3):316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Merchant S.N., Adams J.C., Nadol J.B. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2005;26(2):151–160. doi: 10.1097/00129492-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kudo T., Kure S., Ikeda K., et al. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum Mol Genet. 2003;12(9):995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- 20.Masuda M., Kanzaki S., Minami S., et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33(7):1142–1150. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 21.Chen M.L., Kao W.M., Huang J.Y., Hung Y.M., Wei J.C. Human papillomavirus infection associated with increased risk of new-onset psoriasis: a nationwide population-based cohort study. Int J Epidemiol. 2020;49(3):786–797. doi: 10.1093/ije/dyaa027. [DOI] [PubMed] [Google Scholar]
- 22.Parsons L.S. Performing a 1:n case-control match on propensity score. SUGI. 2004;29:165. –29. [Google Scholar]
- 23.Benchimol E.I., Smeeth L., Guttmann A. RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12(10) doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong P.X., Li I.H., Shih J.H., Yeh C.B., Chiang K.W., Kao L.T. Antidepressants and risk of sudden sensorineural hearing loss: a population-based cohort study. Int J Epidemiol. 2021;50(5):1686–1697. doi: 10.1093/ije/dyab023. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Fu Y.Y., Zhang T.Y. Role of viral infection in sudden hearing loss. J Int Med Res. 2019;47(7):2865–2872. doi: 10.1177/0300060519847860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esaki S., Goshima F., Kimura H., et al. Auditory and vestibular defects induced by experimental labyrinthitis following herpes simplex virus in mice. Acta Otolaryngol. 2011;131(7):684–691. doi: 10.3109/00016489.2010.546808. [DOI] [PubMed] [Google Scholar]
- 27.Furuta Y., Takasu T., Fukuda S., et al. Detection of varicella-zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J Infect Dis. 1992;166(5):1157–1159. doi: 10.1093/infdis/166.5.1157. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto S., Billings P., Harris J.P., Firestein G.S., Keithley E.M. Innate immunity contributes to cochlear adaptive immune responses. Audiol Neurootol. 2005;10(1):35–43. doi: 10.1159/000082306. [DOI] [PubMed] [Google Scholar]
- 29.Wilson W.R. The relationship of the herpesvirus family to sudden hearing loss: a prospective clinical study and literature review. Laryngoscope. 1986;96(8):870–877. doi: 10.1002/lary.1986.96.8.870. [DOI] [PubMed] [Google Scholar]
- 30.Greco A., Fusconi M., Gallo A., Marinelli C., Macri G.F., De Vincentiis M. Sudden sensorineural hearing loss: an autoimmune disease? Autoimmun Rev. 2011;10(12):756–761. doi: 10.1016/j.autrev.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Merchant S.N., Durand M.L., Adams J.C. Sudden deafness: is it viral? ORL J Otorhinolaryngol Relat Spec. 2008;70(1):52–62. doi: 10.1159/000111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherer E.Q., Yang J., Canis M., et al. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41(11):2618–2624. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsinaslanidou Z., Tsaligopoulos M., Angouridakis N., Vital V., Kekes G., Constantinidis J. The expression of TNFα, IL-6, IL-2 and IL-8 in the serum of patients with idiopathic sudden sensorineural hearing loss: possible prognostic factors of response to corticosteroid treatment. Audiol Neurotol Extra. 2016;6:9–19. doi: 10.1159/000442016. [DOI] [Google Scholar]
- 34.Masuda M., Kanzaki S., Minami S., et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33(7):1142–1150. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 35.Brattsand R., Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996;10(Suppl 2):81–92. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz D., Lee K.J., Smith H.W. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope. 1984;94(5 Pt 1):664–666. [PubMed] [Google Scholar]
- 37.McCabe B.F. Autoimmune inner ear disease: therapy. Am J Otol. 1989;10(3):196–197. [PubMed] [Google Scholar]
- 38.Adams J.C. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23(3):316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Rauch S.D. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359(8):833–840. doi: 10.1056/NEJMcp0802129. [DOI] [PubMed] [Google Scholar]
- 40.Lin H.C., Chao P.Z., Lee H.C. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39(10):2744–2748. doi: 10.1161/STROKEAHA.108.519090. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.Y., Hong J.Y., Kim D.K. Association of sudden sensorineural hearing loss with risk of cardiocerebrovascular disease: a study using data from the Korea national health insurance service. JAMA Otolaryngol Head Neck Surg. 2018;144(2):129–135. doi: 10.1001/jamaoto.2017.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin R.J., Krall R., Westerberg B.D., Chadha N.K., Chau J.K. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. 2012;122(3):624–635. doi: 10.1002/lary.22480. [DOI] [PubMed] [Google Scholar]
- 43.Lee H., Cho Y.W. Auditory disturbance as a prodrome of anterior inferior cerebellar artery infarction. J Neurol Neurosurg Psychiatry. 2003;74(12):1644–1648. doi: 10.1136/jnnp.74.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baxter R., Lewis N., Bohrer P., Harrington T., Aukes L., Klein N.P. Sudden-onset sensorineural hearing loss after immunization: a case-centered analysis. Otolaryngol Head Neck Surg. 2016;155(1):81–86. doi: 10.1177/0194599816639043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng C.L., Kao Y.H., Lin S.J., Lee C.H., Lai M.L. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 46.Zhong P.X., Li I.H., Shih J.H., Yeh C.B., Chiang K.W., Kao L.T. Antidepressants and risk of sudden sensorineural hearing loss: a population-based cohort study [published online ahead of print, 2021 Mar 20] Int J Epidemiol. 2021:dyab023. doi: 10.1093/ije/dyab023. [DOI] [PubMed] [Google Scholar]
- 47.Yen Y.C., Lin C., Weng S.F., Lin Y.S. Higher risk of developing sudden sensorineural hearing loss in patients with chronic otitis media. JAMA Otolaryngol Head Neck Surg. 2015;141(5):429–435. doi: 10.1001/jamaoto.2015.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.