Abstract
In order to establish a screening system for xenosiderophores which can be utilized by mycobacteria, we generated a set of mutants of Mycobacterium smegmatis that are blocked in different steps of the well-known iron acquisition system. One mutant with a block in mycobactin biosynthesis was generated from strain mc2155 by chemical mutagenesis. The exochelin biosynthesis gene fxbA and the ferric exochelin uptake gene fxuA, previously identified by Fiss et al. (E. H. Fiss, S. Yu, and W. R. Jacobs, Jr., Mol. Microbiol. 14:557–559, 1994), were knocked out by gene replacement. Adjacent chromosomal fragments were used for homologous recombination in order to replace wild-type genes by the kanamycin resistance gene from transposon Tn903. Gene replacement was confirmed by PCR. The isolated mutants show the expected phenotype: fxbA mutants are defective in exochelin biosynthesis, whereas fxuA mutants excrete a significantly larger amount of exochelin compared to the amount excreted by the parent strain. This is due to their defectiveness in ferriexochelin uptake, as demonstrated in growth promotion assays. This new set of mutants allows differentiation of siderophores that supply mycobacteria with iron by ligand exchange with exochelin or mycobactin, by the use of separate siderophore uptake routes, or by the use of the exochelin permease. All these types of iron uptake routes were identified with 25 exogenous siderophores as test substances. Siderophores that act without ligand exchange are potential candidates as drug vectors that can be used to overcome permeability-mediated resistance.
Increasing numbers of mycobacterial infections and the spread of resistance against the frontline antituberculosis agents urgently demand a search for novel drugs with new mechanisms of action. The low levels of permeability of the extraordinarily thick and hydrophobic cell envelopes of the mycobacteria limit the activities of antimycobacterial agents. The aim of our study was to provide a basis for overcoming the permeability barrier of the mycobacterial envelope by conjugation of drugs to species-specific vectors and by the use of the concomitant transport systems essential for the pathogens. The ability to acquire iron is essential for the survival of mycobacteria and other pathogens and is an important virulence factor. High-affinity iron sequestration systems have therefore evolved (3). Siderophores are part of these systems and are the basis for new antibiotic vectors that are useful as means of circumventing membrane-associated resistance (1). Thus, a diversity of iron-siderophore uptake routes would provide a good basis for realization of the shuttle transport of drugs through the bacterial membrane.
Mycobacteria produce two classes of siderophores, the mycobactins and the extracellular hydrophilic exochelins. Mycobactin was initially isolated from Mycobacterium phlei by Snow in 1965 (19), and a large number of mycobactins which have a common core structure have been isolated (3). The cell wall-associated lipophilic mycobactins are synthesized by most mycobacterial species and are thought to facilitate the transport of iron across the cell wall (9, 14). Structurally related water-soluble mycobactins which are excreted into the environment bind to iron and transfer it to the cell wall-associated mycobactins (5, 7, 8). Water-soluble mycobactins are the only extracellular siderophores expressed by pathogenic mycobacteria, while the saprophytic strains Mycobacterium smegmatis and Mycobacterium neoaurum use exochelins as main siderophores for iron acquisition (12, 16, 17). Exochelin-mediated iron uptake into M. smegmatis is both a receptor-dependent and an energy-dependent process similar to the intensively studied uptake of siderophores in Escherichia coli (2). The genes involved in the biosynthesis and export of exochelin and in the uptake of ferriexochelin have been identified and cloned (4, 21, 22).
Not only those siderophores that are produced by the organism itself can be utilized, but also siderophores that are synthesized by other microorganisms (xenosiderophores). Mycobacteria were found to utilize ferrirhodotorulic acid, isolated from Rhodotorula strains (20); ferricrocin, produced by Aspergillus and Neurospora species (10); and rhizoferrin, produced by Rhizopus strains (11). These results were obtained with wild-type strains and methods that use 55Fe-labeled siderophores and in situ Mössbauer spectroscopy. Our goal is to develop a new efficient screening method for the identification of the iron siderophores utilized by mycobacteria. In this context it is very important to consider the potential ligand exchange with exochelins and mycobactins in the iron supply of wild-type mycobacteria by xenosiderophores.
In order to identify direct uptake routes for siderophores that circumvent ligand exchange with endogenous siderophores, it is necessary to monitor the passage of the siderophore-bound iron from the outside to the inside of the cell by radiolabeled Fe3+ transport experiments or by time-consuming and expensive Mössbauer spectroscopy or electron spin resonance spectroscopy. The latter elaborate methods are useful only for single compounds and not for the screening of numerous samples. To overcome these limitations, it is important to generate mutant strains which lack endogenous siderophore genes.
In order to establish an efficient screening system that can be used to find xenosiderophores which can be used as a possible platform for synthesis of siderophore-antibiotic conjugates, we selected M. smegmatis as a model system because it is a nonpathogenic, fast-growing mycobacterium which is genetically more tractable than Mycobacterium tuberculosis. In this report we describe the generation of mutants blocked in the biosynthesis of exochelin and/or mycobactin or in the transport of exochelin. These mutants provide a basis for the screening of effective siderophores in a growth promotion assay. This set of strains enables determination of whether there is ligand exchange with exochelin and/or mycobactin or uptake by an alternative transport system.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The mycobacterial strains and plasmids used in this study are listed in Table 1. The E. coli strain used in this study was DH5α (Stratagene). The standard plasmid cloning vector pUC118 was from laboratory stock.
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| M. smegmatis strains | ||
| mc2155 | Epta mutant of mc26, the wild-type orange rough clone from ATCC 607 | 18 |
| M24 | Mycobactin mutant of mc2155 | This study |
| B1 | mc2155 ΔfxbA Kmrb | This study |
| B3 | M24 ΔfxbA Kmr | This study |
| U3 | M24 ΔfxuA Kmr | This study |
| Plasmids | ||
| pUC4K | Kmr | Pharmacia |
| pGS22 | pUC118 containing a part of fxuC and fxuB on both ends of the aph gene from pUC4K | This study |
| pGS31 | pUC118 containing fxuB and a chromosomal fragment upstream of fxbA on both ends of the aph gene from pUC4K | This study |
Ept, efficient plasmid transformation phenotype.
Kmr, kanamycin resistance.
Media and growth conditions.
E. coli cultures were routinely grown in Luria-Bertani medium (Difco Laboratories) at 37°C. The following antibiotics, when required, were added at the indicated concentrations: ampicillin, 100 μg/ml for E. coli; kanamycin, 30 μg/ml for E. coli and M. smegmatis. Mycobacterium cultures were grown in Middlebrook 7H9 broth (Difco Laboratories) supplemented with glycerol (0.2%), Tween 80 (0.05%), and albumin-dextrose-catalase enrichment (10%; Difco Laboratories) at 37°C. The solid medium for plating and maintenance of mycobacterial strains was Middlebrook 7H10 agar (Difco Laboratories) supplemented with glycerol (0.5%) and oleic acid-albumin-dextrose-catalase enrichment (10%; Difco Laboratories) or Oxoid medium no. 1.
Genetic methods.
Restriction enzymes (Boehringer Mannheim), T4 DNA ligase (Gibco BRL), and Taq DNA polymerase (Qiagen) were used in accordance with the manufacturers' recommendations. The DNA fragments used in the cloning procedures were gel purified with SeaPlaque GTG low-melting-temperature agarose (FMC), followed by the recovery of DNA from agarose gel with GenElute agarose spin columns (Supelco).
Plasmids were isolated with a QIAprep Spin kit (Qiagen) and transformed into E. coli strains by standard techniques or electroporated into M. smegmatis strains with a Gene Pulser (Bio-Rad Laboratories) according to the manufacture protocol, with modifications for electroporation into M. smegmatis used as described before (18). Electroporation conditions were 1,000 Ω, 2,500 V, and 25 μF in 0.2-cm-electrode-gap cuvettes with 0.2 ml of mycobacteria, which produced time constants of 16 to 21 ms. The mycobacteria were grown to the mid-logarithmic phase, followed by extensive washing with 10% glycerol at 4°C and final resuspension in a 1/100 volume of ice-cold 10% glycerol.
Chromosomal DNA from mycobacterial strains was isolated by using a DNeasy tissue kit (Qiagen). The DNA was eluted with 100 μl of buffer AE (5 mM Tris-HCl, pH 8.5), and 2 μl of the eluant was used in a 100-μl PCR mixture. PCR amplification for the cloning of the products was carried out with Pwo DNA polymerase (Boehringer Mannheim) according to the manufacturer's recommendations and with the addition of 10% dimethyl sulfoxide (DMSO) to the mixtures. Reactions took place as follows: denaturation in a 50-μl mixture containing DNA, Pwo buffer, primer, and DMSO at 98°C for 10 min and addition of 50 μl of a second mixture containing Pwo buffer, deoxynucleoside triphosphates, and Pwo DNA polymerase, followed by 30 cycles of denaturation at 96°C for 1 min, primer annealing at 45 to 56°C for 1 min, and primer extension at 72°C for 1 min. PCR amplification for the analysis of transformants was carried out with Taq DNA polymerase (Qiagen) and Q-solution (Qiagen) instead of DMSO. Oligionucleotide primers were obtained from MWG-Biotech AG, Ebersberg, Germany, and are listed in Table 2.
TABLE 2.
Oligonucleotide primers used for cloning of chromosomal fragments and detection of gene replacement
| Primer purpose and primer | Comment | Sequencea |
|---|---|---|
| For cloning | ||
| B5 | fxuB forward primer | 5′-ACGAATTCACACGCCAGAGAACGC-3′ |
| B6 | fxuB reverse primer | 5′-CAGGATCCTGATCTGGCAATTCCAG-3′ |
| B7 | fxbA upstream reverse primer | 5′-CAGGATCCATTGGTAAGCCTTACC-3′ |
| B8 | fxbA upstream forward primer | 5′-GATCTAGAGCTGGTTCAGGGCGATG-3′ |
| U1 | fxuC forward primer | 5′-ACGAATTCGAGATCCTCGGCATCAAC-3′ |
| U5 | fxuC reverse primer | 5′-CAGGATCCTGGCGCTTCCCGAAATC-3′ |
| U6 | fxuB forward primer | 5′-GAGGATCCTGAAAGAACATATGACTC-3′ |
| U7 | fxuB reverse primer | 5′-TATCTAGACAAGATCGGCCGACATA-3′ |
| For detection of gene replacement | ||
| K1 | Out of 5′ end of aph | 5′-GAGACACAACGTGGCTTTC-3′ |
| K2 | Out of 3′ end of aph | 5′-CACGAGGCAGACCTCAGC-3′ |
| B1 | Upstream of B8 | 5′-GGTACCGACACCTCGCA-3′ |
| B9 | Out of 3′ end of fxuA | 5′-GGCGGGATCTACCTGGTAT-3′ |
| U8 | fxuC, upstream of U1 5′ end of fxb | 5′-TCCAGGCCGTGGGACGTAA-3′ |
| U9 | 5′-GTCGATGATCTGGCAATTCC-3′ |
Restriction sites are in bold face.
The fidelities of the PCR-amplified DNA fragments were established by nucleotide sequencing after subcloning. Automated sequencing was performed with a Licor DNA sequencer with dye-primer cycle sequencing chemistry.
Chemical mutagenesis of M. smegmatis mc2155 was carried out with a culture grown for 2 days in medium TL, which contained (per liter) glycerol, 10 g; meat extract, 5 g; peptone, 5 g; and NaCl, 3 g (pH 7.0). Cells that had been harvested by centrifugation were washed in sterile water and resuspended in medium TL to the density of a no. 3 McFarland standard (9 × 108 CFU per ml; BioMerieux). N-Methyl-N′-nitro-N-nitrosoguanidine was added at 1.5 mg per ml, and the suspension was incubated for 1 h at 37°C. After appropriate dilution the cells were plated onto Oxoid no. 1 agar plates.
Assays.
The production of mycobactin was determined by measurement of the UV fluorescence at 264 nm of patched colonies grown for 3 days at 37°C on the iron-limiting medium GHE, which contained (per liter) yeast extract (Difco), 10 g; glucose, 10 g; ethylendiamin-di-(o-hydroxyphenylacetic acid) (EDDHA), 3.6 mg; and agar (Difco), 15 g.
For the detection of exochelin production, chrome azurol S (CAS) medium (15) was used with the modifications described previously (4).
The utilization of exochelin and heterologous siderophores was determined by growth promotion assays. M. smegmatis mutants were grown for 2 days on Oxoid no. 1 agar, suspended in medium VA (glycerol, 50 ml; NaCl, 8.5 g per liter [pH 7.2]), and diluted to the density of a no. 1 McFarland standard (3 × 108 CFU per ml). A total of 1.5 ml of this suspension was mixed with 99 ml of test medium prepared as described by Hall and Ratledge (6) (glycerol, 20 ml; l-asparagine, 5 g; KH2PO4, 5 g per liter of distilled water [pH 7.5]). After the addition of 20 g of Al2O3 and sterilization at 121°C for 15 min, the suspension was filtered and the pH was adjusted to 6.8. A total of 12 g agar (Oxoid no. 1) was added, and the medium was sterilized again at 121°C for 15 min. Prior to the inoculation, ZnSO4 · 7H2O, 2.03 mg; MnSO4 · 4H2O, 0.405 mg; MgSO4, 0.2 mg; CaCl2 · 2H2O, 1 mg; Na2MoO4 · 2H2O, 0.2 mg; CuSO4 · 5H2O, 0.2 mg; CoCl2 · 6H2O, 0.4 mg; and EDDHA, 3.6 g, were added to the medium as a filter-sterilized solution. Siderophore solutions were applied to assay disks (diameter, 6 mm) the disks were placed onto the surfaces of the inoculated test agar plates, and the zones of growth surrounding the disks were estimated after incubation for 2 days at 37°C.
Siderophores.
The siderophores were kindly supplied as indicated in Table 4.
TABLE 4.
Growth promotion test of various natural siderophores
| Siderophore | Growth zonea
|
||||
|---|---|---|---|---|---|
| mc2155 | M24 | B1 | B3 | U3 | |
| Aerobactinb | +++ | +++ | +++ | +++ | +++ |
| Arthrobactinb | +++ | +++ | +++ | +++ | +++ |
| Amycolachromb | +++ | +++ | +++ | +++ | − |
| Coprogenc | − | − | − | − | − |
| Corynebactind | +++ | +++ | +++ | − | − |
| Enterobactinb | +++ | ++ | +++ | + | + |
| Exocheline | +++ | +++ | ++ | ++ | − |
| Ferricrocinb | +++ | +++ | ++ | − | − |
| Ferrichromeb | +++ | +++ | +++ | +++ | +++ |
| Ferrichrome A†b | +++ | +++ | +++ | ++ | ++ |
| Ferrichrysinb | − | − | − | − | − |
| Ferrioxamine Bb | ++ | ++ | ++ | − | − |
| Ferrioxamine D1b | +++ | ++ | +++ | − | − |
| Ferrioxamine D2b | ++ | ++ | − | − | − |
| Ferrioxamine Eb | ++ | ++ | − | − | − |
| Ferrioxamine Gb | + | − | − | − | − |
| Ferrirubinb | − | − | − | − | − |
| Ferrithiocinb | +++ | +++ | +++ | +++ | − |
| Mycobactin | +++ | +++ | +++ | +++ | +++ |
| Myxochelin Ce | +++ | +++ | +++ | ++ | +++ |
| Myxochelin Ee | − | − | − | − | − |
| Omibactinf | +++ | +++ | +++ | ++ | ++ |
| Triacetylfusarininec | ++ | ++ | + | + | + |
| Rhizoferrinf | ++ | + | ++ | + | + |
| Rhodotorulic acidc | +++ | − | − | − | − |
−, no growth zone; +, growth zones, <12 mm; ++, growth zones, 12 to 20 mm; +++, growth zones, >20 mm.
Siderophores were kindly supplied by H.-P. Fiedler, Tübingen, Germany.
Siderophores were kindly supplied by B. F. Matzanke, Lübeck, Germany.
Siderophores were kindly supplied by H. Budzikiewicz, Cologne, Germany.
Siderophores were kindly supplied by R. Reissbrodt, Wernigerode, Germany.
Siderophores were kindly supplied by G. Winkelmann, Tübingen, Germany.
RESULTS
Construction of mutants.
In the first step, a mycobactin-deficient mutant was isolated from strain mc2155 after chemical mutagenesis with nitrosoguanidine. Mutagenized cells were screened for clones that lacked UV fluorescence on iron-depleted GHE medium. Of 72 colonies tested, one mutant (mutant M24) that lacked UV fluorescence was identified. The loss of mycobactin biosynthesis was confirmed by high-pressure liquid chromatography and mass spectroscopy of ethanolic biomass extracts (data not shown). Because nitrosoguanidine is known to produce multiple mutations, we cannot exclude the possibility that M24 has additional defects other than that which precludes mycobactin synthesis; but our tests have shown that the strain is not affected in relevant characteristics such as growth, production, and excretion of exochelin or utilization of mycobactin- and exochelin-bound iron.
In the second step, mutant strain M24 and wild-type strain mc2155 were used to generate defined mutants with a block in exochelin biosynthesis or ferric exochelin uptake by gene replacement. The exochelin biosynthesis gene fxbA and the ferric exochelin uptake gene fxuA, previously identified by Fiss et al. (4), were replaced by the aminoglycoside phosphotransferase (aph) gene of Tn903, which confers resistance to kanamycin in M. smegmatis. The gene was obtained as a 1,264-bp BamHI fragment from pUC4K. Chromosomal fragments adjacent to the target genes (Fig. 1) were amplified by PCR and cloned into the E. coli vector pUC118 on flanking sites of the aph gene. Restriction sites in the primers used to amplify these fragments (Table 2) allowed targeted insertion in the orientation of the chromosomal context.
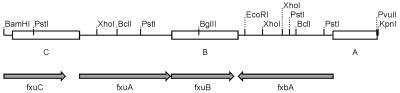
FIG. 1.
Restriction map and genetic organization of the part of exochelin gene cluster of M. smegmatis mc2155 relevant to the present study. The arrows indicate the direction of gene transcription. Boxes represent chromosomal fragments cloned in pGS22 (fragments B and C) and pGS31 (fragments A and B). Fragment A is the 507-bp part upstream of fxbA gene, fragment B (758 bp) contains the entire fxuB gene, and fragment C (768 bp) contains a part of the fxuC gene.
The resulting constructs contain an origin of replication that permits autonomous propagation in E. coli but not in M. smegmatis. The cloned chromosomal fragments permit integration into M. smegmatis genomic DNA by homologous recombination, and a double crossover in both fragments results in the replacement of the target gene by the aph gene (Fig. 2A and 3A).
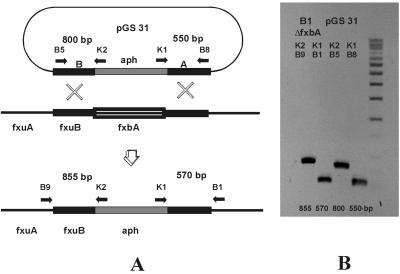
FIG. 2.
Replacement of the fxbA gene in M. smegmatis mc2155. (A) Strategy used to replace the fxbA gene by double crossover. The horizontal arrows indicate annealing sites of primers used to confirm the replacement (B1-K1, B9-K2) by using the chromosomal DNA of mutant strain B1 as the template and to generate control fragments by using plamid pGS31 as the template. The expected sizes of the fragments are indicated between the arrows. (B) Confirmation of replacement of the fxbA gene by PCR analysis of DNA from the B3 ΔfxbA mutant.
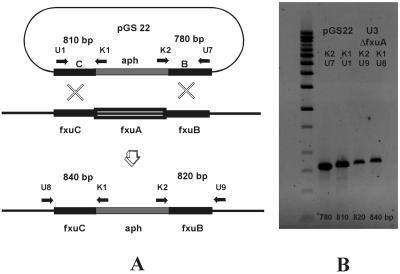
FIG. 3.
Replacement of the fxuA gene in M. smegmatis mc2155 mutant strain M24. (A) Strategy used to replace the fxuA gene by double crossover. The horizontal arrows indicate annealing sites of primers used to confirm the replacement (U3-K1, U9-K2) by using the chromosomal DNA of mutant strain U3 as the template and to generate control fragments by using plasmid pGS22 as the template. The expected sizes of the fragments are indicated between the arrows. (B) Confirmation of replacement of the fxuA gene by PCR analysis of DNA from the U3 ΔfxuA mutant.
M. smegmatis mc2155 and M24 were transformed with these plasmids by electroporation, and kanamycin-resistant clones were selected. For the identification of mutants blocked in exochelin biosynthesis by replacement of the fxbA gene, kanamycin-resistant clones were selected after transformation with pGS31 (Table 1) and were screened on CAS medium (5). A total of 145 transformants of both strains were analyzed, and 4 mutants that lacked an orange halo on CAS medium were identified (Fig. 4). Replacement of fxbA by homologous recombination by double crossover was confirmed by PCR (Fig. 2B). DNA isolated from the mutants was subjected to PCR amplification with primers that anneal at both ends of the aph cassette and at chromosomal sites that flank the cloned fragments. Control fragments were generated with pGS31 as a template and primers which bound at the ends of the cloned fragments.
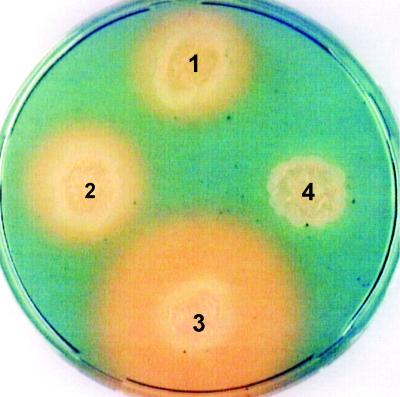
FIG. 4.
CAS plate assay of isolated mutants. The presence of an orange halo indicates the exochelin production. 1, mc2155 wild type; 2, mutant strain M24 (mycobactin negative); 3, mutant strain U3 ΔfxuA; 4, mutant strain B3 ΔfxbA.
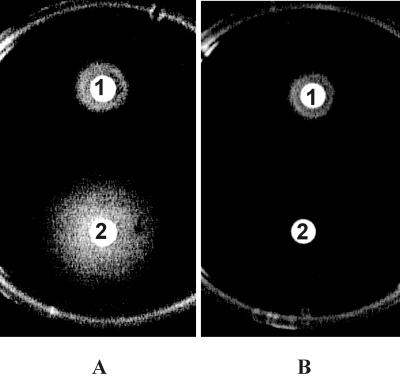
To screen mutants blocked in ferric exochelin uptake, kanamycin-resistant clones selected after transformation with pGS22 (Table 1) were tested in a growth promotion assay (Fig. 5). Among the 75 transformants tested from strain M24 only, 1 mutant which did not grow in the presence of ferric exochelin could be identified. The replacement of fxuA gene, as confirmed by PCR (Fig. 3B), resulted in exochelin hyperproduction, as monitored on CAS agar (Fig. 4).
FIG. 5.
Growth promotion assay with an ΔfxuA mutant. (A) Control plate inoculated with wild-type strain mc2155. (B) Test plate inoculated with mutant strain U3 ΔfxuA. Zones of growth surrounding the disks containing exochelin or mycobactin indicate utilization of siderophores by the tested strains. 1, mycobactin; 2, exochelin.
The various mutants generated during our study showed different growth characteristics. Compared to parent strain mc2155, mutants M24 and B1 are able to grow under conditions of iron depletion with a slightly increased lag phase because M24 can take up exochelin directly via the permease and B1 can use low-molecular-weight iron chelators like citrate via mycobactin (10). B3 and U3 are nearly completely unable to grow in the absence of an alternative iron supply because of their lack of mycobactin and exochelin or exochelin permease. Both strains, however, can grow under conditions of iron depletion when xenosiderophores are added. This observation indicates the existence of additional mechanisms of direct uptake of siderophores besides the exochelin-mycobactin route.
These new mutants and wild-type strains differ in the presence or absence of the endogenous siderophores exochelin and mycobactin as well as in the presence or absence of the exochelin permease (Table 3). By use of these strains in growth promotion experiments, it is possible to differentiate between siderophore iron supply processes that include ligand exchange with exochelin and/or mycobactin and those that exclude ligand exchange. In addition, it is now possible to differentiate between uptake via the exochelin permease and uptake via an alternative transport system. Consequently, the mutants can be used to generate an ideal screening system for the selection of siderophores that are able to enter the mycobacterial cell directly on the basis of simple and efficient growth promotion assays.
TABLE 3.
Phenotypic characteristics of the various strains of M. smegmatis generated and used in the present studya
| Siderophore biosynthesis and uptake system | mc2155 | M24 | B1 | B3 | U3 |
|---|---|---|---|---|---|
| Exochelin | + | + | − | − | + |
| Mycobactin | + | − | + | − | − |
| Exochelin permease | + | + | + | + | − |
+, present; −, deleted.
Several exogenous siderophores of different structural classes were tested with this new screening model. Exochelin and mycobactin were used as controls. The results are shown in Table 4. Six xenosiderophores showed the same effectiveness in growth promotion in all strains tested, indicating the utilization of the siderophore-bound iron which is independent of ligand exchange or exochelin permease. With the other 12 xenosiderophores tested, the growth responses of the wild-type strain and the mutants used in the test were different. These findings imply that ligand exchange with exochelin and/or mycobactin accounts for the process of iron uptake from these substrates or that the exochelin permease is involved in the uptake of the xenosiderophore.
DISCUSSION
Previously, Matzanke et al. (10) have shown that mycobacteria utilize a variety of xenosiderophores. Utilization of siderophore-bound iron occurs by diverse mechanisms which include ligand exchange between citrate and mycobactin or rapid reductive removal from ferricrocin. Whether ferricrocin is transported into the cytoplasm before reduction or the reduction occurs at the membrane remained unclear. The results presented by Matzanke et al. (11) imply a third uptake mechanism for rhizoferrin which excludes mycobactin as a temporary iron carrier. Moreover, the intracellular presence of small amounts of ferric rhizoferrin strongly suggests that the complex was transported into the cytoplasm as a whole. The complete permeation of the cell envelope by the siderophore is a prerequisite for the possible use of siderophore-antibiotic conjugates to circumvent the barrier problem of the relatively impermeable, hydrophobic cell envelopes of mycobacteria. These results were obtained by Mössbauer or electron spin resonance spectroscopy, which is usable only for single compounds.
In the present study, we have generated a set of mycobacterial mutant strains blocked in the biosynthesis of exochelin and mycobactin or in the transport of exochelin in order to establish a screening system. This set of strains allows testing of siderophores in a growth promotion assay for ligand exchange with exochelin and/or mycobactin or uptake by an alternative transport system.
Previously, Fiss et al. (4) identified four contiguous genes that may be involved in exochelin biosynthesis and uptake by M. smegmatis. One of these genes (fxbA) was shown to be involved in exochelin biosynthesis by complementation of a blocked mutant. On the basis of the similarities of the amino acid sequences of known cytoplasm membrane permeases to the deduced amino acid sequences of the three other genes (fxuA, fxuB, fxuC), the investigators suggested that these genes code for the subunits of the ferric exochelin permease.
The published sequences were used to replace fxbA and fxuA by the aminoglycoside phosphotransferase (aph) gene of Tn903. Functional studies confirmed that the isolated mutants represent the expected phenotypes. fxbA mutants are defective in exochelin biosynthesis, whereas fxuA mutants excrete significantly larger amounts of exochelin than the parent strain. This effect is due to defective ferriexochelin uptake, as demonstrated in growth promotion tests.
This new set of mutants were used in combination with their parent strain as well as a mutant blocked in biosynthesis of mycobactin in a growth promotion assay to 25 natural xenosiderophores which exhibit a wide range of different structural building blocks.
On the basis of the results obtained with this assay system, the tested siderophores can be divided into six different groups. (i) Coprogen, ferrichrysin, ferrirubin, and myxochelin E are not utilized by M. smegmatis mc2155. (ii) Iron utilization mediated by corynebactin, ferricrocin, ferrioxamine B, and ferrioxamine D1 depends on ligand exchange with exochelin or mycobactin. Strain B3, which lacks the ability to synthesize exochelin and mycobactin, and strain U3, which lacks the ability to synthesize mycobactin and exochelin permease, are unable to use these substrates. (iii) Iron bound to ferrioxamine D2 and ferrioxamine E could not be used by the strains that lack exochelin biosynthesis or exochelin permease. This indicates ligand exchange with exochelin but not with mycobactin. (iv) The results obtained with rhodotorulic acid and ferrioxamin G also suggest ligand exchange with exochelin but, additionally, suggest mycobactin-dependent utilization. (v) The growth of all strains except strain U3 showed excellent responses to amycolachrom and ferrithiocin. This finding implies that iron utilization via amycolachrom and ferrithiocin depends on the action of exochelin permease. (vi) Iron bound to aerobactin, arthrobactin, enterobactin, ferrichrome, ferrichrome A, myxochelin C, omibactin, rhizoferrin, and triacetylfusarinine was utilized by all strains, although with different levels of effectiveness. These results indicate that no ligand exchange with exochelin or mycobactin takes place and that the exochelin permease is not involved in uptake of these siderophores. In conclusion, this suggests the existence of specific uptake systems for these xenosiderophores, as already indicated for rhizoferrin (11). All these siderophores represent potential candidates for synthetic derivatization toward new antibiotic shuttle vectors.
ACKNOWLEDGMENTS
This work was supported by the German Ministry of Education and Research (BMBF grant 0311232) and the Grünenthal GmbH, Aachen, Germany
We thank G. Mrotzek and K. Hartung from the HKI Department of Cell and Molecular Biology for help with sequencing. We also thank B. F. Matzanke and P. F. Zipfel for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Braun V. Active transport of siderophore-mimicking antibacterials across the outer membrane. Drug Resist Updates. 1999;2:363–369. doi: 10.1054/drup.1999.0107. [DOI] [PubMed] [Google Scholar]
- 2.Braun V, Hantke K. Genetics of bacterial iron transport. In: Winkelman G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 107–138. [Google Scholar]
- 3.De Voss J J, Rutter K, Schroeder B G, Barry C E. Iron acquisition and metabolism by mycobacteria. J Bacteriol. 1999;181:4443–4451. doi: 10.1128/jb.181.15.4443-4451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiss E H, Yu S, Jacobs W R., Jr Identification of genes involved in the sequestration of iron in mycobacteria: the ferric exochelin biosynthetic and uptake pathways. Mol Microbiol. 1994;14:557–569. doi: 10.1111/j.1365-2958.1994.tb02189.x. [DOI] [PubMed] [Google Scholar]
- 5.Gobin J, Moore C H, Reeve J R, Jr, Wong D K, Gibson B W, Horwitz M A. Iron acquisition by Mycobacterium tuberculosis: isolation and characterization of a family of iron-binding exochelins. Proc Natl Acad Sci USA. 1995;92:5189–5193. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall M J, Ratledge C. A simple method for the production of mycobactin, the lipid-soluble siderophore, from mycobacteria. FEMS Microbiol Lett. 1982;15:133–136. [Google Scholar]
- 7.Lane S J, Marshall P S, Upton R J, Ratledge C, Ewing M. Novel extracellular mycobactins, the carboxymycobactins from Mycobacterium avium. Tetrahedron Lett. 1995;36:4129–4132. [Google Scholar]
- 8.Lane S J, Marshall P S, Upton R J, Ratledge C. Isolation and characterisation of caroxymycobactins as the second extracellular siderophores in Mycobacterium smegmatis. BioMetals. 1998;11:13–20. [Google Scholar]
- 9.Macham L P, Ratledge C, Nocton J C. Extracellular iron acquisition by mycobacteria: role of the exochelins and evidence against the participation of mycobactin. Infect Immun. 1975;12:1242–1251. doi: 10.1128/iai.12.6.1242-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzanke B F, Böhnke R, Möllmann U, Reissbrodt R, Schünemann V, Trautwein A X. Iron uptake and intracellular metal transfer in mycobacteria mediated by xenosiderophores. BioMetals. 1997;10:193–203. doi: 10.1023/a:1018351728081. [DOI] [PubMed] [Google Scholar]
- 11.Matzanke B F, Böhnke R, Möllmann U, Schünemann V, Schumann G, Trautwein A X, Winkelmann G. Transport and utilization of rhizoferrin bound iron in Mycobacterium smegmatis. BioMetals. 1999;12:315–321. doi: 10.1023/a:1009274415607. [DOI] [PubMed] [Google Scholar]
- 12.Ratledge C. Iron metabolism. In: Ratledge C, Dale J, editors. Mycobacteria: molecular biology and virulence. Oxford, United Kingdom: Blackwell Science Publishers Ltd.; 1999. pp. 260–286. [Google Scholar]
- 13.Ratledge C, Hall M J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971;108:314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratledge C, Marshall B J. Iron transport in Mycobacterium smegmatis. Role of mycobactin. Biochim Biophys Acta. 1972;279:58–74. doi: 10.1016/0304-4165(72)90241-3. [DOI] [PubMed] [Google Scholar]
- 15.Schwyn B, Nellands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 16.Sharman G J, Williams D H, Ewing D F, Ratledge C. Isolation, purification and structure of exochelin MS, the extracellular siderophore from Mycobacterium smegmatis. Biochem J. 1995;305:187–196. doi: 10.1042/bj3050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharman G J, Williams D H, Ewing D F, Ratledge C. Isolation, purification and structure of exochelin MN, the extracellular siderophore from Mycobacterium neoaurum. Chem Biol. 1995;2:553–561. doi: 10.1016/1074-5521(95)90189-2. [DOI] [PubMed] [Google Scholar]
- 18.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 19.Snow G A. The structure of mycobactin P, a growth factor for Mycobacterium johnei, and the significance of its iron complex. Biochem J. 1965;94:160–165. doi: 10.1042/bj0940160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler R P, Ratledge C. Metabolism of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 353–385. [Google Scholar]
- 21.Yu S, Fiss E, Jacobs W R., Jr Analysis of the exochelin locus in Mycobacterium smegmatis: biosynthesis genes have homology with genes of the peptide synthase family. J Bacteriol. 1998;180:4676–4685. doi: 10.1128/jb.180.17.4676-4685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Arceneaux J E L, Beggs M L, Byers B R, Eisenach K D, Lungrigan M D. Exochelin genes in Mycobacterium smegmatis: identification of an ABC transporter and two non-ribosomal peptide synthase genes. Mol Microbiol. 1998;29:629–639. doi: 10.1046/j.1365-2958.1998.00961.x. [DOI] [PubMed] [Google Scholar]